Cloning, Expression, and Characterization of a Thermophilic Endoglucanase, AcCel12B from Acidothermus cellulolyticus 11B
Abstract
:1. Introduction
2. Results and Discussion
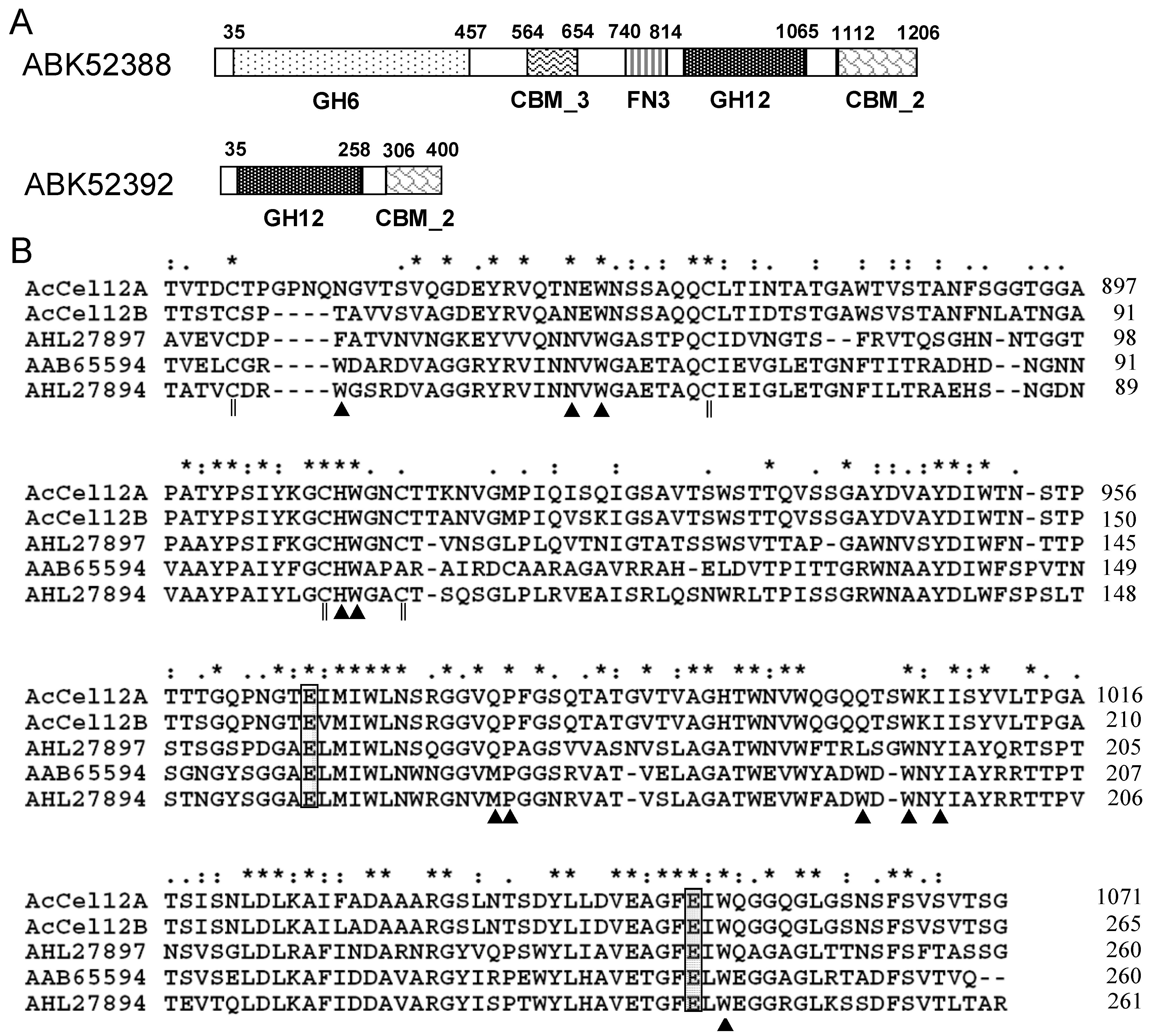
2.1. Architecture Analysis and Sequence Alignment
2.2. Cloning, Expression, and Purification of AcCel12B

2.3. Enzyme Properties of AcCel12B
2.3.1. Effects of pH and Temperature on AcCel12B Activity

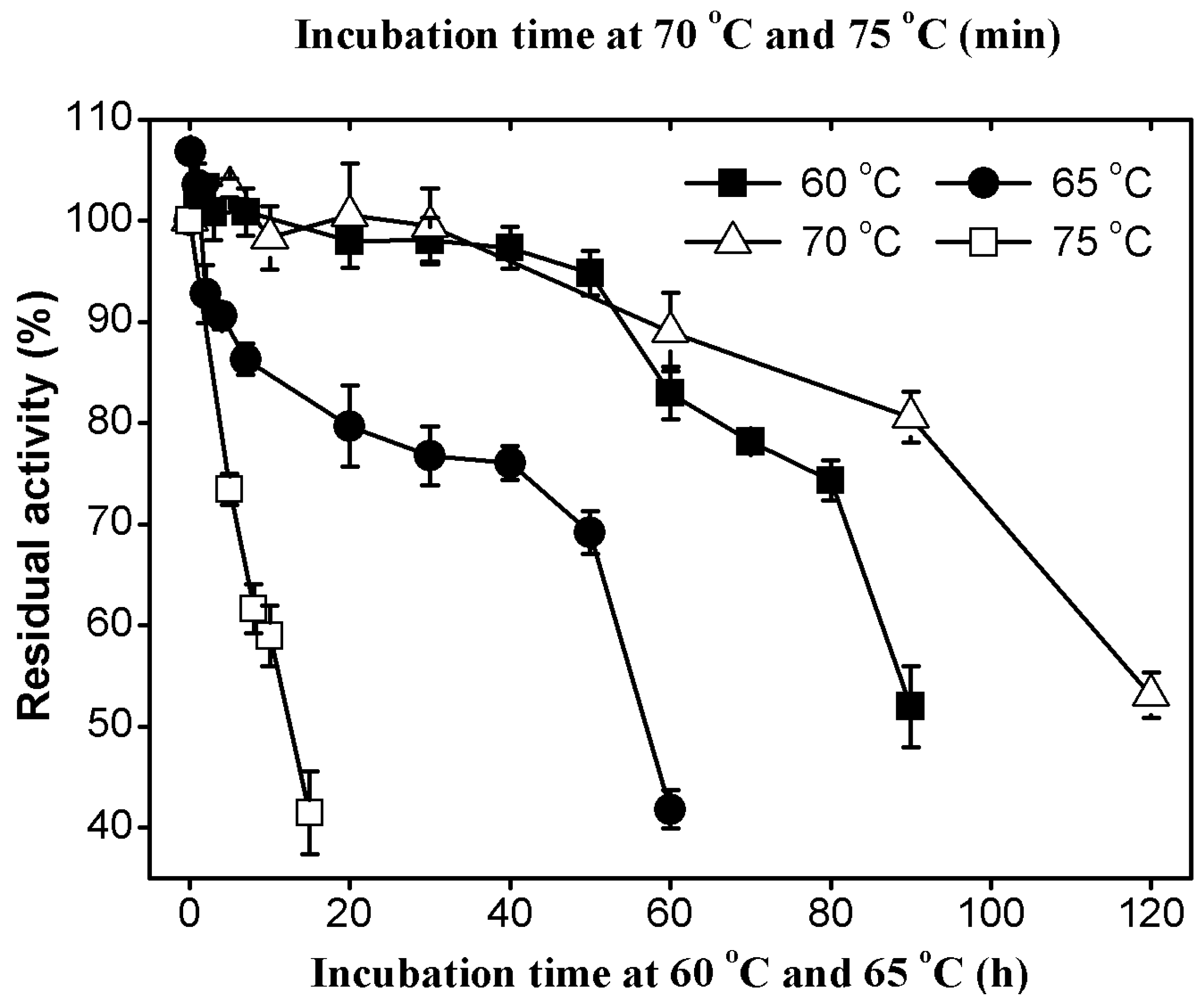
2.3.2. Thermal Stability of AcCel12B
2.3.3. Effects of Metal Ions and Chemical Agents on AcCel12B Activity
2.4. Substrate Specificity and Catalytic Kinetics
| Substrate | Specific Activity (U·mg−1) | Relativity Activity (%) |
|---|---|---|
| CMC (low viscosity) | 118.3 ± 5.18 | 100 |
| CMC (medium viscosity) | 100.7 ± 8.19 | 85 |
| β-d-Glucan (barley) | 271.2 ± 5.79 | 229 |
| RAC | 104.0 ± 6.40 | 88 |
| Avicel® PH101 | 14.7 ± 0.52 | 12 |
| Sigmacell cellulose Type 50 | Trace | - |
| Pachyman | ND | - |
| Starch | ND | - |
| Xylan (birch) | ND | - |
| Galactomannan | ND | - |
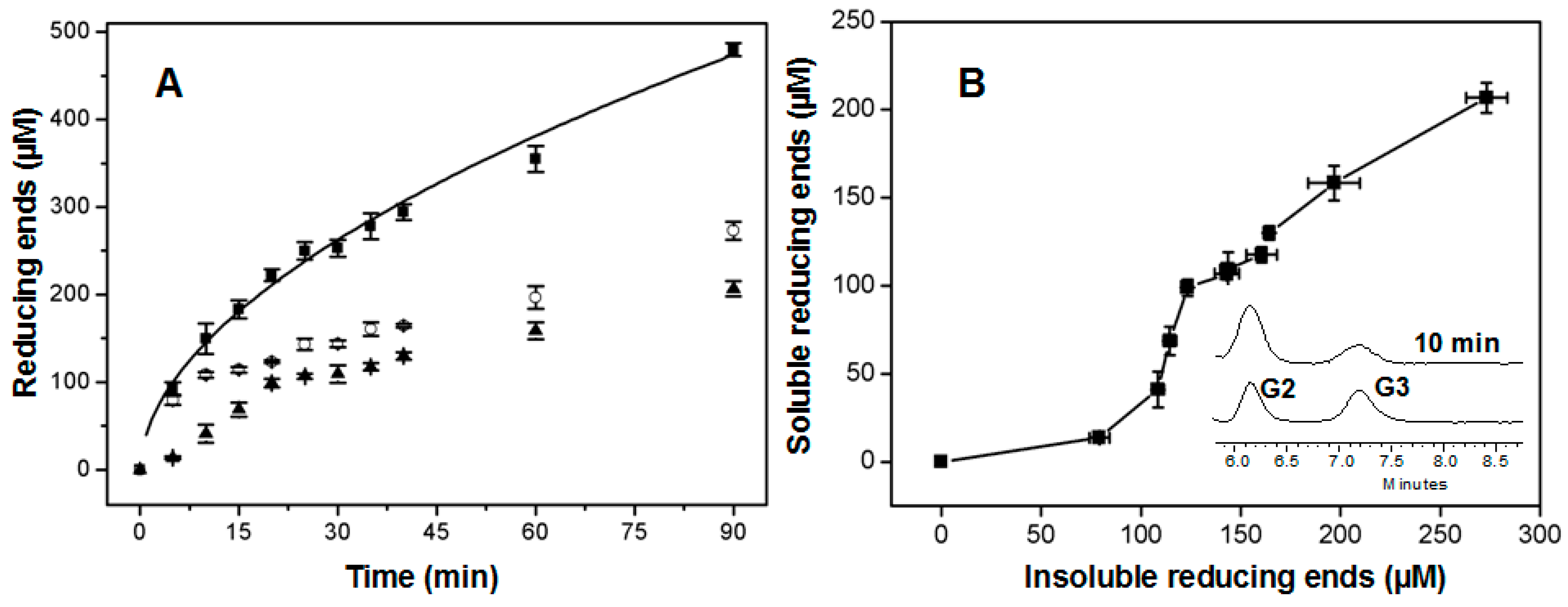
2.5. Time Course of Hydrolysis
3. Experimental Section
3.1. Recombinant DNA Techniques
3.2. Expression and Purification of AcCel12B
3.3. Enzyme Activity Assay
3.4. Enzyme Characterization of AcCel12B
3.5. Time Course of Hydrolysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lynd, L.R.; Weimer, P.J.; van Zyl, W.H.; Pretorius, I.S. Microbial cellulose utilization: Fundamentals and biotechnology. Microbiol. Mol. Biol. Rev. 2002, 66, 506–577. [Google Scholar] [CrossRef] [PubMed]
- Beeson, W.T.; Vu, V.V.; Span, E.A.; Phillips, C.M.; Marletta, M.A. Cellulose degradation by polysaccharide monooxygenases. Annu. Rev. Biochem. 2015, 84, 923–946. [Google Scholar] [CrossRef] [PubMed]
- Dienes, D.; Egyhazi, A.; Reczey, K. Treatment of recycled fiber with Trichoderma cellulases. Ind. Crops Prod. 2004, 20, 11–21. [Google Scholar] [CrossRef]
