Role of Long-Range Protein Dynamics in Different Thymidylate Synthase Catalyzed Reactions
Abstract
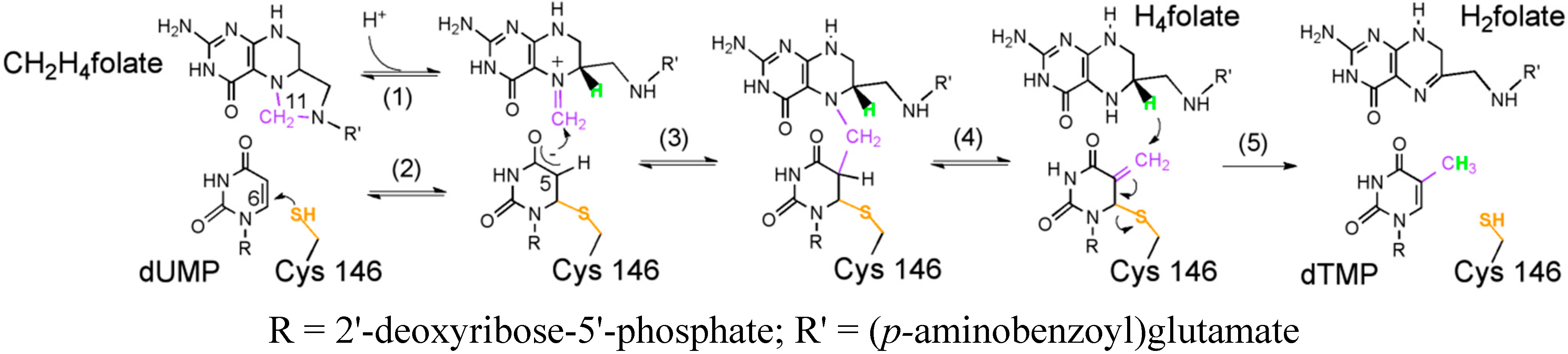
:1. Introduction
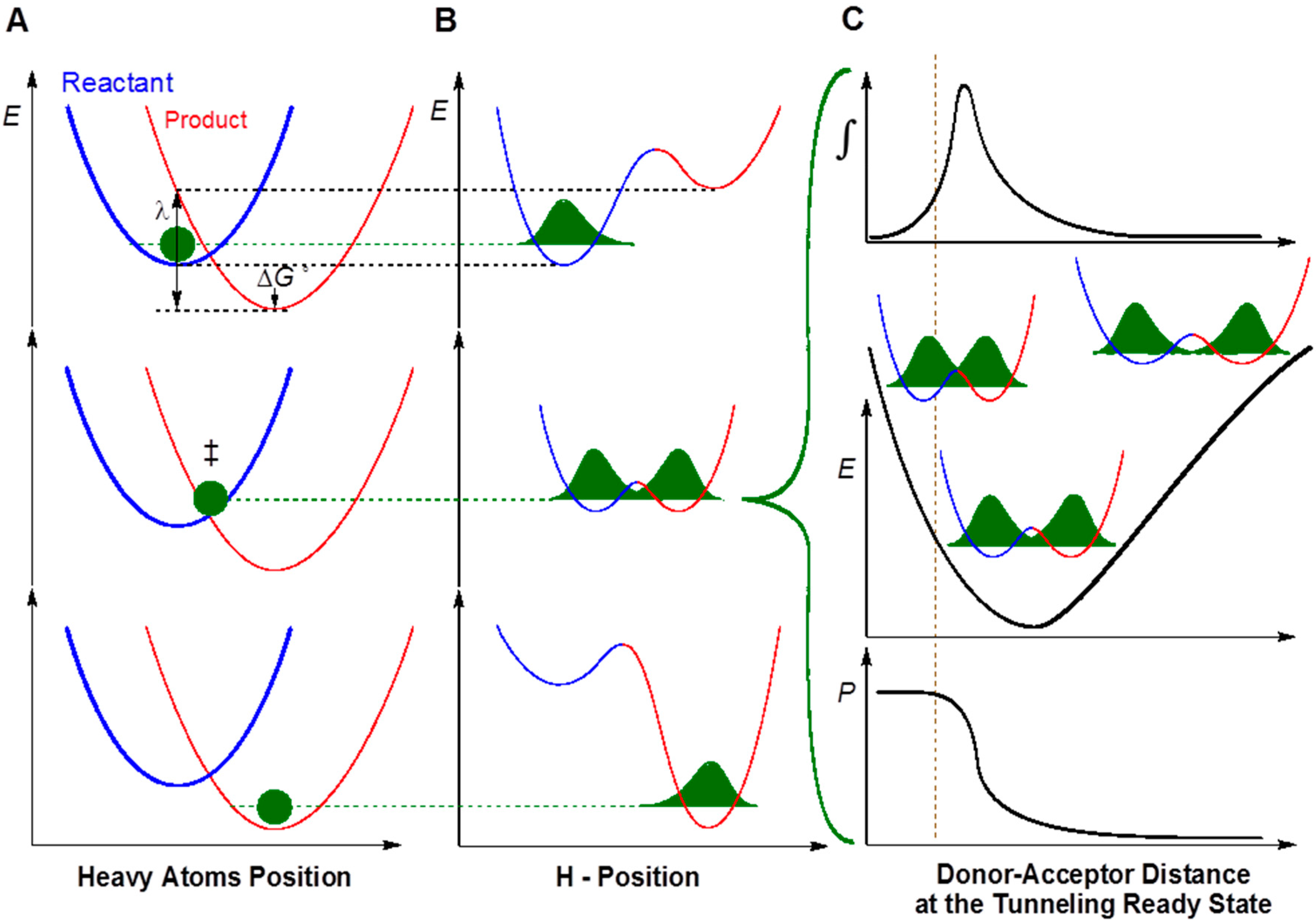
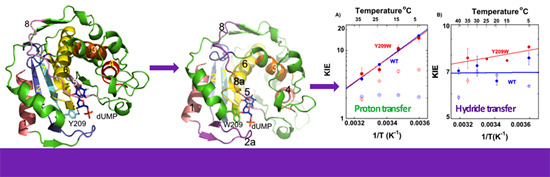
2. Results and Discussion
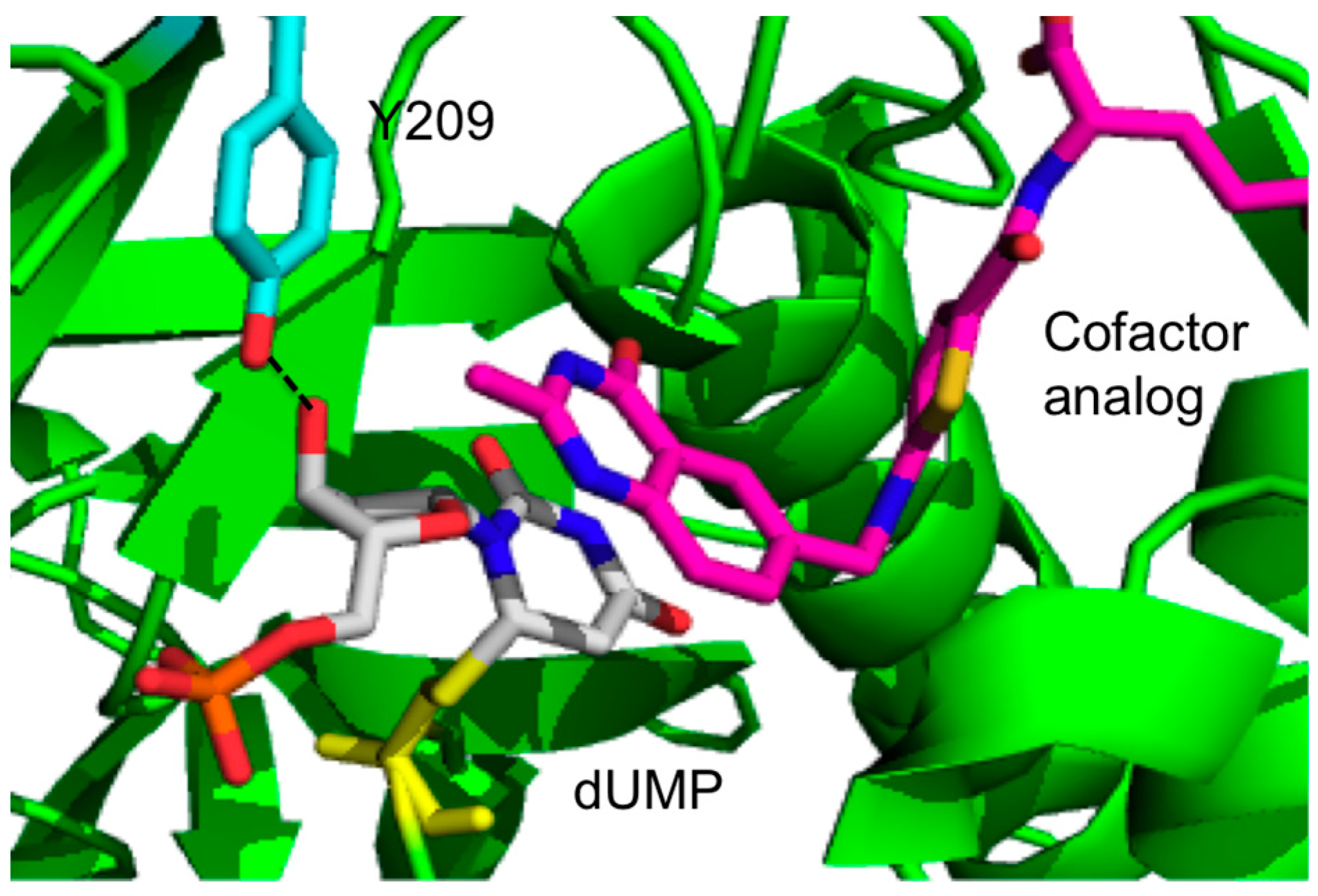
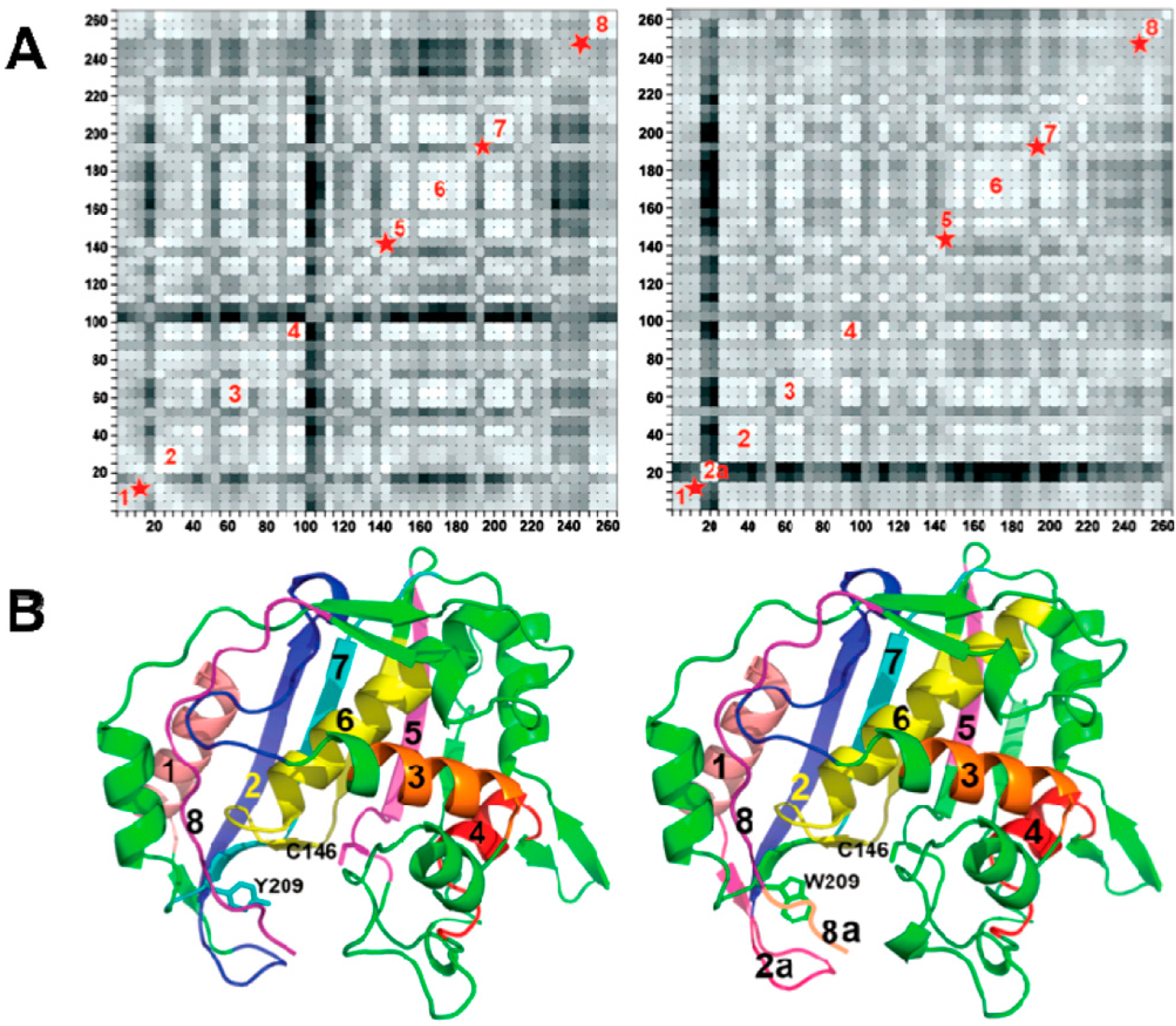
2.1. Binding Mechanism of Y209W Mutant


2.2. Temperature Dependence of Intrinsic KIEs in the Y209W Mutant
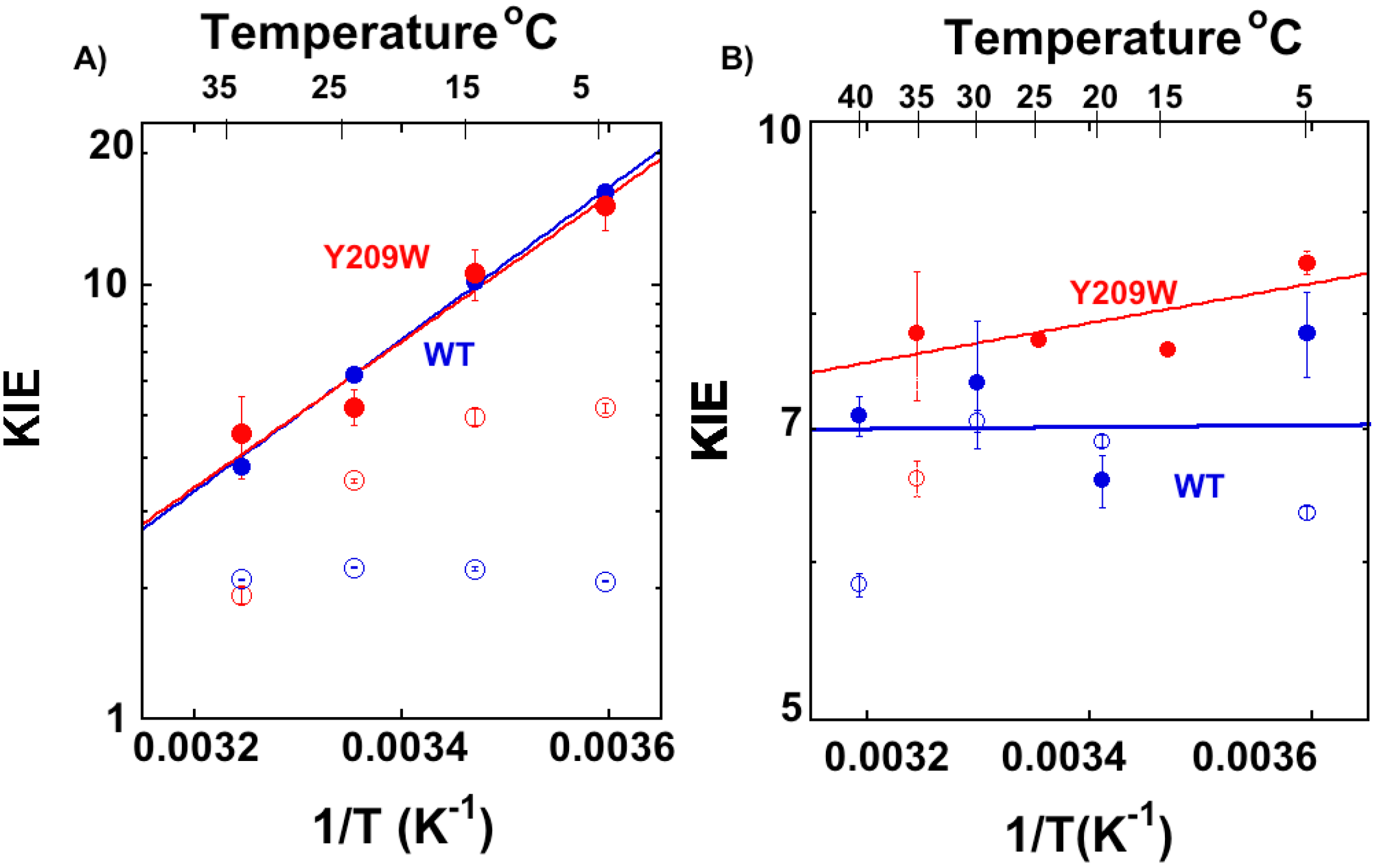
| Proton Abstraction | Hydride Transfer | |||
|---|---|---|---|---|
| WT a | Y209W | WT b | Y209W c | |
| AH/AT | 8.3 (±1.0) ×10−6 | 1.4 (±0.1) ×10−6 | 6.8 (±2.8) | 3.6 (±0.9) |
| ∆Ea H–T (kcal/mol) | −8.0 (±0.1) | −7.70 (±0.40) | −0.02 (±0.25) | −0.5 (±0.1) |
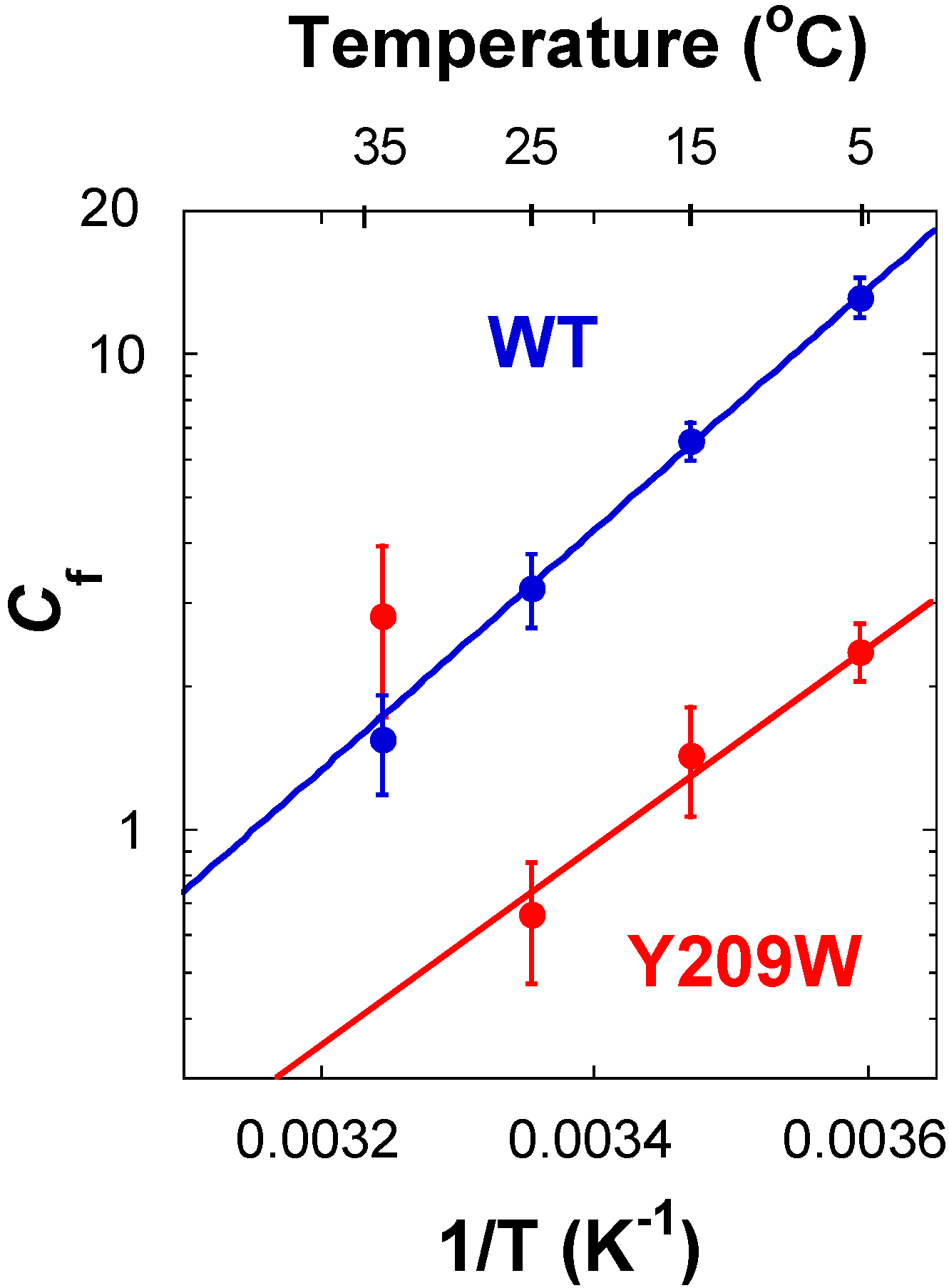
2.3. Effect of Y209W Mutation on Catalytic Steps Other than the Two C–H Activations
3. Experimental Section
3.1. Materials
3.2. Synthesis of [2-14C,5-2H] dUMP(>99.5% D)
3.3. Competitive KIEs on the Proton Abstraction (Step 4 in Scheme 1)
4. Conclusions
Supplementary Files
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wong, K.F.; Selzer, T.; Benkovic, S.J.; Hammes-Schiffer, S. Impact of distal mutations on the network of coupled motions correlated to hydride transfer in dihydrofolate reductase. Proc. Natl. Acad. Sci. USA 2005, 102, 6807–6812. