Ecdysone Receptor (EcR) Is Involved in the Transcription of Cell Cycle Genes in the Silkworm
Abstract
:1. Introduction
2. Results
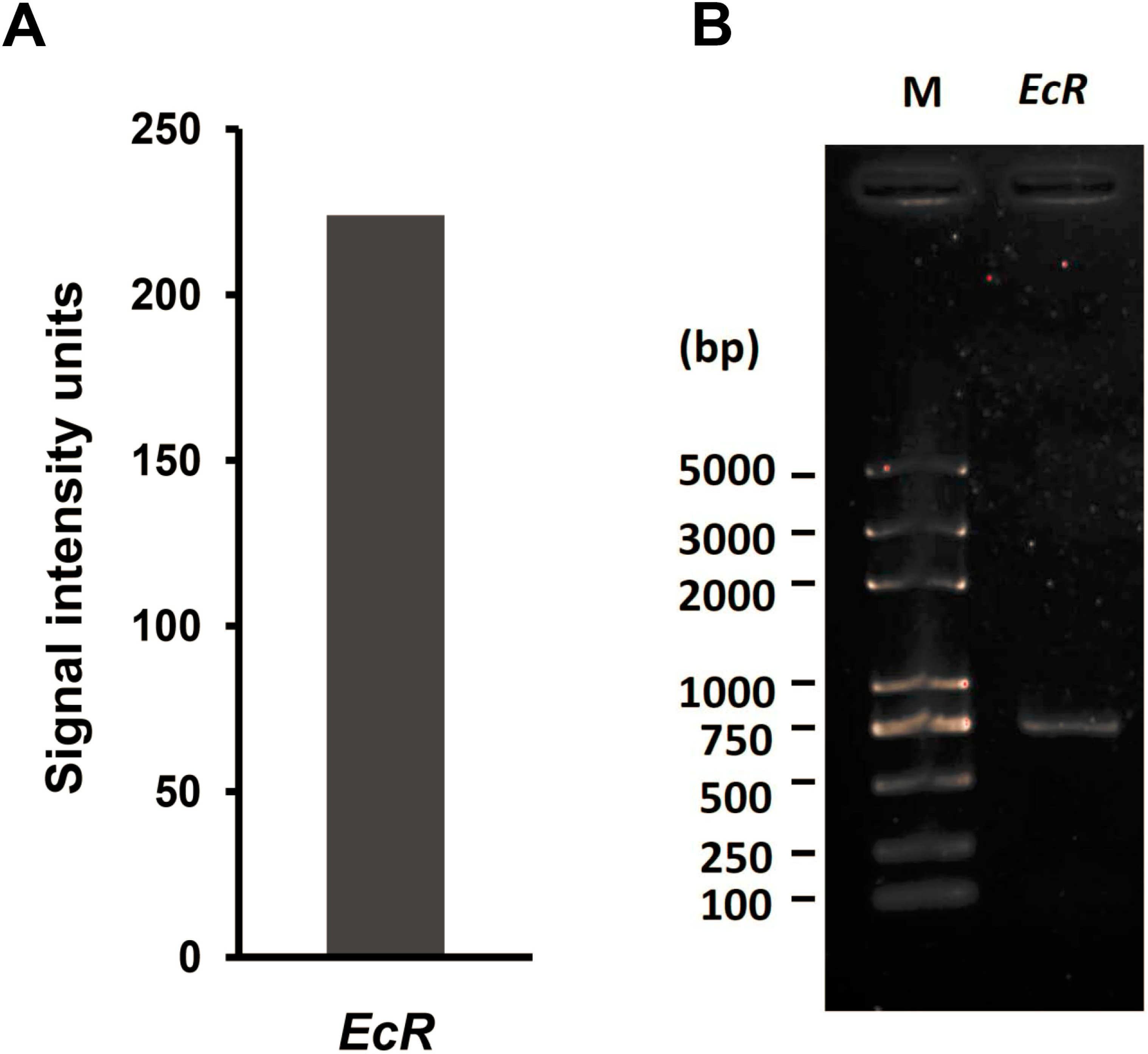
2.1. EcR Was Expressed in Silkworm BmN4 Cells
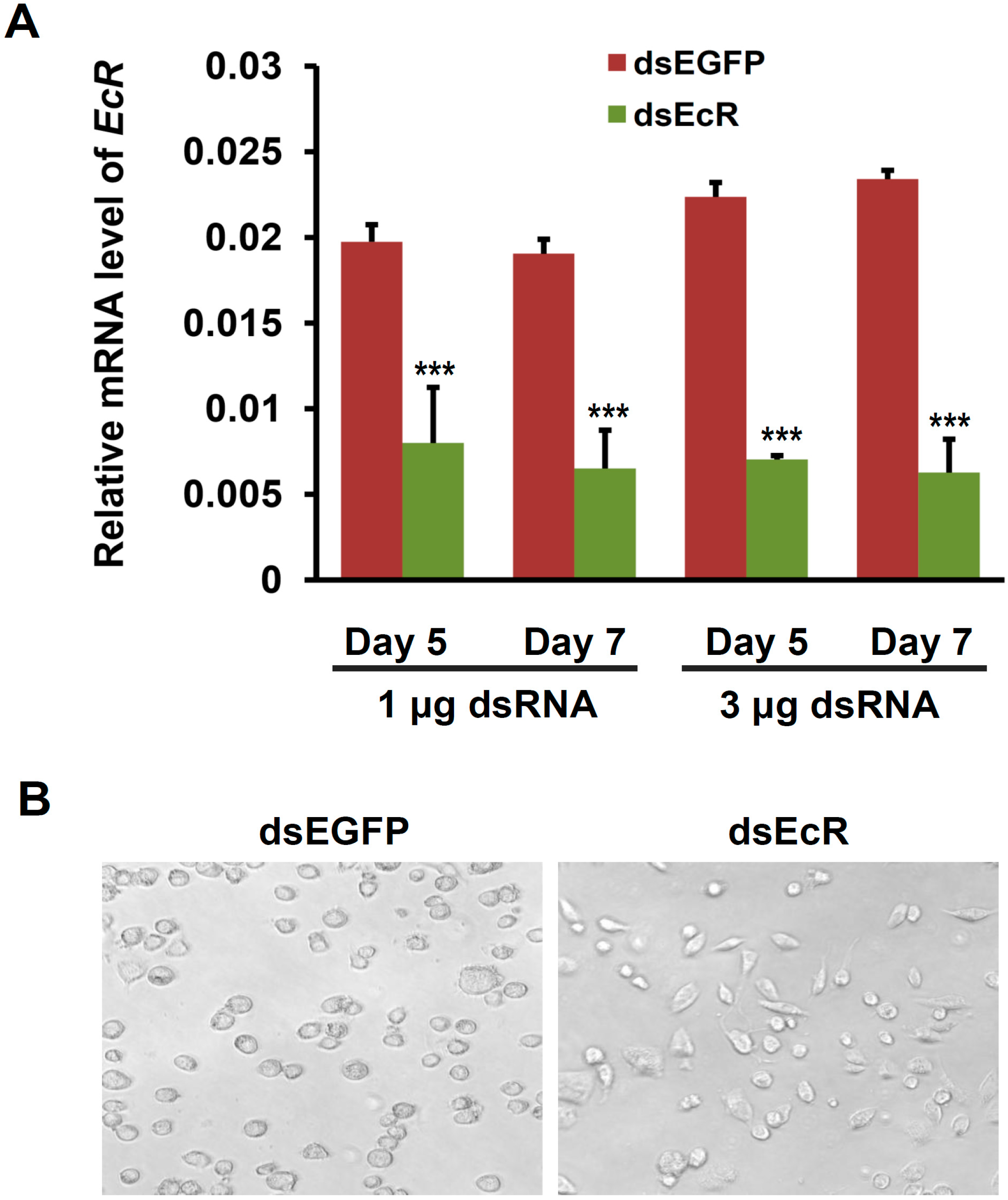
2.2. EcR RNAi Alters the Shape of Silkworm BmN4-SID1 Cells
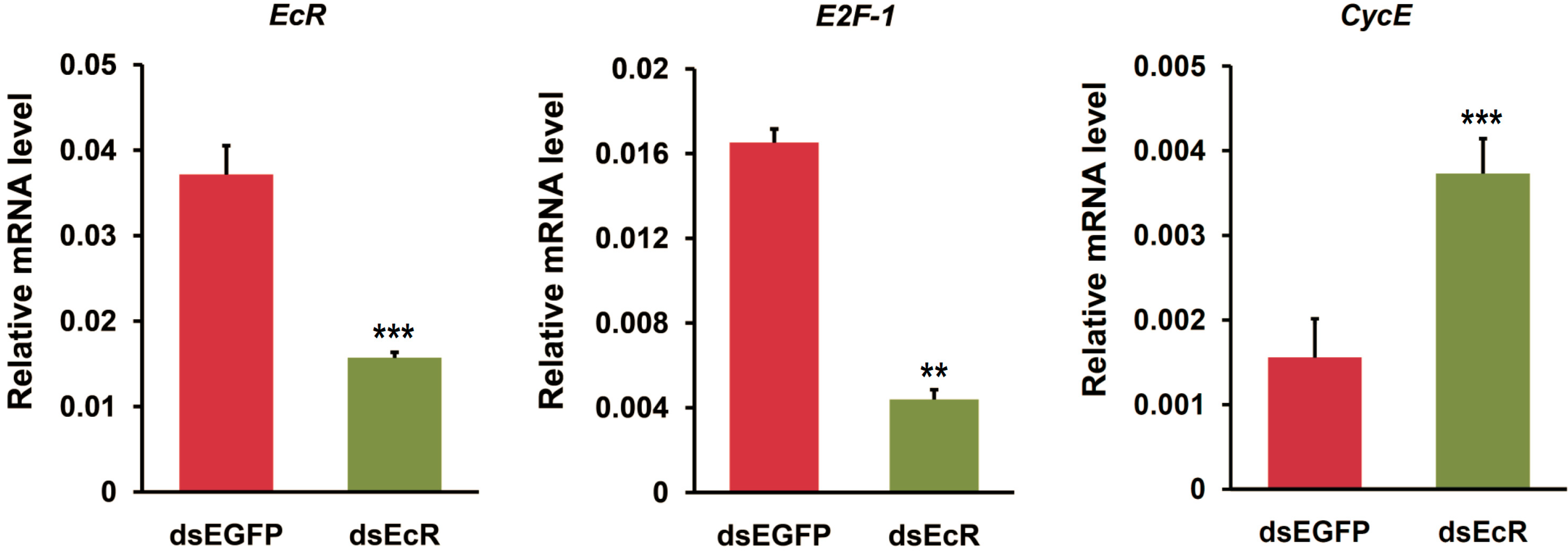
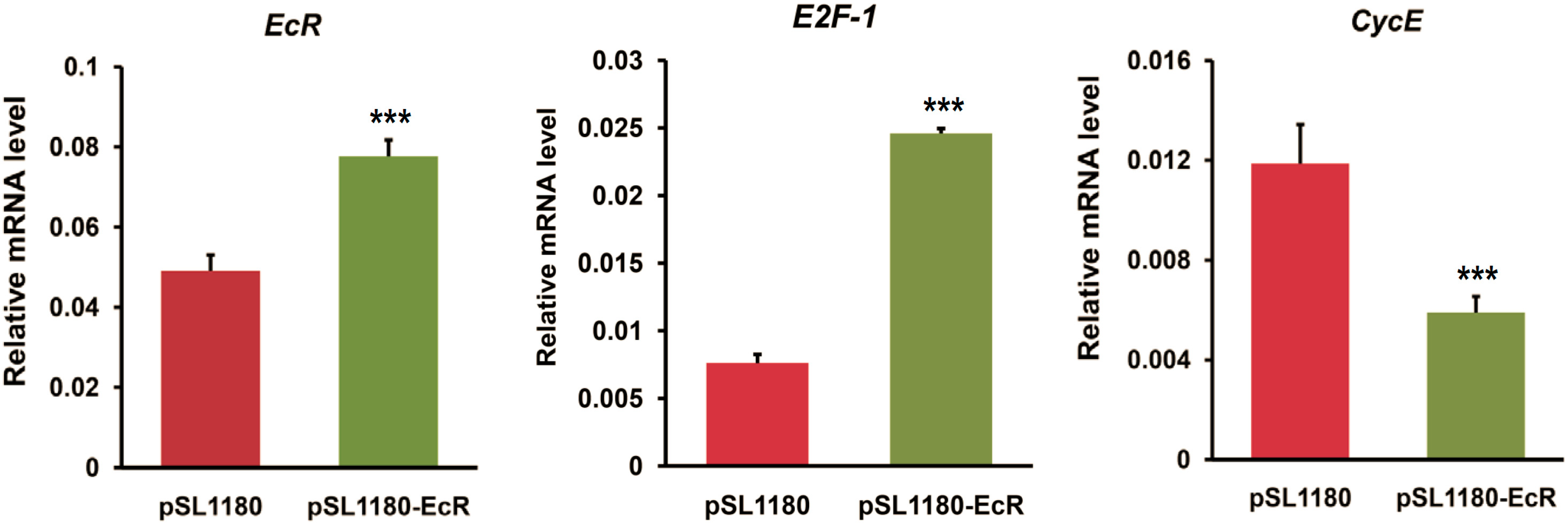
2.3. RNAi or Overexpression of EcR Gene Disrupts the Expression of Cell Cycle Genes in BmN4 Cells
2.4. Ecdysone Treatment Changes the Expression of Cell Cycle Genes in BmN4 Cells

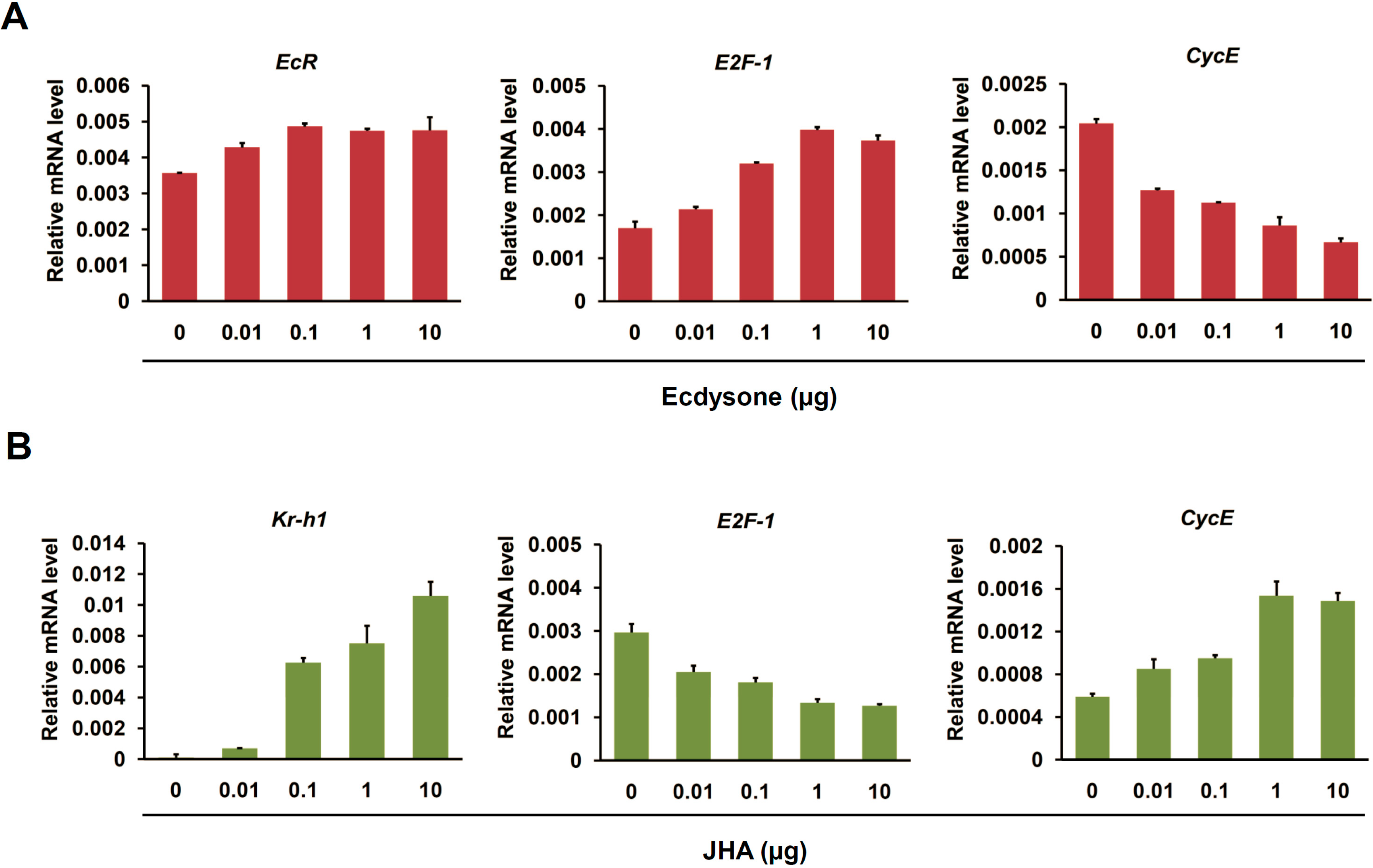
2.5. Cell Cycle Genes Respond to Ecdysone Treatment in Silkworm Silk Gland
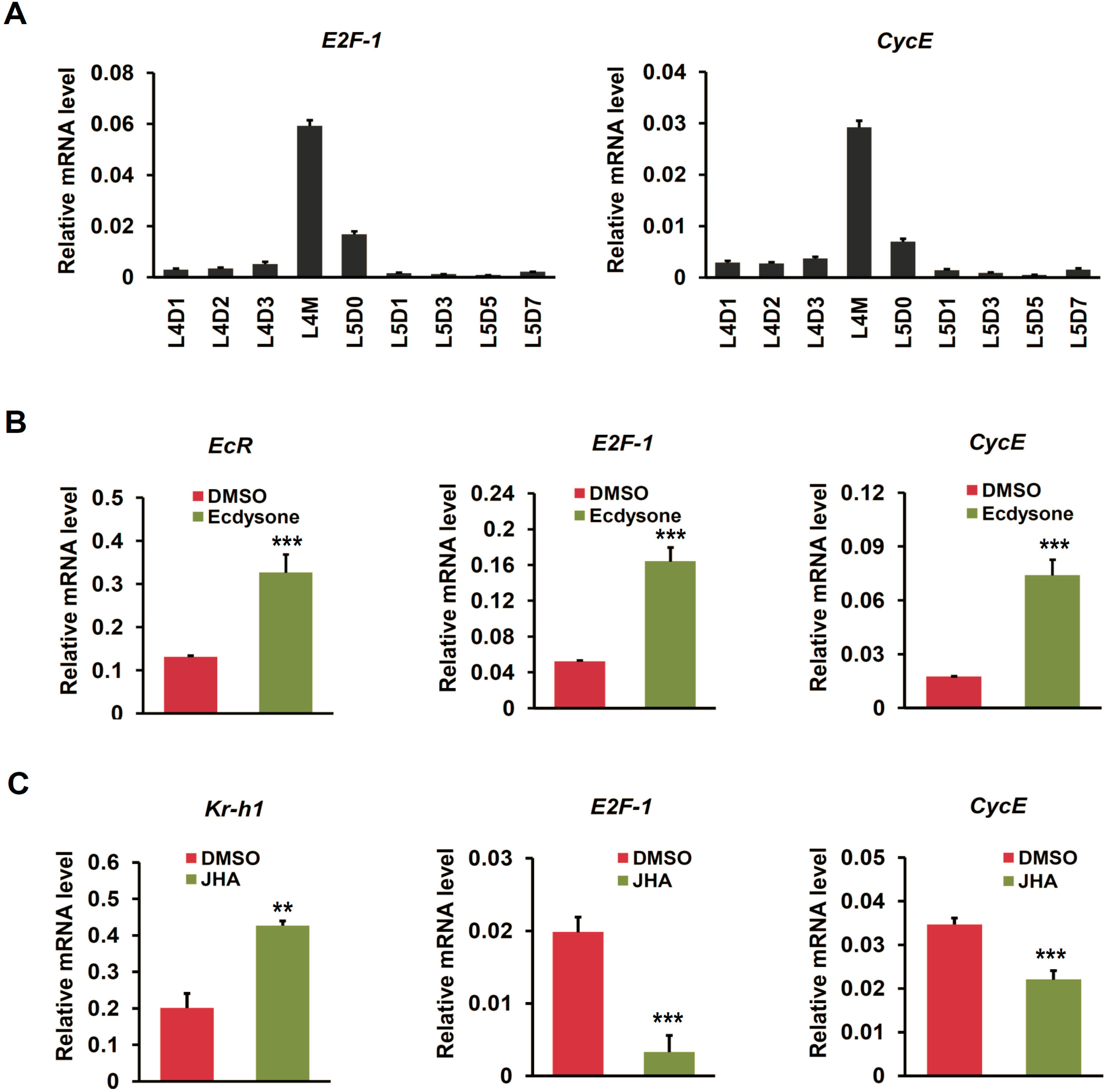
2.6. Ecdysone Treatment Induces Cell Cycle Arrest in BmN4 Cells
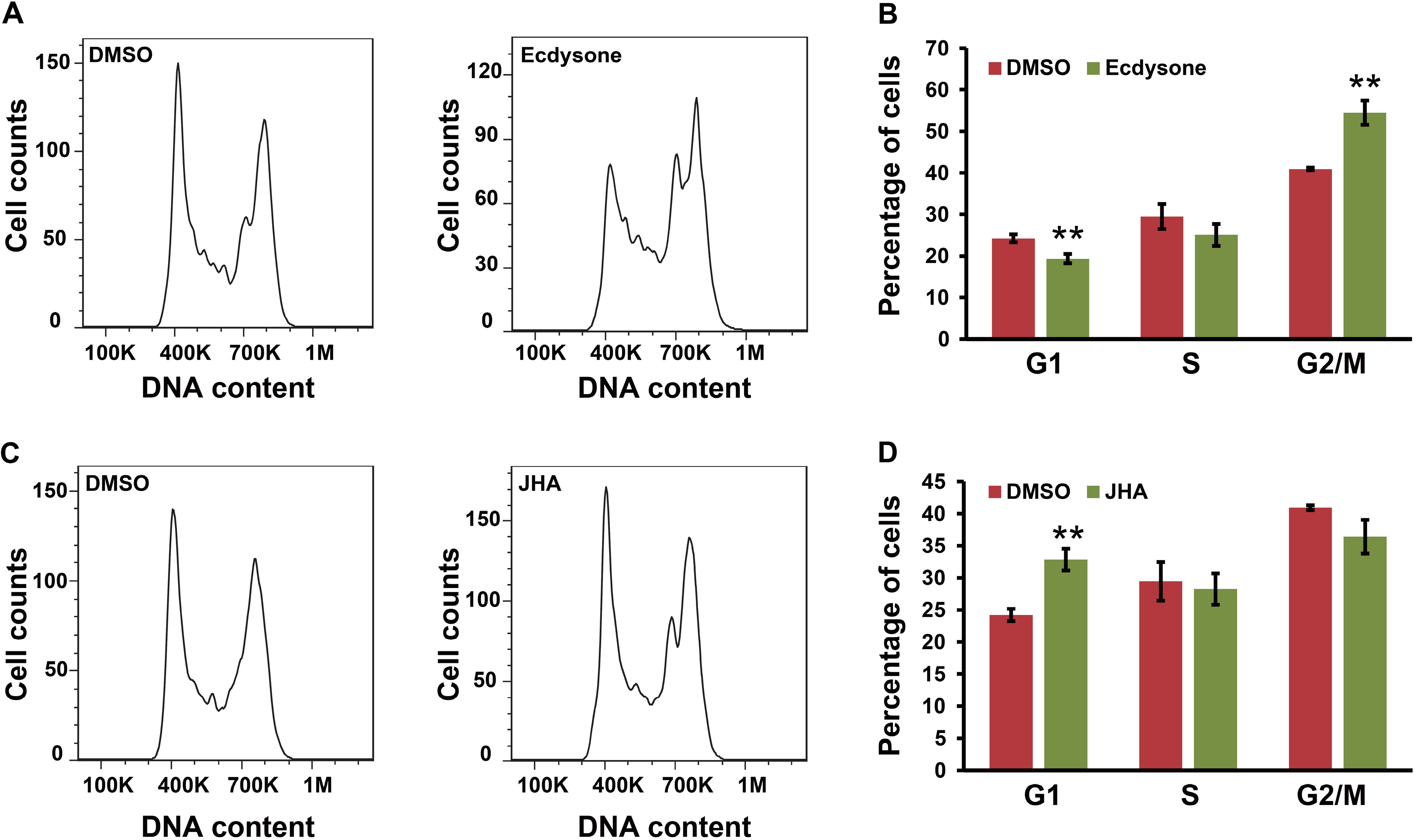
3. Discussion
4. Experimental Section
4.1. Cell Lines
4.2. RNAi Knockdown of EcR Gene in BmN4-SID1 Cells
4.3. RT-PCR Assay
4.4. Overexpression of EcR Gene in BmN4 Cells
4.5. Hormone Treatment in BmN4 Cells
4.6. Gene Expression Assay in Silkworm Silk Gland
4.7. Cell Cycle Analysis by Flow Cytometry
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Koelle, M.R.; Talbot, W.S.; Segraves, W.A.; Bender, M.T.; Cherbas, P.; Hogness, D.S. The Drosophila EcR gene encodes an ecdysone receptor, a new member of the steroid receptor superfamily. Cell 1991, 67, 59–77. [Google Scholar] [CrossRef] [PubMed]
- Fahrbach, S.E.; Smagghe, G.; Velarde, R.A. Insect nuclear receptors. Annu. Rev. Entomol. 2012, 57, 83–106. [Google Scholar] [CrossRef] [PubMed]