- Duan, X.Y.; Liu, S.Y.; Zhang, W.C.; Zhang, Q.X.; Gao, P.J. Volumetric productivity improvement for endoglucanase of Trichoderma pseudokoingii S-38. J. Appl. Microbiol. 2004, 96, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, A.; Bansal, N.; Kumar, S.; Bischoff, K.M.; Sani, R.K. Improved lignocellulose conversion to biofuels with thermophilic bacteria and thermostable enzymes. Bioresour. Technol. 2013, 128, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.P.; Himmel, M.E.; Mielenz, J.R. Outlook for cellulase improvement: Screening and selection strategies. Biotechnol. Adv. 2006, 24, 452–481. [Google Scholar] [CrossRef] [PubMed]
- Sandgren, M.; Stahlberg, J.; Mitchinson, C. Structural and biochemical studies of gh family 12 cellulases: Improved thermal stability, and ligand complexes. Prog. Biophys. Mol. Biol. 2005, 89, 246–291. [Google Scholar] [CrossRef] [PubMed]
- Bok, J.D.; Yernool, D.A.; Eveleigh, D.E. Purification, characterization, and molecular analysis of thermostable cellulases CelA and CelB from Thermotoga neapolitana. Appl. Environ. Microbiol. 1998, 64, 4774–4781. [Google Scholar] [PubMed]
- Halldorsdottir, S.; Thorolfsdottir, E.T.; Spilliaert, R.; Johansson, M.; Thorbjarnardottir, S.H.; Palsdottir, A.; Hreggvidsson, G.O.; Kristjansson, J.K.; Holst, O.; Eggertsson, G. Cloning, sequencing and overexpression of a Rhodothermus marinus gene encoding a thermostable cellulase of glycosyl hydrolase family 12. Appl. Microbiol. Biotechnol. 1998, 49, 277–284. [Google Scholar] [PubMed]
- Bauer, M.W.; Driskill, L.E.; Callen, W.; Snead, M.A.; Mathur, E.J.; Kelly, R.M. An endoglucanase, EglA, from the hyperthermophilic archaeon Pyrococcus furiosus hydrolyzes β-1,4 bonds in mixed-linkage (1→3),(1→4)-β-d-glucans and cellulose. J. Bacteriol. 1999, 181, 284–290. [Google Scholar] [PubMed]
- Huang, Y.; Krauss, G.; Cottaz, S.; Driguez, H.; Lipps, G. A highly acid-stable and thermostable endo-β-glucanase from the thermoacidophilic archaeon Sulfolobus solfataricus. Biochem. J. 2005, 385, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Barabote, R.D.; Xie, G.; Leu, D.H.; Normand, P.; Necsulea, A.; Daubin, V.; Medigue, C.; Adney, W.S.; Xu, X.C.; Lapidus, A.; et al. Complete genome of the cellulolytic thermophile Acidothermus cellulolyticus 11B provides insights into its ecophysiological and evolutionary adaptations. Genome Res. 2009, 19, 1033–1043. [Google Scholar] [PubMed]