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Goodey, N.M.; Benkovic, S.J.; Kohen, A. Coordinated effects of distal mutations on environmentally coupled tunneling in dihydrofolate reductase. Proc. Natl. Acad. Sci. USA 2006, 103, 15753–15758. [Google Scholar] [CrossRef] [PubMed]
- Francis, K.; Stojković, V.; Kohen, A. Preservation of protein dynamics in dihydrofolate reductase evolution. J. Biol. Chem. 2013, 288, 35961–35968. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Sen, A.; Francis, K.; Kohen, A. Extension and limits of the network of coupled motions correlated to hydride transfer in dihydrofolate reductase. J. Am. Chem. Soc. 2014, 136, 2575–2582. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, M.; Li, L.; Wing, C.; Schramm, V.L. Altered thermodynamics from remote mutations altering human toward bovine purine nucleoside phosphorylase. Biochemistry 2008, 47, 2559–2564. [Google Scholar] [CrossRef] [PubMed]
- Saen-Oon, S.; Ghanem, M.; Schramm, V.L.; Schwartz, S.D. Remote mutations and active site dynamics correlate with catalytic properties of purine nucleoside phosphorylase. Biophys. J. 2008, 94, 4078–4088. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.P.; Tomchick, D.R.; Klinman, J.P. Enzyme structure and dynamics affect hydrogen tunneling: The impact of a remote side chain (I553) in soybean lipoxygenase-1. Proc. Natl. Acad. Sci. USA 2008, 105, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Carreras, C.W.; Santi, D.V. The catalytic mechanism and structure of thymidylate synthase. Annu. Rev. Biochem. 1995, 64, 721–762. [Google Scholar] [CrossRef] [PubMed]