- King-Jones, K.; Thummel, C.S. Nuclear receptors—A perspective from Drosophila. Nat. Rev. Genet. 2005, 6, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Andres, A.J.; Thummel, C.S. Hormones, puffs and flies: The molecular control of metamorphosis by ecdysone. Trends Genet. 1992, 8, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Thummel, C.S. Flies on steroids—Drosophila metamorphosis and the mechanisms of steroid hormone action. Trends Genet. 1996, 12, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, N.; Rewitz, K.F.; O’Connor, M.B. Ecdysone control of developmental transitions: Lessons from Drosophila research. Annu. Rev. Entomol. 2013, 58, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Carney, G.E.; Wade, A.A.; Sapra, R.; Goldstein, E.S.; Bender, M. DHR3, an ecdysone-inducible early-late gene encoding a Drosophila nuclear receptor, is required for embryogenesis. Proc. Natl. Acad. Sci. USA 1997, 94, 12024–12029. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Bender, M. A conditional rescue system reveals essential functions for the ecdysone receptor (EcR) gene during molting and metamorphosis in Drosophila. Development 2000, 127, 2897–2905. [Google Scholar] [PubMed]
- Baehrecke, E.H. Ecdysone signaling cascade and regulation of Drosophila metamorphosis. Arch. Insect Biochem. Physiol. 1996, 33, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Schwedes, C.C.; Carney, G.E. Ecdysone signaling in adult Drosophila melanogaster. J. Insect Physiol. 2012, 58, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Tapon, N.; Moberg, K.H.; Hariharan, I.K. The coupling of cell growth to the cell cycle. Curr. Opin. Cell Biol. 2001, 13, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Levine, E. Cell cycling through development. Development 2004, 131, 2241–2246. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, T.P.; Edgar, B.A. Connections between growth and the cell cycle. Curr. Opin. Cell Biol. 1998, 10, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Swanhart, L.; Kupsco, J.; Duronio, R.J. Developmental control of growth and cell cycle progression in Drosophila. Methods Mol. Biol. 2005, 296, 69–94. [Google Scholar] [PubMed]
- Sanchez, I.; Dynlacht, B.D. New insights into cyclins, CDKs, and cell cycle control. Semin. Cell Dev. Biol. 2005, 16, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Kaldis, P. Cdks, cyclins and CKIs: Roles beyond cell cycle regulation. Development 2013, 140, 3079–3093. [Google Scholar] [CrossRef] [PubMed]
- Knoblich, J.A.; Sauer, K.; Jones, L.; Richardson, H.; Saint, R.; Lehner, C.F. Cyclin E controls S phase progression and its down-regulation during Drosophila embryogenesis is required for the arrest of cell proliferation. Cell 1994, 77, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Edgar, B.A.; Lehman, D.A.; O’Farrell, P.H. Transcriptional regulation of string (cdc25): A link between developmental programming and the cell cycle. Development 1994, 120, 3131–3143. [Google Scholar] [PubMed]
- Nigg, E.A. Cyclin-dependent protein kinases: Key regulators of the eukaryotic cell cycle. BioEssays 1995, 17, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, T.P.; de la Cruz, A.F.; Johnston, L.A.; Edgar, B.A. Coordination of growth and cell division in the Drosophila wing. Cell 1998, 93, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Reis, T.; Edgar, B.A. Negative regulation of dE2F1 by