- Barabote, R.D.; Parales, J.V.; Guo, Y.Y.; Labavitch, J.M.; Parales, R.E.; Berry, A.M. Xyn10A, a thermostable endoxylanase from Acidothermus cellulolyticus 11B. Appl. Environ. Microbiol. 2010, 76, 7363–7366. [Google Scholar] [PubMed]
- Baker, J.O.; Adney, W.S.; Nleves, R.A.; Steven, R.T.; David, B.W.; Michael, E.H. A new thermostable endoglucanase, Acidothermus cellulolyticus E1: Synergism with Trichoderma reesei CBH I and comparison to Thermomonospora fusca E-5. Appl. Biochem. Biotechnol. 1994, 45–46, 245–256. [Google Scholar]
- Dai, Z.; Hooker, B.S.; Anderson, D.B.; Thomas, S.R. Expression of Acidothermus cellulolyticus endoglucanase E1 in transgenic tobacco: Biochemical characteristics and physiological effects. Transgenic Res. 2000, 9, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.; Young, J.; Cha, M.; Brunecky, R.; Bomble, Y.J.; Himmel, M.E.; Westpheling, J. Expression of the Acidothermus cellulolyticus E1 endoglucanase in Caldicellulosiruptor bescii enhances its ability to deconstruct crystalline cellulose. Biotechnol. Biofuels 2015, 8, 113. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Yin, X.; Wu, M.; Tang, C.; Zhang, H.; Li, J. Cloning and bioinformatics analysis of an endoglucanase gene (Aucel12A) from Aspergillus usamii and its functional expression in Pichia pastoris. J. Ind. Microbiol. Biotechnol. 2012, 39, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active Enzymes database (CAZy): An expert resource for glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef] [PubMed]
- Linger, J.G.; Adney, W.S.; Darzins, A. Heterologous expression and extracellular secretion of cellulolytic enzymes by Zymomonas mobilis. Appl. Environ. Microbiol. 2010, 76, 6360–6369. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Doerks, T.; Bork, P. Smart: Recent updates, new developments and status in 2015. Nucleic Acids Res. 2015, 43, D257–D260. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Okano, H.; Ozaki, M.; Kanaya, E.; Kim, J.J.; Angkawidjaja, C.; Koga, Y.; Kanaya, S. Structure and stability of metagenome-derived glycoside hydrolase family 12 cellulase (LC-CelA) a homolog of Cel12A from Rhodothermus marinus. FEBS Open Bio. 2014, 4, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, T.; Shibuya, H.; Nojiri, M.; Yoshida, S.; Ishihara, M. Purification, molecular cloning, and enzymatic properties of a family 12 endoglucanase (EG-II) from Fomitopsis palustris: Role of EG-II in larch holocellulose hydrolysis. Appl. Environ. Microbiol. 2008, 74, 5857–5861. [Google Scholar] [CrossRef] [PubMed]
- Van Solingen, P.; Meijer, D.; van der Kleij, W.A.; Barnett, C.; Bolle, R.; Power, S.D.; Jones, B.E. Cloning and expression of an endocellulase gene from a novel streptomycete isolated from an east african soda lake. Extremophiles 2001, 5, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Wicher, K.B.; Abou-Hachem, M.; Halldorsdottir, S.; Thorbjarnadottir, S.H.; Eggertsson, G.; Hreggvidsson, G.O.; Nordberg Karlsson, E.; Holst, O. Deletion of a cytotoxic, N-terminal putatitive signal peptide results in a significant increase in production yields in Escherichia coli and improved specific activity of Cel12A from Rhodothermus marinus. Appl. Microbiol. Biotechnol. 2001, 55, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Kataoka, M.; Ishikawa, K. Atomic resolution of the crystal structure of the hyperthermophilic family 12 endocellulase and stabilizing role of the dxdxdg calcium-binding motif in Pyrococcus furiosus. FEBS Lett. 2012, 586, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Miotto, L.S.; de Rezende, C.A.; Bernardes, A.; Serpa, V.I.; Tsang, A.; Polikarpov, I. The characterization of the endoglucanase Cel12A from Gloeophyllum trabeum reveals an enzyme highly active on β-glucan. PLoS ONE 2014, 9, e108393. [Google Scholar] [CrossRef] [PubMed]
- Picart, P.; Goedegebuur, F.; Diaz, P.; Javier Pastor, F.I. Expression of novel β-glucanase Cel12A from Stachybotrys atra in bacterial and fungal hosts. Fungal Biol. 2012, 116, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.H.; Quyen, D.T.; Nghiem, N.M.; Vu, T.D. Cloning, expression, purification, and properties of an endoglucanase gene (glycosyl hydrolase family 12) from Aspergillus niger VTCC-F021 in Pichia pastoris. J. Microbiol. Biotechnol. 