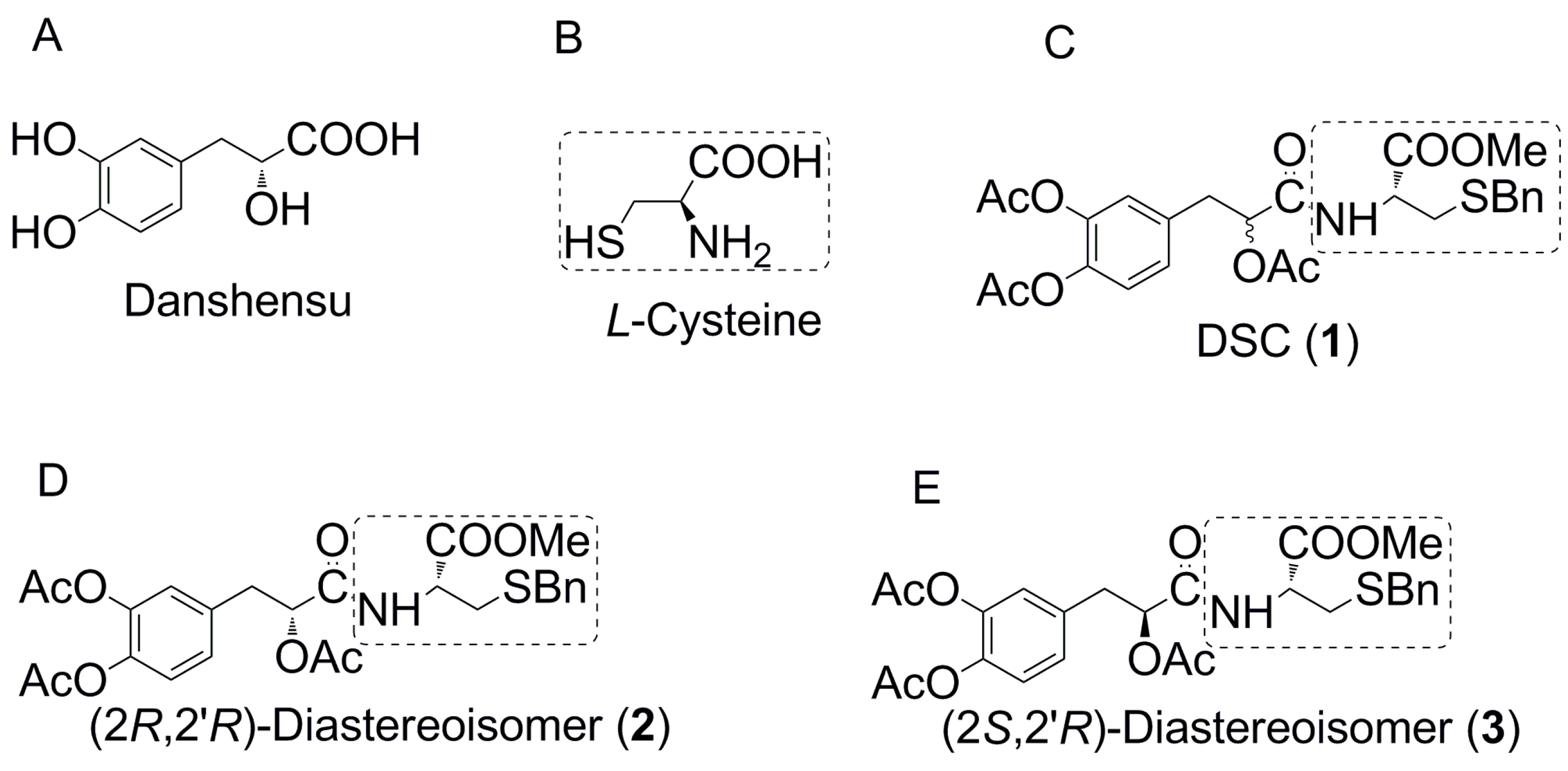
2.1. The Asymmetric Synthesis of Danshensu-Cysteine Conjugates
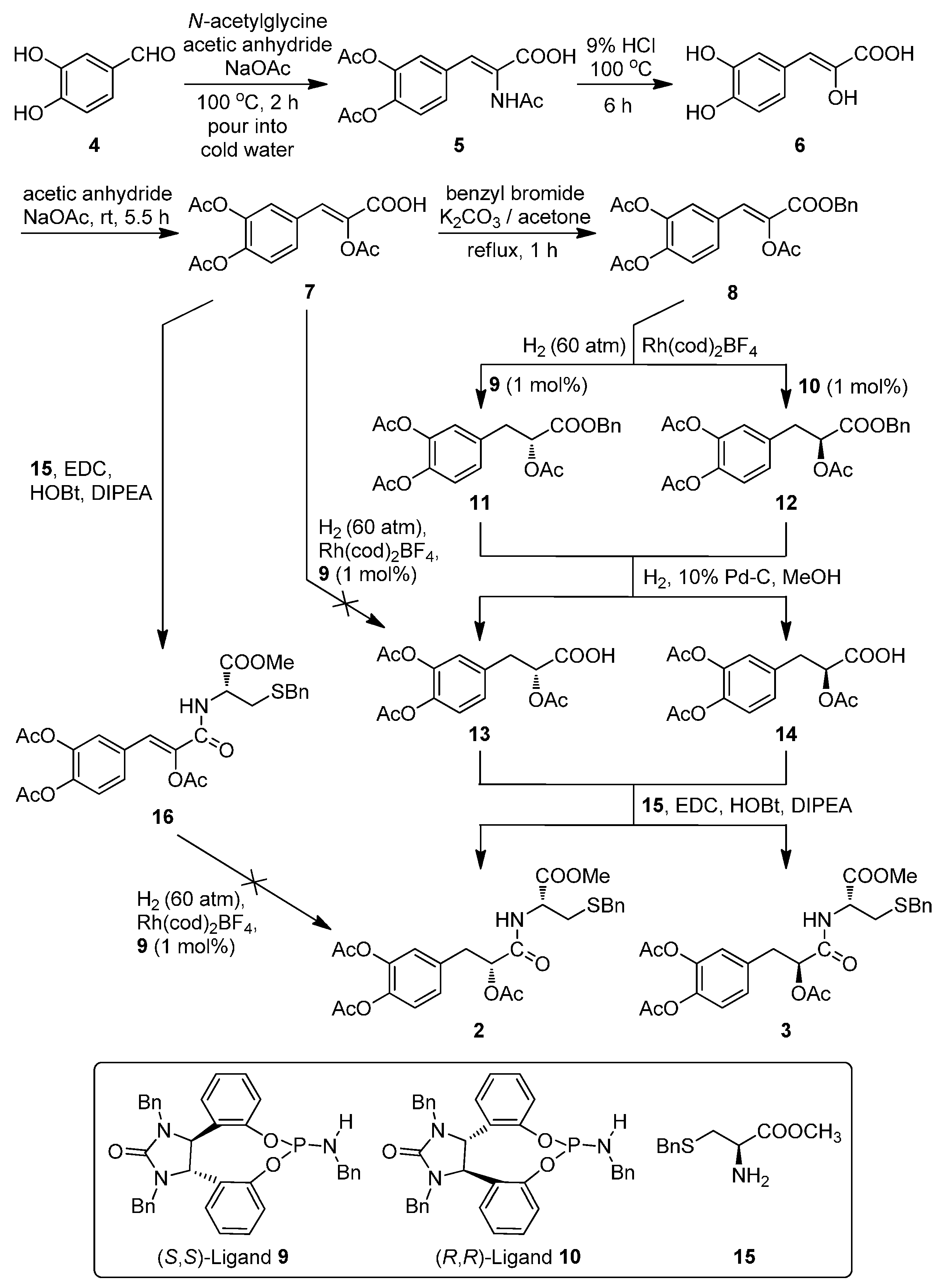
As outlined in
Figure 2, the designed conjugates were synthesized efficiently using 3,4-dihydroxybenzaldehyde (
4) as the starting material. The key intermediate
7 and
l-cysteine derivatives
15 were synthesized according to the reported procedures [
12,
13]. The condensation of
4 with
N-acetylglycine followed by hydrolysis in hydrochloric acid and protection of the hydroxyl groups in acetic anhydride afforded
7 in 33% total yield. Esterization of
7 with benzyl bromide in the presence of K
2CO
3 in acetone provided the thoroughly protected compound
8 in 90% yield. The asymmetric hydrogenation of
8 using
9/
10 as the chiral ligand and [Rh(cod)
2]BF
4 as the catalyst precursor gave excellent enantioselectivities (>97% enantiomeric excess) and good yields (>89%) of compounds
11/
12 respectively under the modified conditions of the literature procedures [
14]. The deprotection of
11/
12 by hydrogenolysis under the catalysis of 10% Pd-C in methanol attained (
R)/(
S)-2-acetoxy-3-(3,4-diacetoxyphenyl) propanoic acid (
13/
14) in >95% yields, which were then subjected to the condensation with
S-benzyl-
l-cysteine methyl ester (
15) in the presence of 1-hydroxybenzotriazole (HOBt), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI) and diisopropylethylamine (DIPEA) in CH
2Cl
2, affording the target amide conjugates
2/
3 in 79% and 81% yields respectively.
Another synthetic route to 2/3 was also tried via the direct asymmetric hydrogenation of 7 to give 13 or condensation of 7 with 15 followed by asymmetric hydrogenation of 16, but both of the asymmetric hydrogenation of 7/16 failed which may be attributed to the reactive hydrogen in the carboxy group of 7 and the sulphur atom or steric-hindrance in 16.
Figure 2.
The synthesis of target diastereoisomers 2 and 3.
Figure 2.
The synthesis of target diastereoisomers 2 and 3.