- Kanaan, N.; Marti, S.; Moliner, V.; Kohen, A. A quantum mechanics/molecular mechanics study of the catalytic mechanism of the thymidylate synthase. Biochemistry 2007, 46, 3704–3713. [Google Scholar] [CrossRef] [PubMed]
- Phan, J.; Steadman, D.J.; Koli, S.; Ding, W.C.; Minor, W.; Dunlap, R.B.; Berger, S.H.; Lebioda, L. Structure of human thymidylate synthase suggests advantages of chemotherapy with noncompetitive inhibitors. J. Biol. Chem. 2001, 276, 14170–14177. [Google Scholar] [PubMed]
- Kanaan, N.; Marti, S.; Moliner, V.; Kohen, A. QM/MM study of thymidylate synthase: Enzymatic Motions and the temperature dependence of the rate limiting step. J. Phys. Chem. A 2009, 113, 2176–2182. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sapienza, P.J.; Abeysinghe, T.; Luzum, C.; Lee, A.L.; Finer-Moore, J.S.; Stroud, R.M.; Kohen, A. Mg2+ binds to the surface of thymidylate synthase and affects hydride transfer at the interior active site. J. Am. Chem. Soc. 2013, 135, 7583–7592. [Google Scholar] [CrossRef] [PubMed]
- Finer-Moore, J.S.; Santi, D.V.; Stroud, R.M. Lessons and conclusions from dissecting the mechanism of a bisubstrate enzyme: Thymidylate synthase mutagenesis, function, and structure. Biochemistry 2002, 42, 248–256. [Google Scholar] [CrossRef]