cyclin-dependent kinases controls cell cycle timing. Cell 2004, 117, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Edgar, B.A.; Lehner, C.F. Developmental control of cell cycle regulators: A fly’s perspective. Science 1996, 274, 1646–1652. [Google Scholar] [CrossRef] [PubMed]
- Cranna, N.; Quinn, L. Impact of steroid hormone signals on Drosophila cell cycle during development. Cell Div. 2009, 4. [Google Scholar] [CrossRef]
- Ninov, N.; Manjon, C.; Martin-Blanco, E. Dynamic control of cell cycle and growth coupling by ecdysone, EGFR, and PI3K signaling in Drosophila histoblasts. PLoS Biol. 2009, 7, e1000079. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.; Alvarez, C.M.; Bohman, R.; O’Connor, J.D. An ecdysteroid-induced alteration in the cell cycle of cultured Drosophila cells. Cell 1980, 22, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Fallon, A.M.; Gerenday, A. Ecdysone and the cell cycle: Investigations in a mosquito cell line. J. Insect Physiol. 2010, 56, 1396–1401. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, N.C.; Lin, J.I.; Zaytseva, O.; Cranna, N.; Lee, A.; Quinn, L.M. The Ecdysone receptor constrains wingless expression to pattern cell cycle across the Drosophila wing margin in a Cyclin B-dependent manner. BMC Dev. Biol. 2013, 13. [Google Scholar] [CrossRef]
- Mitchell, N.; Cranna, N.; Richardson, H.; Quinn, L. The Ecdysone-inducible zinc-finger transcription factor Crol regulates Wg transcription and cell cycle progression in Drosophila. Development 2008, 135, 2707–2716. [Google Scholar] [CrossRef] [PubMed]
