High Insulin Levels in KK-Ay Diabetic Mice Cause Increased Cortical Bone Mass and Impaired Trabecular Micro-Structure
Abstract
:1. Introduction
2. Results
2.1. Serum Insulin, Glucose and Osteocalcin Levels in KK-Ay Mice

2.2. Areal BMD in KK-Ay Diabetic Mice
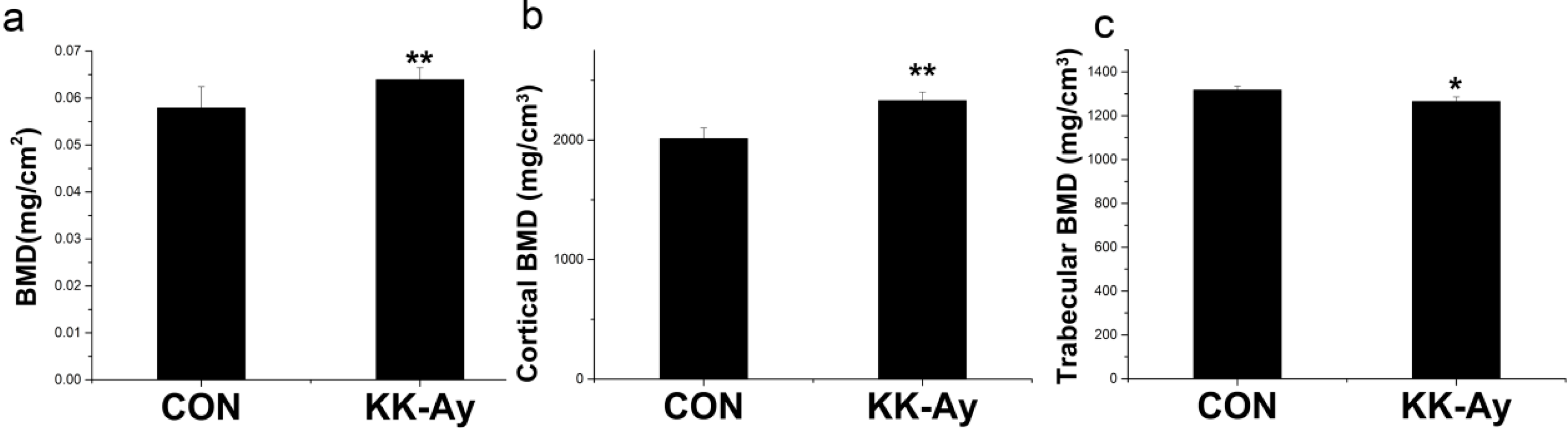
2.3. Volumetric BMD in KK-Ay Diabetic Mice
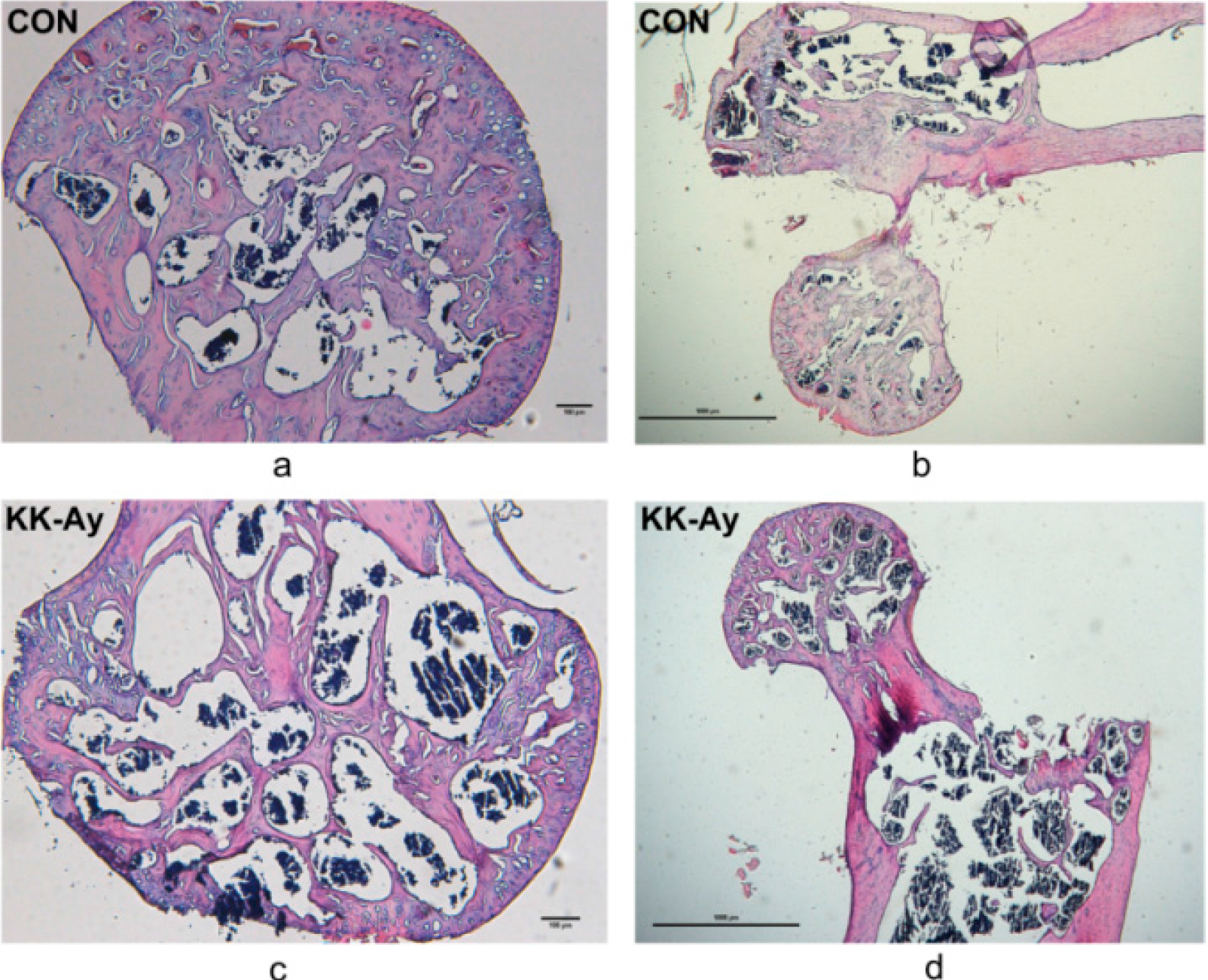
2.4. Histological Analyses
2.5. Bone Micro-Structure Analyses

2.6. Bone Metabolism
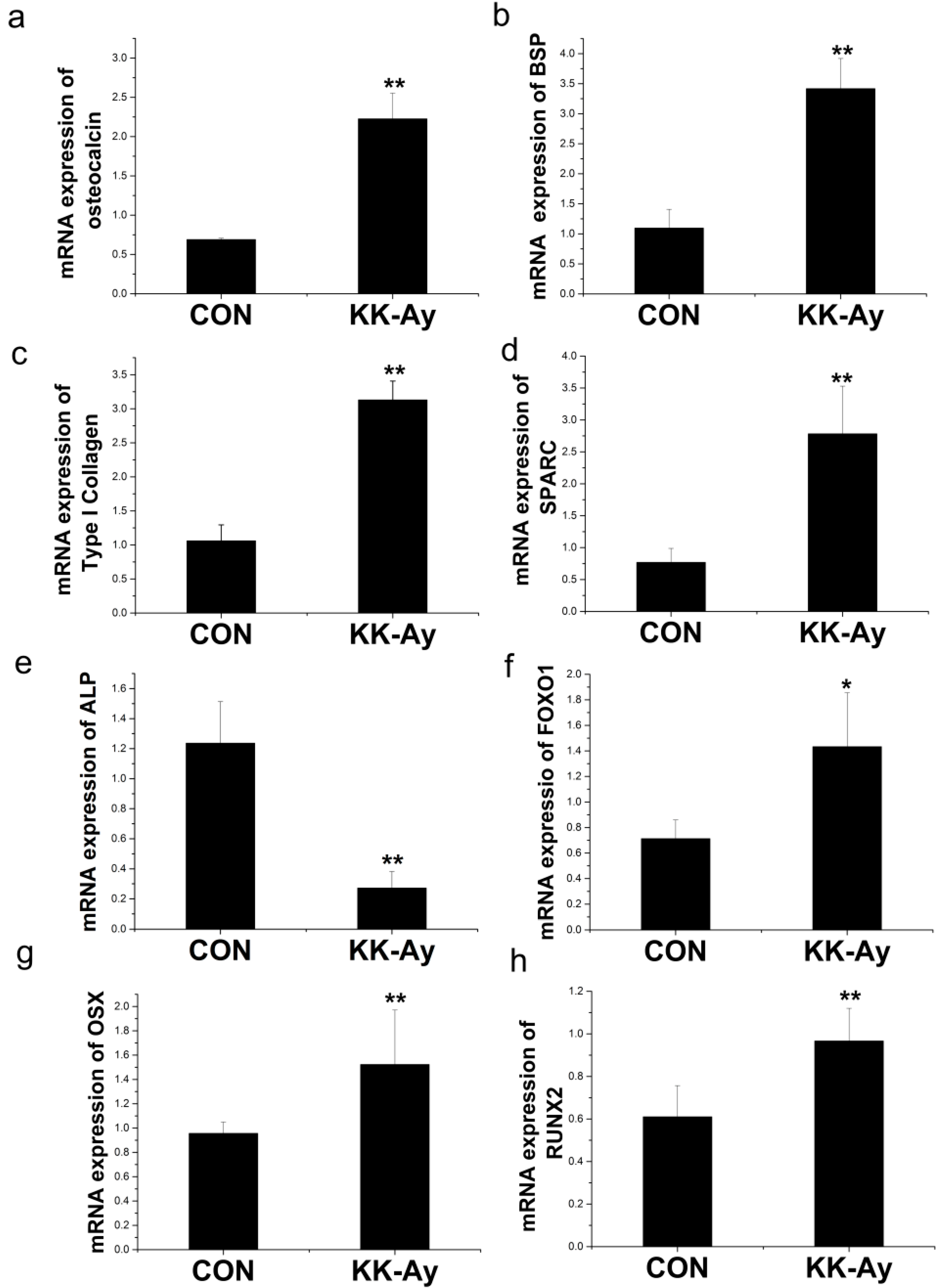
2.6.1. Enhanced Osteoblast-Related Gene Expression in KK-Ay Diabetic Mice
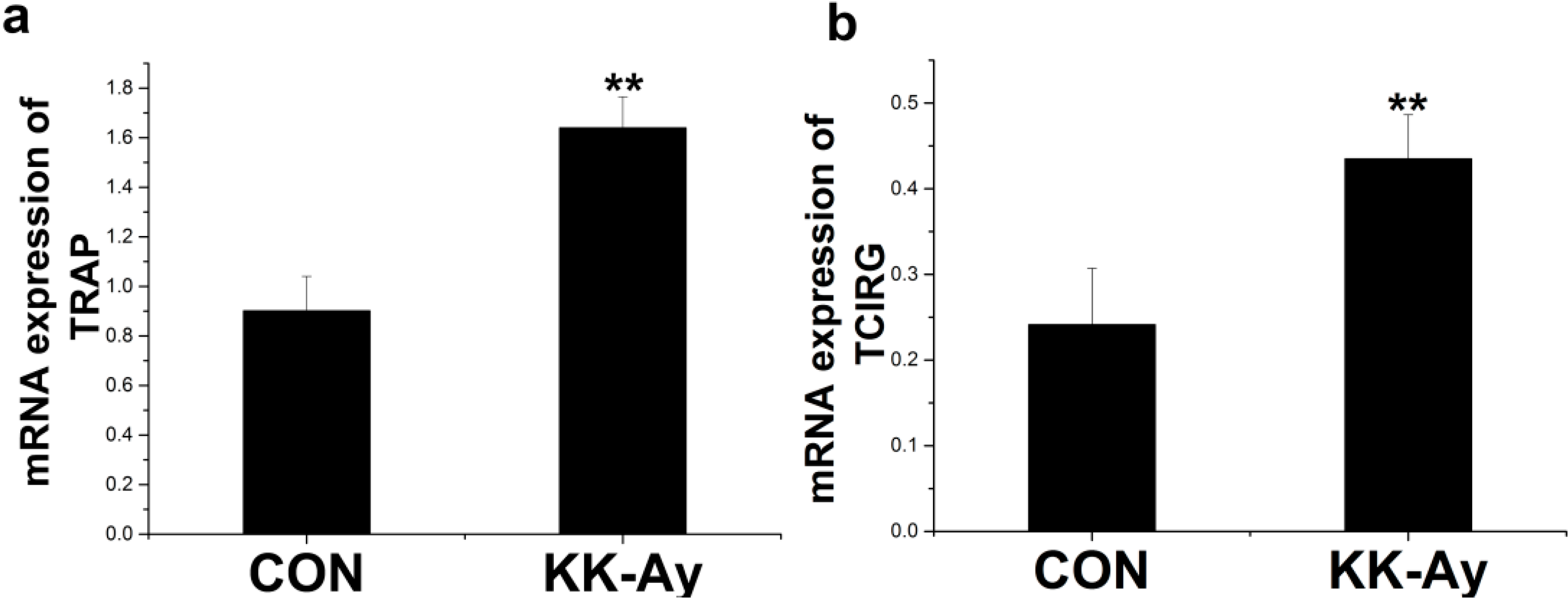
2.6.2. Enhanced Osteoclast-Related Gene Expression in KK-Ay Diabetic Mice
2.7. Correlation Analyses
| Parameter | Insulin | Glucose |
|---|---|---|
| n = 17 | n = 17 | |
| aBMD of the total femur | 0.62 ** | 0.59 * |
| Cortical vBMD | 0.90 ** | |
| Trabecular vBMD | −0.82 * | |
| Ct Th | 0.75 * | |
| Tb Th | −0.91 * | −0.73 * |
| BV/TV | −0.84 ** | −0.75 ** |
| Tb N | −0.88 * | |
| Tb Sp | 0.77 * |
| Parameter | Serum Insulin ( n = 10) | Serum Glucose ( n = 10) |
|---|---|---|
| Osteoblast-related gene | ||
| Osteocalcin | 0.96 ** | |
| ALP | −0.94 ** | |
| BSP | 0.96 ** | |
| Collagen I | 0.98 ** | |
| SPARC | 0.96 ** | 0.76 * |
| FOXO1 | 0.82 * | |
| RUNX2 | 0.80 * | |
| OSX | 0.69 * | |
| Osteoclast-related gene | ||
| TRAP | 0.97 ** |
3. Discussion
4. Experiential Section
4.1. Animals and Treatment
4.2. Biochemical Analyses
4.3. Bone Mineral Density Analyses
4.4. Micro-Computed Tomography Measurements
4.5. Histological Sections and Staining