- Newby, Z.; Lee, T.T.; Morse, R.J.; Liu, Y.; Liu, L.; Venkatraman, P.; Santi, D.V.; Finer-Moore, J.S.; Stroud, R.M. The role of protein dynamics in thymidylate synthase catalysis: Variants of conserved 2'-deoxyuridine 5'-monophosphate (dUMP)-binding Tyr-261. Biochemistry 2006, 45, 7415–7428. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Abeysinghe, T.; Finer-Moore, J.S.; Stroud, R.M.; Kohen, A. A remote mutation affects the hydride transfer by disrupting concerted protein motions in thymidylate synthase. J. Am. Chem. Soc. 2012, 134, 17722–17730. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kohen, A. Thymidylate synthase catalyzed H-transfers: Two chapters in one tale. J. Am. Chem. Soc. 2010, 132, 9820–9825. [Google Scholar] [CrossRef] [PubMed]
- Mishanina, T.V.; Koehn, E.M.; Conrad, J.A.; Palfey, B.A.; Lesley, S.A.; Kohen, A. Trapping of an intermediate in the reaction catalyzed by flavin-dependent thymidylate synthase. J. Am. Chem. Soc. 2012, 134, 4442–4448. [Google Scholar] [CrossRef] [PubMed]
- Nagel, Z.D.; Klinman, J.P. Update 1 of: Tunneling and dynamics in enzymatic hydride transfer. Chem. Rev. 2010, 110, PR41–PR67. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Roston, D.; Kohen, A. Experimental and theoretical studies of enzyme-catalyzed hydrogen-transfer reactions. Adv. Protein Chem. Struct. Biol. 2012, 87, 155–180. [Google Scholar] [PubMed]
