Hair-Growth-Promoting Effect of Conditioned Medium of High Integrin α6 and Low CD 71 (α6bri/CD71dim) Positive Keratinocyte Cells
Abstract
:1. Introduction
2. Results
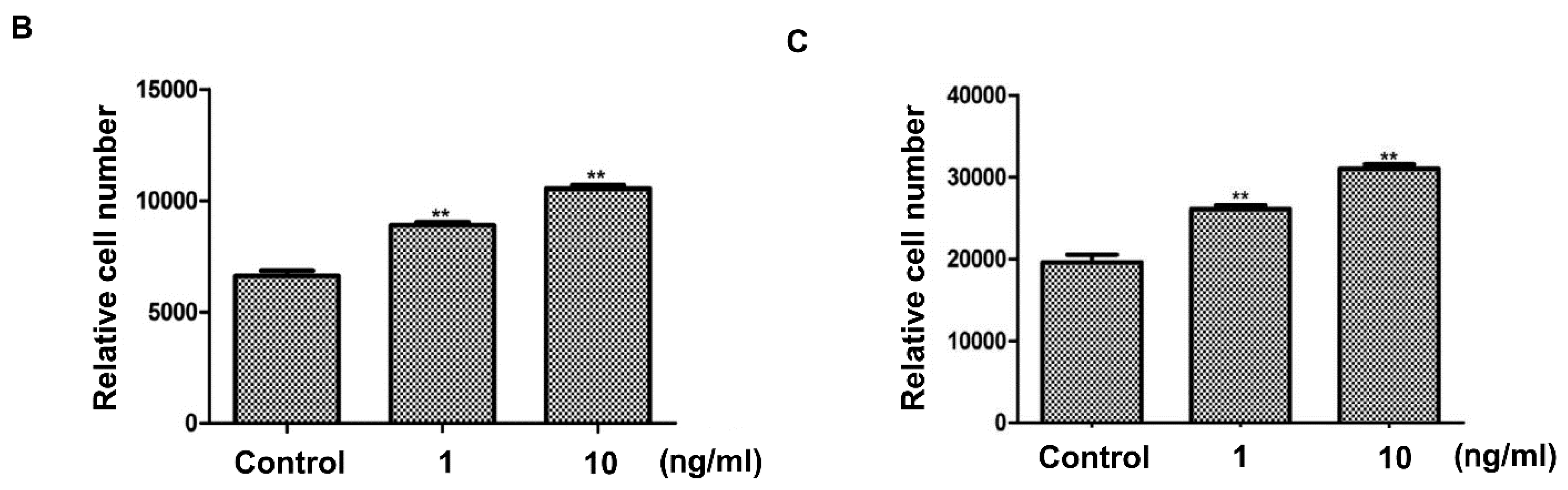
2.1. Effects of Keratinocyte Stem/Progenitor Cells Conditioned Medium (KSC-CM) on Hair Follicle Dermal Papilla Cells (HFDPCs)
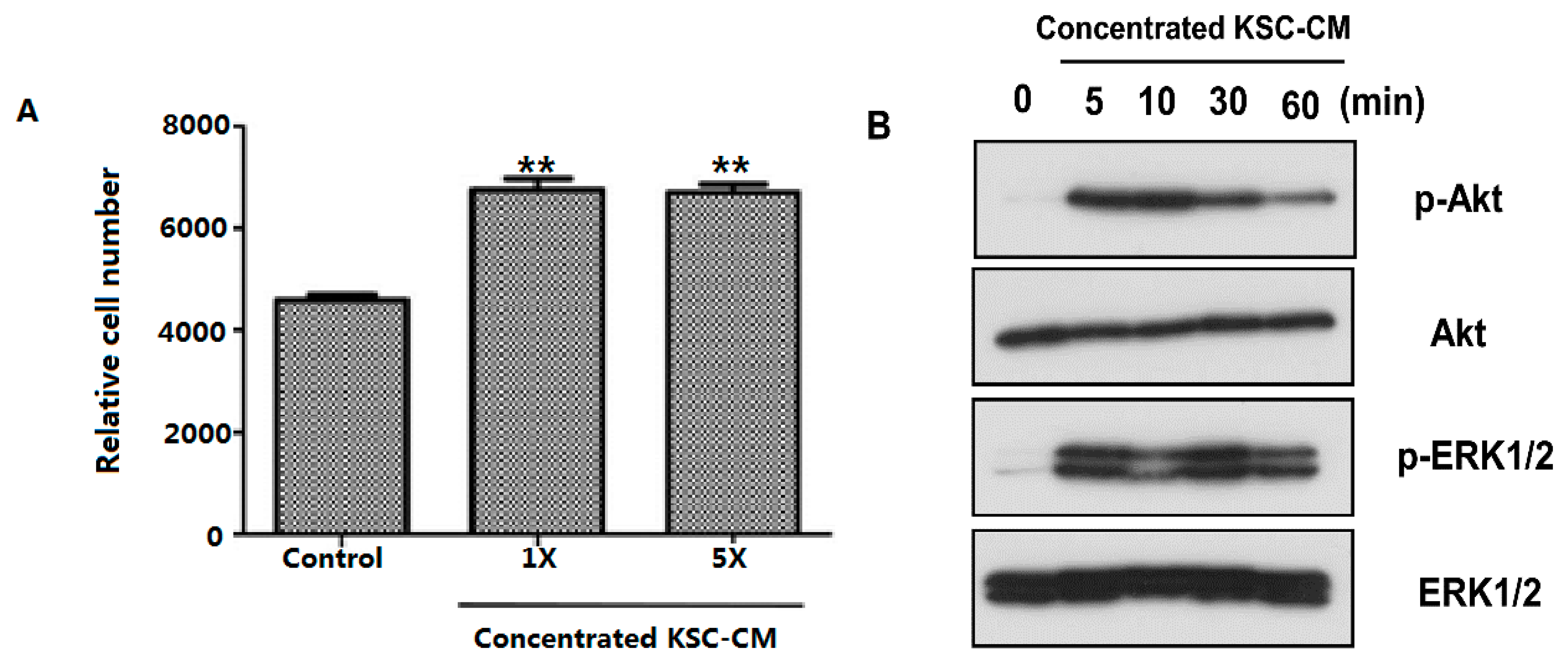

2.2. Effects of KSC-CM on Outer Root Sheath Cells
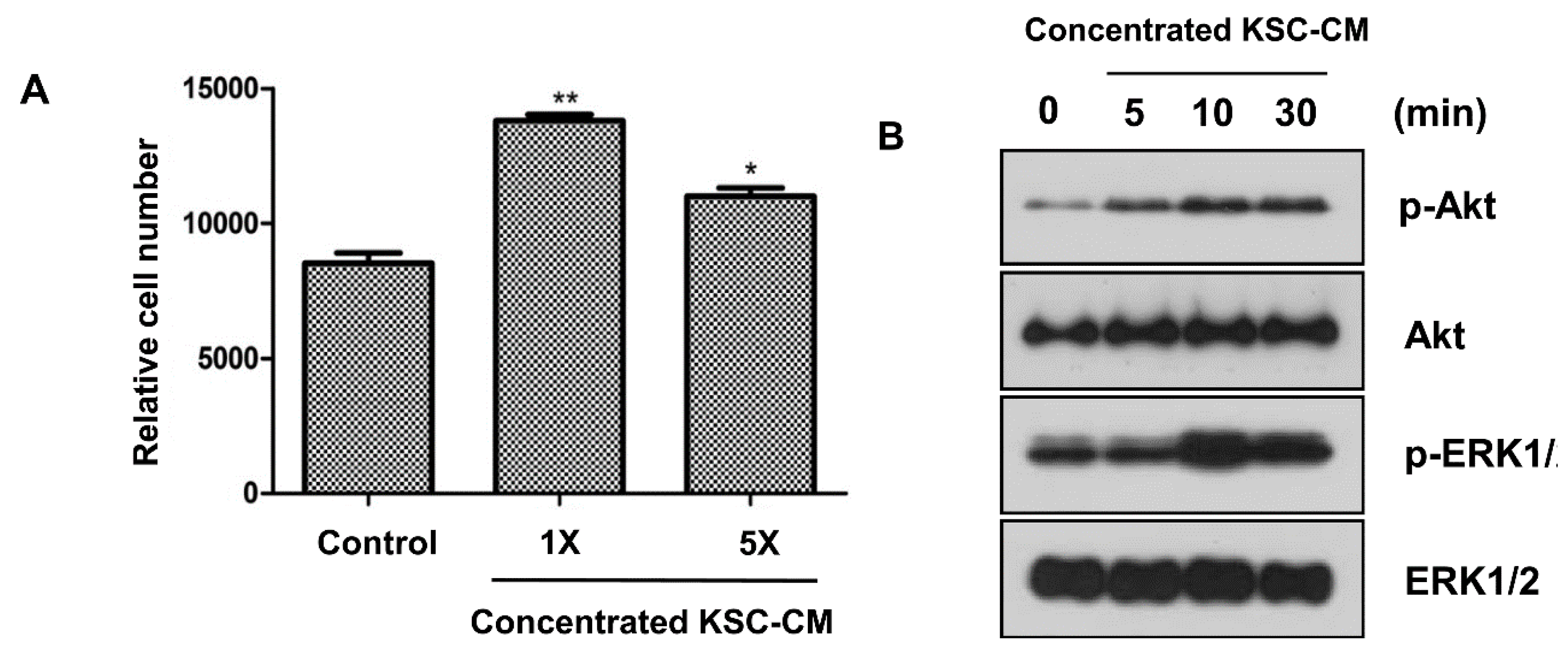
2.3. Hair-Growth-Promoting Effects of KSC-CM in a C3H/HeN Mouse Model

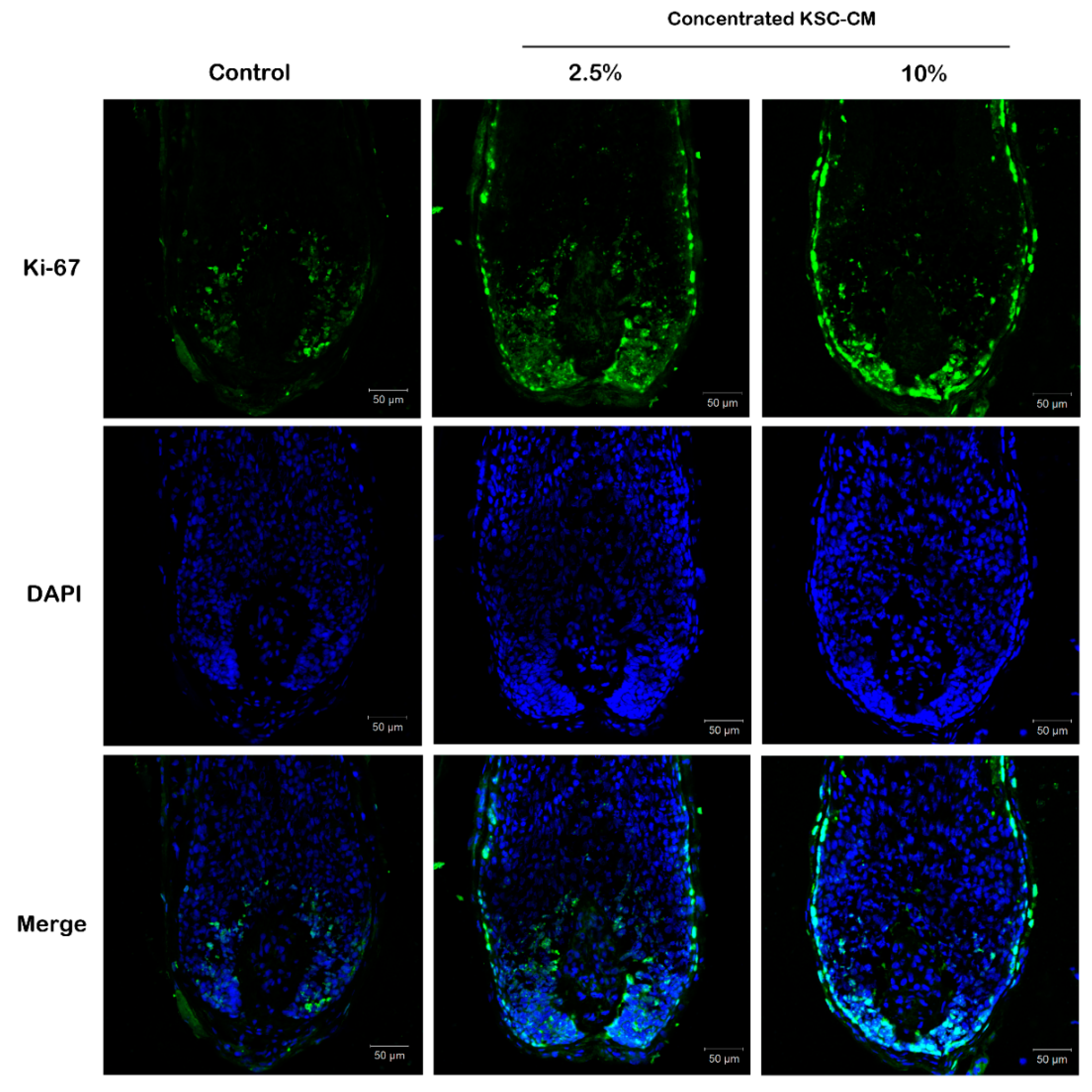
2.4. KSC-CM Increased Proliferation of Hair Matrix Keratinocytes Ex Vivo
2.5. Identification of Proteins Involved in the Hair-Growth Promotion by KSC-CM
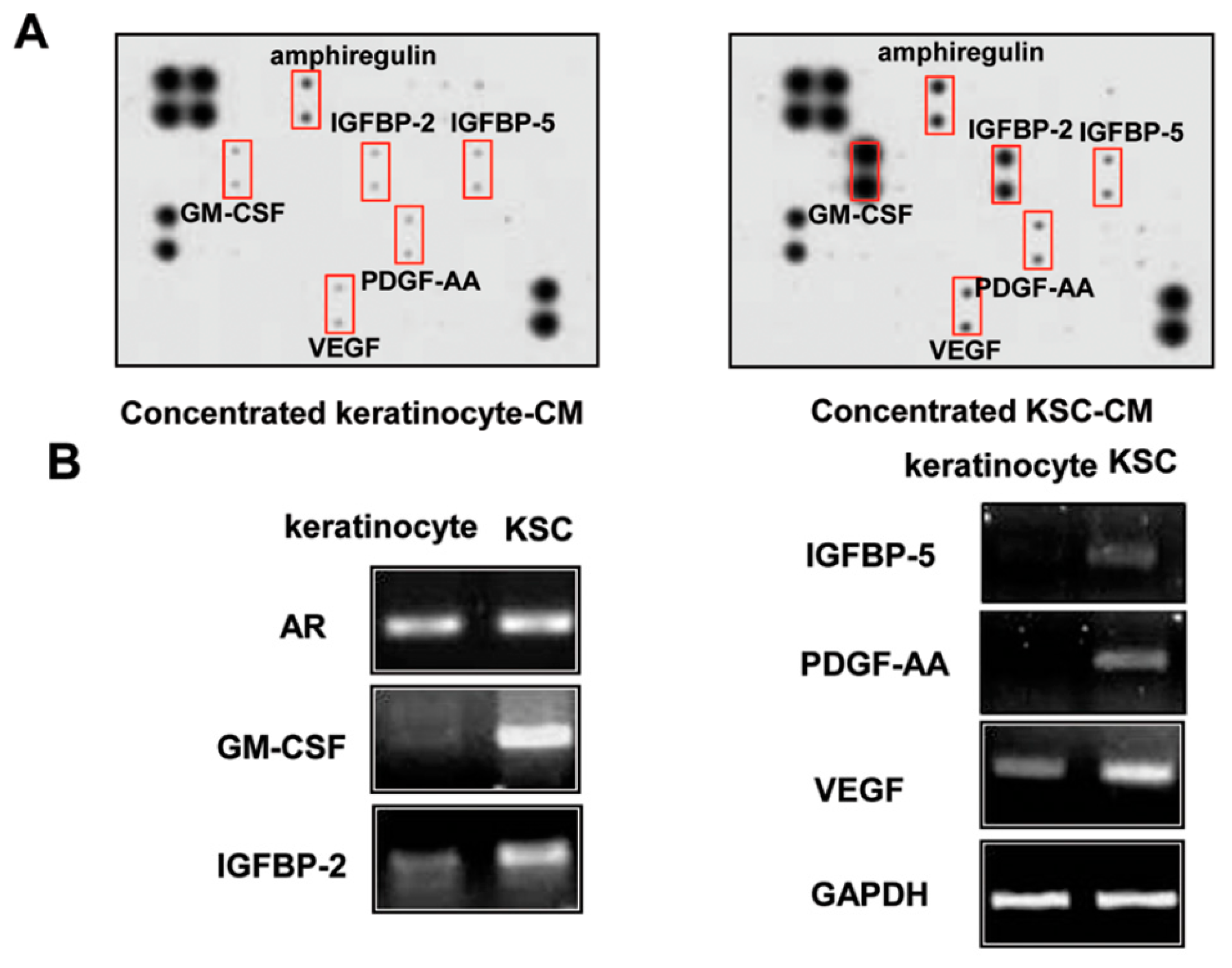
2.6. Hair-Growth-Promotion Effect of the Growth-Factor Complex

3. Discussion
4. Experimental Section
4.1. Cell Cultures
4.2. Preparation and Concentration of KSC-CM
4.3. Proliferation Assay
4.4. FACS Analysis
4.5. Western Blotting
4.6. Animal Studies
4.7. Ex Vivo Human HF Organ Culture Study
4.8. Human Growth Factor Chip Array
4.9. Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) Analysis
4.10. Statistics
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ozeki, M.; Tabata, Y. Promoted growth of murine hair follicles through controlled release of vascular endothelial growth factor. Biomaterials 2002, 23, 2367–2373. [Google Scholar] [CrossRef] [PubMed]