4.6. RNA Isolation and Quantitative Polymerase Chain Reaction (qPCR) Analyses
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| (Sequence 5'–3') | (Sequence 5'–3') | |
| Osteocalcin | TGCAAAGCCCAGCGACTCT | AGTCCATTGTTGAGGTAGCG |
| ALP | TCTCCAGACCCTGCAACCTC | CATCCTGAGCAGACCTGGTC |
| BSP | GAAAATGGAGACGGCGATAG | ACCCGAGAGTGTGGAAAGTG |
| Collagen I | TTGACCCTAACCAAGGATGC | CACCCCTTCTGCGTTGTATT |
| SPARC | ATCCAGAGCTGTGGCACACA | GGAAAGAAACGCCCGAAGA |
| TCIRG | GATCATGGGCTCTATGTTCCG | ACCTGCCCGCTGCACTTCTT |
| TRAP | CAGCAGCCAAGGAGGACTAC | ACATAGCCCACACCGTTCTC |
| FOXO1 | AGAGGCTCACCCTGTCGCAGA | GTGAAGGGACAGATTGTGGCGA |
| RUNX2 | TCCTGGTCACAATGGGATACC | ATCTCCTGGGTCACCCTTAGG |
| OSX | ACTGGCTAGGTGGTGGTCAG | GGTAGGGAGCTGGGTTAAGG |
| β-Actin | GCTCTTTTCCAGCCTTCCTT | AGGTCTTTACGGATGTCAACG |
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Statement of Human and Animal Rights
Conflicts of Interest
References
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [PubMed]
- Takagi, S.; Miura, T.; Yamashita, T.; Ando, N.; Nakao, H.; Ishihara, E.; Ishida, T. Characteristics of diabetic osteopenia in KK-Ay diabetic mice. Biol. Pharm. Bull. 2012, 35, 438–443. [Google Scholar] [PubMed]
- Burghardt, A.J.; Issever, A.S.; Schwartz, A.V.; Davis, K.A.; Masharani, U.; Majumdar, S.; Link, T.M. High-resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2010, 95, 5045–5055. [Google Scholar] [PubMed]
- Bhattoa, H.P.; Onyeka, U.; Kalina, E.; Balogh, A.; Paragh, G.; Antal-Szalmas, P.; Kaplar, M. Bone metabolism and the 10-year probability of hip fracture and a major osteoporotic fracture using the country-specific frax algorithm in men over 50 years of age with type 2 diabetes mellitus: A case-control study. Clin. Rheumatol. 2013, 32, 1161–1167. [Google Scholar] [PubMed]