- Klinman, J.P.; Kohen, A. Hydrogen tunneling links protein dynamics to enzyme catalysis. Annu. Rev. Biochem. 2013, 82, 471–496. [Google Scholar] [CrossRef] [PubMed]
- Loveridge, E.J.; Allemann, R.K. The temperature dependence of the kinetic isotope effects of dihydrofolate reductase from thermotoga maritima is influenced by intersubunit interactions. Biochemistry 2010, 49, 5390–5396. [Google Scholar] [CrossRef] [PubMed]
- Sutcliffe, M.J.; Masgrau, L.; Roujeinikova, A.; Johannissen, L.O.; Hothi, P.; Basran, J.; Ranaghan, K.E.; Mulholland, A.J.; Leys, D.; Scrutton, N.S. Hydrogen tunnelling in enzyme-catalyzed H-transfer reactions: Flavoprotein and quinoprotein systems. Philos. Trans. R. Soc. B 2006, 361, 1375–1386. [Google Scholar] [CrossRef]
- Sen, A.; Kohen, A. Enzymatic tunneling and kinetic isotope effects: Chemistry at the crossroads. J. Phys. Org. Chem. 2010, 23, 613–619. [Google Scholar] [CrossRef]
- Roston, D.; Islam, Z.; Kohen, A. Isotope effects as probes for enzyme catalyzed hydrogen-transfer reactions. Molecules 2013, 18, 5543–5567. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Hong, B.; Mihai, C.; Kohen, A. Vibrationally enhanced hydrogen tunneling in the Escherichia coli thymidylate synthase catalyzed reaction. Biochemistry 2004, 43, 1998–2006. [Google Scholar] [CrossRef] [PubMed]