- Yano, K.; Brown, L.F.; Detmar, M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J. Clin. Investig. 2001, 107, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Akiyama, M.; Shimizu, H. PDGF isoforms induce and maintain anagen phase of murine hair follicles. J. Dermatol. Sci. 2006, 43, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Jindo, T.; Tsuboi, R.; Takamori, K.; Ogawa, H. Local injection of hepatocyte growth factor/scatter factor (HGF/SF) alters cyclic growth of murine hair follicles. J. Investig. Dermatol. 1998, 110, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Lindner, G.; Menrad, A.; Gherardi, E.; Merlino, G.; Welker, P.; Handjiski, B.; Roloff, B.; Paus, R. Involvement of hepatocyte growth factor/scatter factor and met receptor signaling in hair follicle morphogenesis and cycling. FASEB J. 2000, 14, 319–332. [Google Scholar] [PubMed]
- Su, H.Y.; Hickford, J.G.; Bickerstaffe, R.; Palmer, B.R. Insulin-like growth factor 1 and hair growth. Dermatol. Online J. 1999, 5, 1. [Google Scholar] [PubMed]
- Weger, N.; Schlake, T. IGF-I signalling controls the hair growth cycle and the differentiation of hair shafts. J. Investig. Dermatol. 2005, 125, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P. Interfollicular epidermal stem cells: Identification, challenges, potential. J. Investig. Dermatol. 