2.1.1. (Z)-2-Acetoxy-3-(3,4-diacetoxyphenyl)acrylic Acid Benzyl Ester (8)
(
Z)-2-Acetoxy-3-(3,4-diacetoxyphenyl)acrylic acid (
7) was prepared by using aldehyde
4 as the starting material according to the method in the literature [
13]. To a solution of a mixture of
7 (1.5 g, 4.65 mmol) and anhydrous K
2CO
3 (1.28 g, 9.31 mmol) in acetone (30 mL) was dropped benzyl bromide (1.5 mL, 12.6 mmol). The mixture was refluxed for 7 h, then filtered and concentrated. The residue was purified by silica gel column chromatography (eluent:PE-EtOAc, 6:1~3:1), providing 1.72 g of
8 as a white solid in 90% yield, mp 79–82 °C.
1H NMR (400 MHz, CDCl
3) δ 2.28 (s, 9H, –OCOCH
3), 5.28 (s, 2H, –CH
2Ph), 7.21 (d,
J = 8.6 Hz, 1H), 7.27 (d,
J = 5.5 Hz, 1H), 7.35–7.40 (m, 5H), 7.43 (d,
J = 8.8 Hz, 1H), 7.47 (s, 1H). ESI-MS
m/
z (%): 435.2 (M + Na
+, 100). High Resolution Mass Spectrum (HRMS) calculated mass for C
22H
20O
8Na [M + Na
+] 435.1056, found 435.1050.
2.1.2. (R)/(S)-2-Acetoxy-3-(3,4-diacetoxyphenyl)propanoic Acid Benzyl Ester (11/12)
[Rh(cod)2]BF4 (2.0 mg, 0.005 mmol), ligand 9 (6 mg, 0.011 mmol) in CH2Cl2 (1 mL) were dissolved under nitrogen and the solution was stirred at room temperature for 10 min. The substrate 8 (206 mg, 0.5 mmol) in CH2Cl2 (2 mL) was added to the above catalyst solution. The mixture was then transferred to a stainless steel autoclave under nitrogen atmosphere, and then sealed. After purging with hydrogen three times, final H2 pressure was adjusted to 60 atm. After stirring at room temperature for 24 h, H2 was released. After removal of the solvent under the reduced pressure, the residue of the product was passed through a pad of Celite and purified by silica gel column chromatography (eluent:PE-EtOAc, 5:1~3:1), providing 184 mg of 11 as a colorless oil in 89% yield and 97% ee, = +4.8° (c = 1.0 mg/mL, CHCl3). The enantiomeric excess was determined by HPLC on Chiralcel OJ column, hexane:isopropanol = 95:5; flow rate = 1.0 mL/min; UV detection at λ = 230 nm; tR = 16.1 min (minor), 19.8 min (major), respectively. Using 10 as chiral ligand, 186 mg of product 12 could be obtained as a colorless oil in 90% yield and 98% ee, = −4.8° (c = 1.0 mg/mL, CHCl3). 1H NMR (400 MHz, CDCl3), δ 2.1 (s, 3H, –OCOCH3), 2.29 (s, 6H, –OCOCH3), 3.06–3.19 (m, 2H, –CH2Ar), 5.17 (s, 2H, –CH2Ph), 5.20–5.25 (m, 1H, –CHOCOCH3), 7.05–7.11 (m, 3H, –Ar), 7.27–7.39 (m, 5H, –Ph). ESI-MS m/z (%): 437.3 (M + Na+, 100). HRMS calculated mass for C22H22O8Na [M + Na+] 437.1212, found 437.1207.
2.1.3. (R)/(S)-2-Acetoxy-3-(3,4-diacetoxyphenyl)propanoic Acid (13/14)
Compounds 11/12 (200 mg, 0.48 mmol) were deprotected by hydrogenolysis under the catalysis of 10% Pd-C (200 mg) at atmospheric pressure in MeOH (5 mL) for 24 h. The mixture was filtered and concentrated, and the residue was purified by silica gel column chromatography (eluent:PE-EtOAc, 10:1~5:1) to give 13 (149 mg)/14 (148 mg) as sticky colorless oils in 96%/95% yields respectively. 1H NMR (400 MHz, CDCl3), δ 2.17 (s, 3H, –OCOCH3), 2.27 (s, 6H, –OCOCH3), 3.12–3.18 (m, 2H, –CH2–), 5.21 (br, 1H, –COOH), 7.09–7.12 (m, 3H, Ph–H).