2011, 21, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Grishutin, S.G.; Gusakov, A.V.; Dzedzyulya, E.I.; Sinitsyn, A.P. A lichenase-like family 12 endo-(1→4)-β-glucanase from Aspergillus japonicus: Study of the substrate specificity and mode of action on β-glucans in comparison with other glycoside hydrolases. Carbohydr. Res. 2006, 341, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Liebl, W.; Ruile, P.; Bronnenmeier, K.; Riedel, K.; Lottspeich, F.; Greif, I. Analysis of a Thermotoga maritima DNA fragment encoding two similar thermostable cellulases, CelA and CelB, and characterization of the recombinant enzymes. Microbiology 1996, 142 (Pt 9), 2533–2542. [Google Scholar] [CrossRef] [PubMed]
- Saarilahti, H.T.; Henrissat, B.; Palva, E.T. CelS: A novel endoglucanase identified from Erwinia carotovora subsp. carotovora. Gene 1990, 90, 9–14. [Google Scholar] [CrossRef]
- Byeong-Cheol, S.; Kim, K.Y.; Yoon, J.J.; Sim, S.H.; Lee, K.; Kim, Y.S.; Kim, Y.K.; Cha, C.J. Functional analysis of a gene encoding endoglucanase that belongs to glycosyl hydrolase family 12 from the brown-rot basidiomycete Fomitopsis palustris. J. Microbiol. Biotechnol. 2008, 18, 404–409. [Google Scholar]
- Wang, Y.G.; Wang, X.N.; Tang, R.T.; Yu, S.S.; Zheng, B.S.; Feng, Y. A novel thermostable cellulase from Fervidobacterium nodosum. J. Mol. Catal. B Enzym. 2010, 66, 294–301. [Google Scholar] [CrossRef]
- Telke, A.A.; Zhuang, N.; Ghatge, S.S.; Lee, S.H.; Ali Shah, A.; Khan, H.; Um, Y.; Shin, H.D.; Chung, Y.R.; Lee, K.H. Engineering of family-5 glycoside hydrolase (Cel5A) from an uncultured bacterium for efficient hydrolysis of cellulosic substrates. PLoS ONE 2013, 8, e65727. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; Tang, R.T.; Tao, J.; Wang, X.N.; Zheng, B.S.; Feng, Y. Chimeric cellulase matrix for investigating intramolecular synergism between non-hydrolytic disruptive functions of carbohydrate-binding modules and catalytic hydrolysis. J. Biol. Chem. 2012, 287, 29568–29578. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Suzuki, M.R.; Hammel, K.E. Processive endoglucanase active in crystalline cellulose hydrolysis by the brown rot basidiomycete Gloeophyllum trabeum. Appl. Environ. Microbiol. 2005, 71, 2412–2417. [Google Scholar] [CrossRef] [PubMed]