2006, 126, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.; Li, A.; Kaur, P. Location and phenotype of human adult keratinocyte stem cells of the skin. Differentiation 2004, 72, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, J.; Lee, D.; Baden, H.P.; Brissette, J.; Dotto, G.P. Primary mouse keratinocyte cultures contain hair follicle progenitor cells with multiple differentiation potential. J. Investing. Dermatol. 1997, 109, 534–540. [Google Scholar] [CrossRef]
- Taylor, G.; Lehrer, M.S.; Jensen, P.J.; Sun, T.T.; Lavker, R.M. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 2000, 102, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Tumbar, T.; Guasch, G.; Greco, V.; Blanpain, C.; Lowry, W.E.; Rendl, M.; Fuchs, E. Defining the epithelial stem cell niche in skin. Science 2004, 303, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.H.; Park, S.H.; Song, S.Y.; Lee, S.J.; Lee, H.W.; Kim, S.H.; A Lee, M.; Yoon, I.S.; Kim, D.D.; Kang, S.; et al. Epidermal regeneration by ENT-16α, 17-dihydroxy-kauran-19-oic acid isolated from Siegesbeckia pubescens. Cell Prolif. 2011, 44, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Won, C.H.; Yoo, H.G.; Kwon, O.S.; Sung, M.Y.; Kang, Y.J.; Chung, J.H.; Park, B.S.; Sung, J.H.; Kim, W.S.; Kim, K.H.; et al. Hair growth promoting