2.1.4. (R)-N-((R)-3-Benzylthio-1-methoxy-1-oxo-2-propanyl)-2-acetoxy-3-(3,4-diacetoxyphenyl)propanamide (2) and (S)-N-((R)-3-Benzylthio-1-methoxy-1-oxo-2-propanyl)-2-acetoxy-3-(3,4-diacetoxyphenyl)propanamide (3)
To a solution of carboxylic acid 13/14 (162 mg, 0.5 mmol) in CH2Cl2 (8 mL) was added HOBt (82 mg, 0.6 mmol) and EDCI (116 mg, 0.6 mmol). The mixture was stirred at room temperature for 15 min, then methyl S-benzyl-l-cysteine hydrochloride (15) (244 mg, 0.55 mmol) followed by diisopropylethylamine (0.1 mL, 0.6 mmol) were added. After 2 h, The mixture was filtered and concentrated, and the residue was purified by silica-gel column chromatography to give the corresponding amide conjugates 2 (210 mg)/3 (215 mg) as white viscous oils in 79%/81% yields and 97%/73% diastereoisomeric excess, respectively. Compound 2: = +12.8° (c = 1.0 mg/mL, CHCl3). 1H NMR (400 MHz, CDCl3) δ 2.11, 2.13 (s, s, 3H, –OCOCH3), 2.25–2.27 (m, 6H, –OCOCH3), 2.78–2.87 (m, 2H, –CH2SBn), 3.11–3.21 (m, 2H, ArCH2CH–), 3.65 (s, 2H, –SCH2Ph), 3.74 (s, 3H, –COOCH3), 4.71–4.75 (m, 1H, –NCH–), 5.36–5.40 (m, 1H, ArCH2CH–), 6.75 (t, J = 7.9 Hz, 1H, –NH–), 7.05–7.09 (m, 3H, Ph–H), 7.24–7.33 (m, 5H, Ph–H). 13C NMR (100 MHz, CDCl3), δ 170.8, 169.5, 168.8, 168.2, 168.1, 141.8, 141.0, 137.5, 134.5, 129.0, 128.9, 128.6, 127.6, 127.3, 124.7, 123.3, 73.7, 52.7, 51.2, 36.9, 36.4, 33.3, 33.0, 20.8, 20.6. ESI-MS m/z (%): 554.8 (M + Na+, 100). HRMS calculated mass for C26H30NO9S [M + H+] 532.1636, found 532.1633. Compound 3: = +8.9° (c = 1.0 mg/mL, CHCl3).
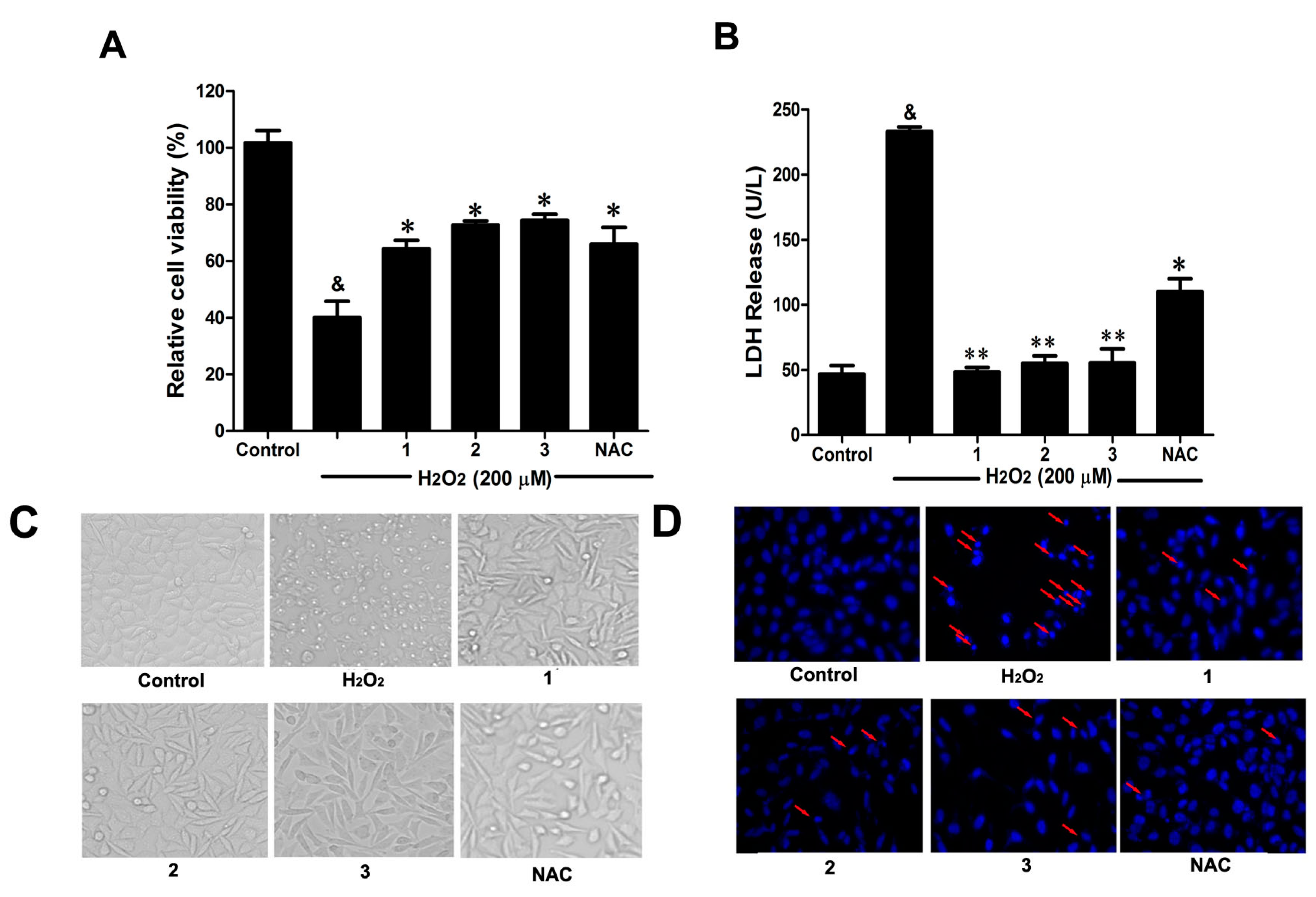
2.2. Effects of Danshensu-Cysteine Conjugates (DSC (1,N-((R)-3-Benzylthio-1-methoxy-1-oxo-2-propanyl)-2-acetoxy-3-(3,4-diacetoxyphenyl)), 2 and 3) on H2O2-Induced Cellular Injury in SH-SY5Y Cells
Consistent with our previous study [
6], H
2O
2 (200 μM) stimulation for 12 h significantly decreased cell viability, accompanied by serious lactate dehydrogenase (LDH) release (
Figure 3A,B). However, pretreatment with the three Danshensu-cysteine conjugates (DSC,
2 and
3) (all at 50 μM), showed similar protective effects on H
2O