- Kostylev, M.; Wilson, D. Two-parameter kinetic model based on a time-dependent activity coefficient accurately describes enzymatic cellulose digestion. Biochemistry 2013, 52, 5656–5664. [Google Scholar] [CrossRef] [PubMed]
- Cruys-Bagger, N.; Elmerdahl, J.; Praestgaard, E.; Tatsumi, H.; Spodsberg, N.; Borch, K.; Westh, P. Pre-steady-state kinetics for hydrolysis of insoluble cellulose by cellobiohydrolase Cel7A. J. Biol. Chem. 2012, 287, 18451–18458. [Google Scholar] [CrossRef] [PubMed]
- Bansal, P.; Hall, M.; Realff, M.J.; Lee, J.H.; Bommarius, A.S. Modeling cellulase kinetics on lignocellulosic substrates. Biotechnol. Adv. 2009, 27, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Praestgaard, E.; Elmerdahl, J.; Murphy, L.; Nymand, S.; McFarland, K.C.; Borch, K.; Westh, P. A kinetic model for the burst phase of processive cellulases. FEBS J. 2011, 278, 1547–1560. [Google Scholar] [CrossRef] [PubMed]
- Murphy, L.; Cruys-Bagger, N.; Damgaard, H.D.; Baumann, M.J.; Olsen, S.N.; Borch, K.; Lassen, S.F.; Sweeney, M.; Tatsumi, H.; Westh, P. Origin of initial burst in activity for Trichoderma reesei endo-glucanases hydrolyzing insoluble cellulose. J. Biol. Chem. 2012, 287, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.; Wang, Y.; An, L.; Yao, L. The slowdown of the endoglucanase Trichoderma reesei Cel5A-catalyzed cellulose hydrolysis is related to its initial activity. Biochemistry 2014, 53, 7650–7658. [Google Scholar] [CrossRef] [PubMed]
- Kurasin, M.; Valjamae, P. Processivity of cellobiohydrolases is limited by the substrate. J. Biol. Chem. 2011, 286, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Chiriac, A.I.; Cadena, E.M.; Vidal, T.; Torres, A.L.; Diaz, P.; Pastor, F.I. Engineering a family 9 processive endoglucanase from Paenibacillus barcinonensis displaying a novel architecture. Appl. Microbiol. Biotechnol. 