- Osório, J. Bone: BMD and fracture risk in T2DM—Clarifying a paradox. Nat. Rev. Endocrinol. 2011, 7, 376–376. [Google Scholar] [PubMed]
- Sosa, M.; Dominguez, M.; Navarro, M.C.; Segarra, M.C.; Hernandez, D.; de Pablos, P.; Betancor, P. Bone mineral metabolism is normal in non-insulin-dependent diabetes mellitus. J. Diabetes Complicat. 1996, 10, 201–205. [Google Scholar] [PubMed]
- Zhang, Y.; Yang, J.H. Activation of the PI3k/Akt pathway by oxidative stress mediates high glucose-induced increase of adipogenic differentiation in primary rat osteoblasts. J. Cell. Biochem. 2013, 114, 2595–2602. [Google Scholar] [PubMed]
- Wittrant, Y.; Gorin, Y.; Woodruff, K.; Horn, D.; Abboud, H.E.; Mohan, S.; Abboud-Werner, S.L. High d(+)glucose concentration inhibits rankl-induced osteoclastogenesis. Bone 2008, 42, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Ling, O.; Zillikens, M.C.; Dehghan, A.; Buitendijk, G.H.; Castaño-Betancourt, M.C.; Estrada, K.; Stolk, L.; Oei, E.H. High bone mineral density and fracture risk in type 2 diabetes as skeletal complications of inadequate glucose control. Diabetes Care 2013, 36, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, X.; Wang, W.; Liu, J. Insulin stimulates osteoblast proliferation and differentiation through ERK and PI3K in MG-63 cells. Cell Biochem. Funct. 