2-induced cell death and LDH release in SH-SY5Y cells. Meanwhile, we also checked the effects of these three compounds on H
2O
2-induced changes of cellular morphology and nuclear morphological changes. As shown in
Figure 3C,D, pretreatment with the three derivatives (DSC,
2 and
3) (all at 50 μM) also showed similar protective effects on H
2O
2-induced cellular morphological changes and nuclear morphological changes. As a positive control,
N-acetyl-
l-cysteine (NAC, 5 mM) could markedly attenuate H
2O
2-mediated cellular events mentioned above. Collectively, DSC and its two diastereoisomers (
2 and
3) showed similar protective effects in H
2O
2-induced cellular injury in SH-SY5Y cells.
Figure 3.
Effects of Danshensu-cysteine conjugates on H2O2-induced cellular injury in SH-SY5Y cells. SH-SY5Y cells were incubated with Danshensu-cysteine conjugates (DSC, 2 and 3) (all at 50 μM) or N-acetyl-l-cysteine (NAC, 5 mM) for 4 h, then stimulated with H2O2 (200 μM) for 12 h, the cell viability (A) and lactate dehydrogenase (LDH) (B) release were measured, respectively. Data shown are means ± SEM, & p < 0.05 compared with unstimulated cells, * p < 0.05, ** p < 0.01 compared with H2O2-stimulated cells. Data were from at least three independent experiments, each performed in duplicate. Representative picture shows cell morphology (C) and nuclear morphology (D) detected by microscope or fluorescence microscope (magnification, 200×), respectively.
Figure 3.
Effects of Danshensu-cysteine conjugates on H2O2-induced cellular injury in SH-SY5Y cells. SH-SY5Y cells were incubated with Danshensu-cysteine conjugates (DSC, 2 and 3) (all at 50 μM) or N-acetyl-l-cysteine (NAC, 5 mM) for 4 h, then stimulated with H2O2 (200 μM) for 12 h, the cell viability (A) and lactate dehydrogenase (LDH) (B) release were measured, respectively. Data shown are means ± SEM, & p < 0.05 compared with unstimulated cells, * p < 0.05, ** p < 0.01 compared with H2O2-stimulated cells. Data were from at least three independent experiments, each performed in duplicate. Representative picture shows cell morphology (C) and nuclear morphology (D) detected by microscope or fluorescence microscope (magnification, 200×), respectively.