2010, 86, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Ding, S. Processivity and enzymatic mode of a glycoside hydrolase family 5 endoglucanase from Volvariella volvacea. Appl. Environ. Microbiol. 2013, 79, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Rollin, J.A.; Zhu, Z.; Sathitsuksanoh, N.; Zhang, Y.H. Increasing cellulose accessibility is more important than removing lignin: A comparison of cellulose solvent-based lignocellulose fractionation and soaking in aqueous ammonia. Biotechnol. Bioeng. 2011, 108, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, D.; Zhou, Y.; Liu, L.; Han, W.; Zheng, B.; Wang, Z.; Zhang, Z. Cloning, expression and characterization of a novel thermophilic polygalacturonase from Caldicellulosiruptor bescii DSM 6725. Int. J. Mol. Sci. 2014, 15, 5717–5729. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein using the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zhang, Y.H.P.; Cui, J.B.; Lynd, L.R.; Kuang, L.R. A transition from cellulose swelling to cellulose dissolution by o-phosphoric acid: Evidence from enzymatic hydrolysis and supramolecular structure. Biomacromolecules 2006, 7, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Gao, G.; Li, Y.; Yang, L.; Liang, Y.; Jin, H.; Han, W.; Feng, Y.; Zhang, Z. Cloning, Expression, and Characterization of a Thermophilic Endoglucanase, AcCel12B from Acidothermus cellulolyticus 11B. Int. J. Mol. Sci. 2015, 16, 25080-25095. https://doi.org/10.3390/ijms161025080
Wang J, Gao G, Li Y, Yang L, Liang Y, Jin H, Han W, Feng Y, Zhang Z. Cloning, Expression, and Characterization of a Thermophilic Endoglucanase, AcCel12B from Acidothermus cellulolyticus 11B. International Journal of Molecular Sciences. 2015; 16(10):25080-25095. https://doi.org/10.3390/ijms161025080
Chicago/Turabian StyleWang, Junling, Gui Gao, Yuwei Li, Liangzhen Yang, Yanli Liang, Hanyong Jin, Weiwei Han, Yan Feng, and Zuoming Zhang. 2015. "Cloning, Expression, and Characterization of a Thermophilic Endoglucanase, AcCel12B from Acidothermus cellulolyticus 11B" International Journal of Molecular Sciences 16, no. 10: 25080-25095. https://doi.org/10.3390/ijms161025080