2010, 28, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Freude, T.; Braun, K.F.; Haug, A.; Pscherer, S.; Stockle, U.; Nussler, A.K.; Ehnert, S. Hyperinsulinemia reduces osteoblast activity in vitro via upregulation of TGF-β. J. Mol. Med. 2012, 90, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, R.J.; Karim, L.; Calley, V.I.; Bouxsein, M.L. A review of rodent models of type 2 diabetic skeletal fragility. J. Bone Miner. Res. 2014, 29, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Nordentoft, I.; Jeppesen, P.B.; Hong, J.; Abudula, R.; Hermansen, K. Increased insulin sensitivity and changes in the expression profile of key insulin regulatory genes and β cell transcription factors in diabetic KKAY-mice after feeding with a soy bean protein rich diet high in isoflavone content. J. Agric. Food Chem. 2008, 56, 4377–4385. [Google Scholar] [CrossRef] [PubMed]
- Hadjidakis, D.J.; Androulakis, I. Bone remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Shu, A.; Yin, M.T.; Stein, E.; Cremers, S.; Dworakowski, E.; Ives, R.; Rubin, M.R. Bone structure and turnover in type 2 diabetes mellitus. Osteoporos. Int. 2012, 23, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Melton, L.J., 3rd.; Riggs, B.L.; Leibson, C.L.; Achenbach, S.J.; Camp, J.J.; Bouxsein, M.L.; Atkinson, E.J.; Robb, R.A.; Khosla, S. A bone structural basis for fracture risk in diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 4804–4809. [Google Scholar] [CrossRef] [PubMed]
- Petit, M.A.; Paudel, M.L.; Taylor, B.C.; Hughes, J.M.; Strotmeyer, E.S.; Schwartz, A.V.; Cauley, J.A.; Zmuda, J.M.; Hoffman, A.R.; Ensrud, K.E. Bone mass and strength in older men with type 2 diabetes: The osteoporotic fractures in men study. J. Bone