- Roston, D.; Cheatum, C.M.; Kohen, A. Hydrogen donor–acceptor fluctuations from kinetic isotope effects: A phenomenological model. Biochemistry 2012, 51, 6860–6870. [Google Scholar] [CrossRef] [PubMed]
- Stojkovic, V.; Perissinotti, L.L.; Willmer, D.; Benkovic, S.J.; Kohen, A. Effects of the donor–acceptor distance and dynamics on hydride tunneling in the dihydrofolate reductase catalyzed reaction. J. Am. Chem. Soc. 2012, 134, 1738–1745. [Google Scholar] [CrossRef] [PubMed]
- Kohen, A.; Cannio, R.; Bartolucci, S.; Klinman, J.P.; Klinman, J.P. Enzyme dynamics and hydrogen tunnelling in a thermophilic alcohol dehydrogenase. Nature 1999, 399, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Yahashiri, A.; Howell, E.E.; Kohen, A. Tuning of the H-transfer coordinate in primitive versus well-evolved enzymes. Chem. Phys. Chem. 2008, 9, 980–982. [Google Scholar] [PubMed]
- Stroud, R.M.; Finer-Moore, J.S. Conformational dynamics along an enzymatic reaction pathway: Thymidylate synthase, “the movie”. Biochemistry 2003, 42, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Northrop, D.B. Enzyme Mechanisms from Isotope Effects; Cook, P.F., Ed.; CRC Press: Boca Raton, FL, USA, 1991; pp. 181–202. [Google Scholar]
- Cook, P.F.; Cleland, W.W. In Enzyme Kinetics and Mechanism; Garland Science: London, UK; New York, NY, USA, 2007; pp. 253–324. [Google Scholar]