- Johnston, L.A.; Edgar, B.A. Wingless and Notch regulate cell-cycle arrest in the developing Drosophila wing. Nature 1998, 394, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Johnston, L.A.; Sanders, A.L. Wingless promotes cell survival but constrains growth during Drosophila wing development. Nat. Cell Biol. 2003, 5, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cheng, D.; Mon, H.; Tatsuke, T.; Zhu, L.; Xu, J.; Lee, J.M.; Xia, Q.; Kusakabe, T. Genome-wide identification of polycomb target genes reveals a functional association of Pho with Scm in Bombyx mori. PLoS One 2012, 7, e34330. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, G.; Fallon, A.M. Evidence for expression of EcR and USP components of the 20-hydroxyecdysone receptor by a mosquito cell line. Arch. Insect Biochem. Physiol. 2000, 43, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Mon, H.; Kobayashi, I.; Ohkubo, S.; Tomita, S.; Lee, J.; Sezutsu, H.; Tamura, T.; Kusakabe, T. Effective RNA interference in cultured silkworm cells mediated by overexpression of Caenorhabditis elegans SID-1. RNA Biol. 2012, 9, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Truman, J.W.; Riddiford, L.M. Endocrine insights into the evolution of metamorphosis in insects. Annu. Rev. Entomol. 2002, 47, 467–500. [Google Scholar] [CrossRef] [PubMed]
- Dubrovsky, E.B. Hormonal cross talk in insect development. Trends Endocrinol. Metab. 2005, 16, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Minakuchi, C.; Zhou, X.; Riddiford, L.M. Kruppel homolog 1 (Kr-h1) mediates juvenile hormone action during metamorphosis of Drosophila melanogaster. Mech. Dev. 2008, 125, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Kayukawa, T.; Minakuchi, C.; Namiki, T.; Togawa, T.; Yoshiyama, M.; Kamimura, M.; Mita, K.; Imanishi, S.; Kiuchi, M.; Ishikawa, Y.; et