effects of adipose tissue-derived stem cells. J. Dermatol. Sci. 2010, 57, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Park, B.S.; Sung, J.H. Protective role of adipose-derived stem cells and their soluble factors in photoaging. Arch. Dermatol. Res. 2009, 301, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Jang, K.A.; Sung, J.H.; Park, J.S.; Kwon, Y.H.; Kim, K.J.; Kim, W.S. Adipose-derived stem cells and their secretory factors as a promising therapy for skin aging. Dermatol. Surg. 2008, 34, 1323–1326. [Google Scholar] [PubMed]
- Song, S.Y.; Chung, H.M.; Sung, J.H. The pivotal role of VEGF in adipose-derived-stem-cell-mediated regeneration. Expert Opin. Biol. Ther. 2010, 10, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Xia, Y.; Kim, W.S.; Kim, M.H.; Kim, T.H.; Kim, K.J.; Park, B.S.; Sung, J.H. Hypoxia-enhanced wound-healing function of adipose-derived stem cells: Increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen. 2009, 17, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Batch, J.A.; Mercuri, F.A.; Werther, G.A. Identification and localization of insulin-like growth factor-binding protein (IGFBP) messenger RNAs in human hair follicle dermal papilla. J. Investig. Dermatol. 1996, 106, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Kim, W.S.; Choi, J.S.; Kim, H.K.; Won, J.H.; Ohkubo, F.; Fukuoka, H. Hair growth stimulated by conditioned medium of adipose-derived stem cells is enhanced by hypoxia: Evidence of increased growth factor secretion. Biomed. Res. 2010, 31, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Shin, S.H.; Kim, S.R.; Im, S.U.; Han, I.S.; Kim, M.K.; Kim, J.C.; Sung, Y.K. l-Ascorbic acid 2-phosphate promotes elongation of hair shafts via the secretion of insulin-like growth factor-1 from dermal papilla cells through phosphatidylinositol 3-kinase. Br. J. Dermatol. 2009, 160, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Park, B.S.; Park, S.H.; Kim, H.K.; Sung, J.H. Antiwrinkle effect of adipose-derived stem cell: Activation of dermal fibroblast by secretory factors. J. Dermatol. Sci. 2009, 53, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Park, B.S.; Kim, H.K.; Park, J.S.; Kim, K.J.; Choi, J.S.; Chung, S.J.; Kim, D.D.; Sung, J.H. Evidence supporting antioxidant action of adipose-derived stem cells: Protection of human dermal fibroblasts from oxidative stress. J. Dermatol. Sci. 2008, 49, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Krugluger, W.; Rohrbacher, W.; Laciak, K.; Moser, K.; Moser, C.; Hugeneck, J. Reorganization of hair follicles in human skin organ culture induced by cultured human follicle-derived cells. Exp. Dermatol. 2005, 14, 580–585. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Won, C.H.; Jeong, Y.