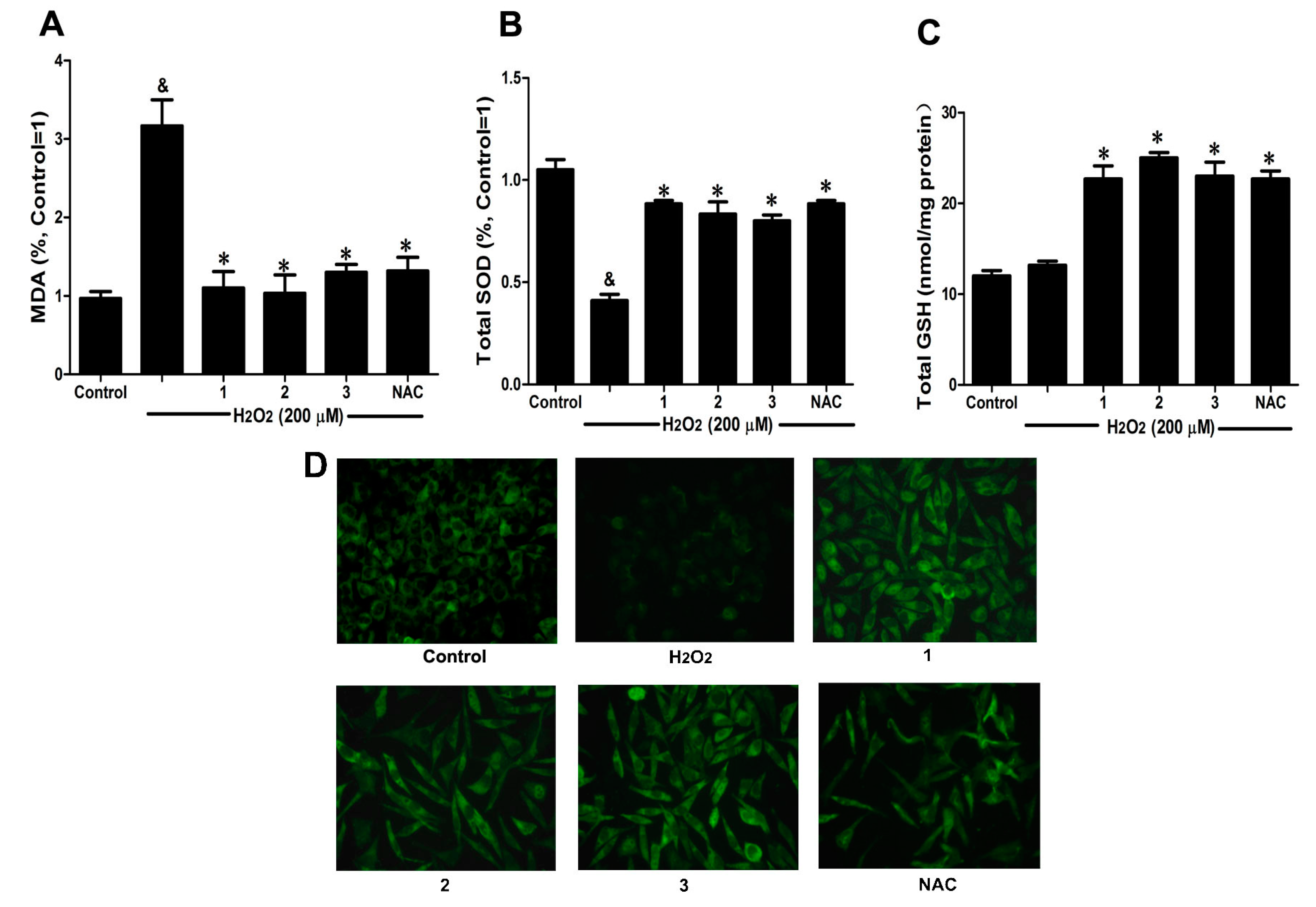
2.3. Effects of Danshensu-Cysteine Conjugates on Intercellular Anti-Oxidative Capacity in H2O2-Stimulated SH-SY5Y Cells
To further elucidate the protective effects of Danshensu-cysteine conjugates on H
2O
2-induced cellular injury, malondialdehyde (MDA) production, intracellular total superoxide dismutase (SOD) and reduced glutathione (GSH) activity as well as glutathione peroxidase (GPx) expression were measured by commercial kits and immunofluorescence, respectively. As shown in
Figure 4A,B, H
2O
2 stimulation significantly decreased intercellular anti-oxidative capacity as evidenced by the increase of MDA production and reduction of SOD activity, which were evidently ameliorated by pretreatment with Danshensu-cysteine conjugates (DSC,
2 and
3) (all at 50 μM) or NAC (5 mM). Although H
2O
2 stimulation did not decrease GSH activity, incubation with the three derivatives (DSC,
2 and
3) (all at 50 μM) or NAC (5 mM) significantly increased GSH activity in H
2O
2-stimulated cells (
Figure 4C). In addition, three conjugates (DSC,
2 and
3) (all at 50 μM) or NAC (5 mM) also evidently blocked H
2O
2-induced decrease of GPx expression in SH-SY5Y cells as detected by immunofluorescent staining (
Figure 4D). These data strongly suggested that DSC and its two diastereoisomers (
2 and
3) could improve intercellular anti-oxidative capacity in H
2O
2-stimulated SH-SY5Y cells, but these effects were not statistically significant between the three compounds at the dosage used in this study.
Figure 4.
Effects of Danshensu-cysteine conjugates on intercellular anti-oxidative capacity in H2O2-stimulated SH-SY5Y cells. SH-SY5Y cells were incubated with Danshensu-cysteine conjugates (all at 50 μM) or NAC (5 mM) for 4 h, then stimulated with H2O2 (200 μM) for 12 h; the malondialdehyde (MDA) (A) production, superoxide dismutase (SOD) (B) and reduced glutathione (GSH) (C) activities were measured, respectively. Data shown are means ± SEM, & p < 0.05 compared with unstimulated cells, * p < 0.05 compared with H2O2-stimulated cells. Data were from at least three independent experiments, each performed in duplicate; (D) Representative pictures showing glutathione peroxidase (GPx) expression by immunofluorescent staining (magnification, 200×).