- Cook, P.F. Enzyme Mechanisms from Isotope Effects; Cook, P.F., Ed.; CRC Press: Boca Raton, FL, USA, 1991; pp. 203–320. [Google Scholar]
- Spencer, H.T.; Villafranca, J.E.; Appleman, J.R. Kinetic scheme for thymidylate synthase from Escherichia coli: Determination from measurements of ligand binding, primary and secondary isotope effects, and pre-steady-state catalysis. Biochemistry 1997, 36, 4212–4222. [Google Scholar] [CrossRef] [PubMed]
- Northrop, D.B. Determining the Absolute Magnitude of Hydrogen Isotope Effects; University Park Press: Baltimore, MD, USA, 1977; pp. 122–152. [Google Scholar]
- Changchien, L.-M.; Garibian, A.; Frasca, V.; Lobo, A.; Maley, G.F.; Maley, F. High-level expression of Escherichia coli and Bacillus subtilis thymidylate synthases. Protein Expr. Purif. 2000, 19, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Hayatsu, H.; Wataya, Y.; Kai, K.; Iida, S. Reaction of sodium bisulfite with uracil, cytosine, and their derivatives. Biochemistry 1970, 9, 2858–2865. [Google Scholar] [CrossRef] [PubMed]
- Wataya, Y.; Hayatsu, H. Effect of amines on the bisulfite-catalyzed hydrogen isotope exchange at the 5 position of uridine. Biochemistry 1972, 11, 3583–3588. [Google Scholar] [CrossRef] [PubMed]
- Wataya, Y.; Hayatsu, H.; Kawazoe, Y. Cysteine-catalyzed hydrogen isotope exchange at the 5 position of uridylic acid. J. Am. Chem. Soc. 1972, 94, 8927–8928. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.; Maley, F.; Kohen, A. Role of Y94 in proton and hydride transfers catalyzed by thymidylate synthase. Biochemistry 2007, 46, 14188–14197. [Google Scholar] [CrossRef] [PubMed]