Miner. Res. 2010, 25, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.M.; Giangregorio, L.M.; Atkinson, S.A.; Beattie, K.A.; Inglis, D.; Ioannidis, G.; Punthakee, Z.; Adachi, J.D.; Papaioannou, A. Association of larger holes in the trabecular bone at the distal radius in postmenopausal women with type 2 diabetes mellitus compared to controls. Arthritis Care Res. 2012, 64, 83–91. [Google Scholar] [CrossRef]
- Lecka-Czernik, B.; Stechschulte, L.A.; Czernik, P.J.; Dowling, A.R. High bone mass in adult mice with diet-induced obesity results from a combination of initial increase in bone mass followed by attenuation in bone formation:Implications for high bone mass and decreased bone quality in obesity. Mol. Cell. Endocrinol. 2015, 7, 2–7. [Google Scholar]
- Malvi, P.; Piprode, V.; Chaube, B.; Pote, S.T.; Mittal, M.; Chattopadhyay, N.; Wani, M.R.; Bhat, M.K. High fat diet promotes achievement of peak bone mass in young rats. Biochem. Biophys. Res. Commun. 2014, 455, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Patsch, J.M.; Kiefer, F.W.; Varga, P.; Pail, P.; Rauner, M.; Stupphann, D.; Resch, H.; Moser, D.; Zysset, P.K.; Stulnig, T.M.; et al. Increased bone resorption and impaired bone microarchitecture in short-term and extended high-fat diet-induced obesity. Metabolism 2011, 60, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Kook, S.H.; Heo, J.S.; Lee, J.C. Crucial roles of canonical runx2-dependent pathway on wnt1-induced osteoblastic differentiation of human periodontal ligament fibroblasts. Mol. Cell. Biochem. 