al. Transcriptional regulation of juvenile hormone-mediated induction of Kruppel homolog 1, a repressor of insect metamorphosis. Proc. Natl. Acad. Sci. USA 2012, 109, 11729–11734. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, A.; Ohashi, Y.; Hosoda, K.; Ishibashi, J.; Kataoka, H. Developmental profile of the changes in the prothoracicotropic hormone titer in hemolymph of the silkworm Bombyx mori: Correlation with ecdysteroid secretion. Insect Biochem. Mol. Biol. 2001, 31, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Siu, K.T.; Rosner, M.R.; Minella, A.C. An integrated view of cyclin E function and regulation. Cell Cycle 2012, 11, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Keyomarsi, K.; Herliczek, T.W. The role of cyclin E in cell proliferation, development and cancer. Prog. Cell Cycle Res. 1997, 3, 171–191. [Google Scholar] [PubMed]
- Johnson, D.G.; Ohtani, K.; Nevins, J.R. Autoregulatory control of E2F1 expression in response to positive and negative regulators of cell cycle progression. Genes Dev. 1994, 8, 1514–1525. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.R.; Sears, R.; Nuckolls, F.; Leone, G.; Nevins, J.R. Complex transcriptional regulatory mechanisms control expression of the E2F3 locus. Mol. Cell. Biol. 2000, 20, 3633–3639. [Google Scholar] [CrossRef] [PubMed]
- Gerenday, A.; Fallon, A.M. Ecdysone-induced accumulation of mosquito cells in the G1 phase of the cell cycle. J. Insect Physiol. 