Figure 4.
Effects of Danshensu-cysteine conjugates on intercellular anti-oxidative capacity in H2O2-stimulated SH-SY5Y cells. SH-SY5Y cells were incubated with Danshensu-cysteine conjugates (all at 50 μM) or NAC (5 mM) for 4 h, then stimulated with H2O2 (200 μM) for 12 h; the malondialdehyde (MDA) (A) production, superoxide dismutase (SOD) (B) and reduced glutathione (GSH) (C) activities were measured, respectively. Data shown are means ± SEM, & p < 0.05 compared with unstimulated cells, * p < 0.05 compared with H2O2-stimulated cells. Data were from at least three independent experiments, each performed in duplicate; (D) Representative pictures showing glutathione peroxidase (GPx) expression by immunofluorescent staining (magnification, 200×).
2.4. DSC Attenuated H2O2-Induced Cell Damage in Human Umbilical Vein Endothelial Cells (HUVEC)
Oxidative stress is one of the mechanisms that cause widespread endothelial dysfunction in cardiovascular diseases and disorders [
15]. To examine the endothelial protective effects of Danshensu-cysteine conjugates on oxidative stress-induced cytotoxicity, HUVEC were conditioned with Danshensu-cysteine conjugates for 4 h prior to H
2O
2 stimulation. Taking into account the difficulty of chemical synthesis, only DSC was chosen to study the protective effects of Danshensu-cysteine conjugates on H
2O
2-induced cellular injury. As shown in
Figure 5A, H
2O
2 (200 μM) stimulation for 12 h significantly decreased cell viability, which was attenuated by DSC (5–100 μM) in a concentration-dependent manner. Meanwhile, DSC (5–100 μM) also decreased H
2O
2-induced LDH release in a concentration dependent manner (
Figure 5B). In addition, DSC (5–200 μM) treatment for 24 h did not affect cell viability of HUVEC as checked by MTT assay (data not shown). As a positive control, NAC also markedly reduced H
2O
2-induced cellular damage in HUVEC (
Figure 5A,B).
Figure 5.
The danshensu-cysteine conjugate N-((R)-3-benzylthio-1-methoxy-1-oxo-2-propanyl)-2-acetoxy-3-(3,4-diacetoxyphenyl) propanamide (DSC) attenuated H2O2-induced cell damage in human umbilical vein endothelial cells (HUVEC). HUVEC were incubated with indicated concentrations of DSC or NAC for 4 h, then stimulated with H2O2 (200 μM) for 12 h; the cell viability (A) and LDH (B) release were measured, respectively. Data shown are means ± SEM, & p < 0.05 compared with unstimulated cells, * p < 0.05 compared with H2O2-stimulated cells. Data were from at least three independent experiments, each performed in duplicate.
Figure 5.
The danshensu-cysteine conjugate N-((R)-3-benzylthio-1-methoxy-1-oxo-2-propanyl)-2-acetoxy-3-(3,4-diacetoxyphenyl) propanamide (DSC) attenuated H2O2-induced cell damage in human umbilical vein endothelial cells (HUVEC). HUVEC were incubated with indicated concentrations of DSC or NAC for 4 h, then stimulated with H2O2 (200 μM) for 12 h; the cell viability (A) and LDH (B) release were measured, respectively. Data shown are means ± SEM, & p < 0.05 compared with unstimulated cells, * p < 0.05 compared with H2O2-stimulated cells. Data were from at least three independent experiments, each performed in duplicate.