-M.; Kang, S.; Koo, T.-S.; Park, S.-H.; Park, K.-Y.; Sung, Y.-K.; Sung, J.-H. Hair-Growth-Promoting Effect of Conditioned Medium of High Integrin α6 and Low CD 71 (α6bri/CD71dim) Positive Keratinocyte Cells. Int. J. Mol. Sci. 2015, 16, 4379-4391. https://doi.org/10.3390/ijms16034379
Won CH, Jeong Y-M, Kang S, Koo T-S, Park S-H, Park K-Y, Sung Y-K, Sung J-H. Hair-Growth-Promoting Effect of Conditioned Medium of High Integrin α6 and Low CD 71 (α6bri/CD71dim) Positive Keratinocyte Cells. International Journal of Molecular Sciences. 2015; 16(3):4379-4391. https://doi.org/10.3390/ijms16034379
Chicago/Turabian StyleWon, Chong Hyun, Yun-Mi Jeong, Sangjin Kang, Tae-Sung Koo, So-Hyun Park, Ki-Young Park, Young-Kwan Sung, and Jong-Hyuk Sung. 2015. "Hair-Growth-Promoting Effect of Conditioned Medium of High Integrin α6 and Low CD 71 (α6bri/CD71dim) Positive Keratinocyte Cells" International Journal of Molecular Sciences 16, no. 3: 4379-4391. https://doi.org/10.3390/ijms16034379
APA StyleWon, C. H., Jeong, Y.-M., Kang, S., Koo, T.-S., Park, S.-H., Park, K.-Y., Sung, Y.-K., & Sung, J.-H. (2015). Hair-Growth-Promoting Effect of Conditioned Medium of High Integrin α6 and Low CD 71 (α6bri/CD71dim) Positive Keratinocyte Cells. International Journal of Molecular Sciences, 16(3), 4379-4391. https://doi.org/10.3390/ijms16034379