2015, 402, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C. Wnt signaling and osteoporosis. Maturitas 2014, 78, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Hamann, C.; Kirschner, S.; Günther, K.-P.; Hofbauer, L.C. Bone, sweet bone—Osteoporotic fractures in diabetes mellitus. Nat. Rev. Endocrinol. 2012, 8, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Emrich, L.J.; Shlossman, M.; Genco, R.J. Periodontal disease in non-insulin-dependent diabetes mellitus. J. Periodontol. 1991, 62, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Gautam, J.; Choudhary, D.; Khedgikar, V.; Kushwaha, P.; Singh, R.S.; Singh, D.; Tiwari, S.; Trivedi, R. Micro-architectural changes in cancellous bone differ in female and male c57bl/6 mice with high-fat diet-induced low bone mineral density. Br. J. Nutr. 2014, 111, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, C.; Zhang, X.; Ye, F.; Yang, J. High Insulin Levels in KK-Ay Diabetic Mice Cause Increased Cortical Bone Mass and Impaired Trabecular Micro-Structure. Int. J. Mol. Sci. 2015, 16, 8213-8226. https://doi.org/10.3390/ijms16048213
Fu C, Zhang X, Ye F, Yang J. High Insulin Levels in KK-Ay Diabetic Mice Cause Increased Cortical Bone Mass and Impaired Trabecular Micro-Structure. International Journal of Molecular Sciences. 2015; 16(4):8213-8226. https://doi.org/10.3390/ijms16048213
Chicago/Turabian StyleFu, Cen, Xiaolin Zhang, Fei Ye, and Jianhong Yang. 2015. "High Insulin Levels in KK-Ay Diabetic Mice Cause Increased Cortical Bone Mass and Impaired Trabecular Micro-Structure" International Journal of Molecular Sciences 16, no. 4: 8213-8226. https://doi.org/10.3390/ijms16048213