- Melander, L.; Saunders, W.H. Reaction Rates of Isotopic Molecules; R.E. Krieger Publication Co.: Malabar, FL, USA, 1987. [Google Scholar]
- Northrop, D.B. Steady-state analysis of kinetic isotope effects in enzymic reactions. Biochemistry 1975, 14, 2644–2651. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abeysinghe, T.; Kohen, A. Role of Long-Range Protein Dynamics in Different Thymidylate Synthase Catalyzed Reactions. Int. J. Mol. Sci. 2015, 16, 7304-7319. https://doi.org/10.3390/ijms16047304
Abeysinghe T, Kohen A. Role of Long-Range Protein Dynamics in Different Thymidylate Synthase Catalyzed Reactions. International Journal of Molecular Sciences. 2015; 16(4):7304-7319. https://doi.org/10.3390/ijms16047304
Chicago/Turabian StyleAbeysinghe, Thelma, and Amnon Kohen. 2015. "Role of Long-Range Protein Dynamics in Different Thymidylate Synthase Catalyzed Reactions" International Journal of Molecular Sciences 16, no. 4: 7304-7319. https://doi.org/10.3390/ijms16047304
APA StyleAbeysinghe, T., & Kohen, A. (2015). Role of Long-Range Protein Dynamics in Different Thymidylate Synthase Catalyzed Reactions. International Journal of Molecular Sciences, 16(4), 7304-7319. https://doi.org/10.3390/ijms16047304