2004, 50, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Siaussat, D.; Bozzolan, F.; Porcheron, P.; Debernard, S. The 20-hydroxyecdysone-induced signalling pathway in G2/M arrest of Plodia interpunctella imaginal wing cells. Insect Biochem. Mol. Biol. 2008, 38, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Giraudo, M.; Califano, J.; Hilliou, F.; Tran, T.; Taquet, N.; Feyereisen, R.; le Goff, G. Effects of hormone agonists on Sf9 cells, proliferation and cell cycle arrest. PLoS One 2011, 6, e25708. [Google Scholar] [CrossRef] [PubMed]
- Siaussat, D.; Bozzolan, F.; Queguiner, I.; Porcheron, P.; Debernard, S. Cell cycle profiles of EcR, USP, HR3 and B cyclin mRNAs associated to 20E-induced G2 arrest of Plodia interpunctella imaginal wing cells. Insect Mol. Biol. 2005, 14, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Zelhof, A.C.; Ghbeish, N.; Tsai, C.C.; Evans, R.M.; McKeown, M. A role for ultraspiracle, the Drosophila RXR, in morphogenetic furrow movement and photoreceptor cluster formation. Development 1997, 124, 2499–2506. [Google Scholar] [PubMed]
- Sala, A.; Nicolaides, N.C.; Engelhard, A.; Bellon, T.; Lawe, D.C.; Arnold, A.; Grana, X.; Giordano, A.; Calabretta, B. Correlation between E2f-1 requirement in the S-Phase and E2f-1 transactivation of cell cycle-related genes in human cells. Cancer Res. 1994, 54, 1402–1406. [Google Scholar] [PubMed]
- Duronio, R.J.; Ofarrell, P.H.; Xie, J.E.; Brook, A.; Dyson, N. The transcription factor E2f is required for S-Phase during Drosophila embryogenesis. Gene Dev. 1995, 9, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Gauhar, Z.; Sun, L.V.; Hua, S.; Mason, C.E.; Fuchs, F.; Li, T.R.; Boutros, M.; White, K.P. Genomic mapping of binding regions for the Ecdysone receptor protein complex. Genome Res. 2009, 19, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Duronio, R.J.; Ofarrell, P.H. Developmental control of the G(1) to S-transition in Drosophila—Cyclin-E Is a limiting downstream target of E2f. Gene Dev. 1995, 9, 1456–1468. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tatsuke, T.; Sakashita, K.; Zhu, L.; Xu, J.; Mon, H.; Lee, J.M.; Kusakabe, T. Identification and characterization of Polycomb group genes in the silkworm, Bombyx mori. Mol. Biol. Rep. 2012, 39, 5575–5588. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, W.; Kang, L.; Zhang, T.; Meng, M.; Wang, Y.; Li, Z.; Xia, Q.; Cheng, D. Ecdysone Receptor (EcR) Is Involved in the Transcription of Cell Cycle Genes in the Silkworm. Int. J. Mol. Sci. 2015, 16, 3335-3349. https://doi.org/10.3390/ijms16023335
Qian W, Kang L, Zhang T, Meng M, Wang Y, Li Z, Xia Q, Cheng D. Ecdysone Receptor (EcR) Is Involved in the Transcription of Cell Cycle Genes in the Silkworm. International Journal of Molecular Sciences. 2015; 16(2):3335-3349. https://doi.org/10.3390/ijms16023335
Chicago/Turabian StyleQian, Wenliang, Lixia Kang, Tianlei Zhang, Meng Meng, Yonghu Wang, Zhiqing Li, Qingyou Xia, and Daojun Cheng. 2015. "Ecdysone Receptor (EcR) Is Involved in the Transcription of Cell Cycle Genes in the Silkworm" International Journal of Molecular Sciences 16, no. 2: 3335-3349. https://doi.org/10.3390/ijms16023335
APA StyleQian, W., Kang, L., Zhang, T., Meng, M., Wang, Y., Li, Z., Xia, Q., & Cheng, D. (2015). Ecdysone Receptor (EcR) Is Involved in the Transcription of Cell Cycle Genes in the Silkworm. International Journal of Molecular Sciences, 16(2), 3335-3349. https://doi.org/10.3390/ijms16023335