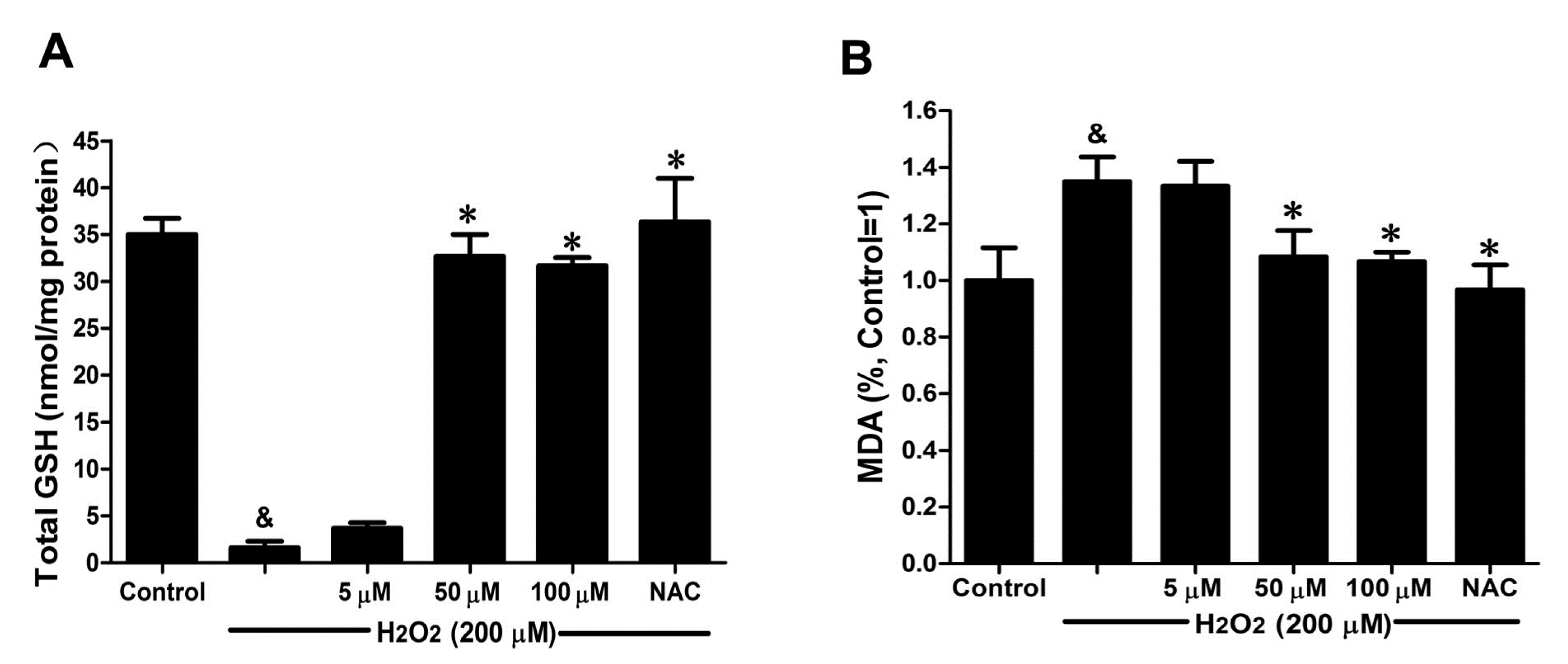
2.5. DSC Improved Anti-Oxidative Capacity in H2O2-Stimulated HUVEC
GSH activity and MDA production were chosen to evaluate the effects of DSC on anti-oxidative capacity in H
2O
2-stimulated HUVEC. As shown in
Figure 6A, H
2O
2 (200 μM) stimulation for 12 h markedly decreased GSH activity (
p < 0.05), which was reversed by DSC pretreatment in a concentration-dependent manner. Meanwhile, a significant increase in MDA release was observed after stimulation with H
2O
2 for 12 h (
p < 0.05), reflecting a decrease in anti-oxidative ability. However, pretreatment with DSC also resulted in a decrease of H
2O
2-induced MDA levels in HUVEC (
Figure 6B). Meanwhile, NAC markedly enhanced intercellular anti-oxidative capacity in H
2O
2-stimulated HUVEC.
Figure 6.
DSC improved anti-oxidative capacity in H2O2-stimulated HUVEC. HUVEC were incubated with indicated concentrations of DSC or NAC for 4 h, then stimulated with H2O2 (200 μM) for 12 h; the GSH activity (A) and MDA (B) production were measured, respectively. Data shown are means ± SEM, & p < 0.05 compared with unstimulated cells, * p < 0.05 compared with H2O2-stimulated cells. Data were from at least three independent experiments, each performed in duplicate.
Figure 6.
DSC improved anti-oxidative capacity in H2O2-stimulated HUVEC. HUVEC were incubated with indicated concentrations of DSC or NAC for 4 h, then stimulated with H2O2 (200 μM) for 12 h; the GSH activity (A) and MDA (B) production were measured, respectively. Data shown are means ± SEM, & p < 0.05 compared with unstimulated cells, * p < 0.05 compared with H2O2-stimulated cells. Data were from at least three independent experiments, each performed in duplicate.
2.6. DSC Inhibited H2O2-Induced Mitochondrial Membrane Potential (ΔΨm) Loss and Apoptosis in HUVEC
Loss of mitochondrial membrane potential (ΔΨ
m) played a pivotal role in H
2O
2-induced apoptosis [
6]. The effect of DSC on H
2O
2-induced ΔΨ
m loss was analyzed by flow cytometry using JC-1 staining. As shown in
Figure 7A, stimulation with H
2O
2 significantly induced the loss of ΔΨ
m, which was attenuated by pretreatment with DSC in a concentration dependent manner. Meanwhile, cell apoptosis induced by H
2O
2 was also inhibited by DSC as observed by Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) assay. NAC, a non-targeted ROS scavenger, also markedly inhibited H
2O
2-induced ΔΨ
m loss and apoptosis in HUVEC.
Figure 7.
DSC inhibited H2O2-induced mitochondrial membrane potential (ΔΨm) loss and apoptosis in HUVEC. HUVEC were incubated with indicated concentrations of DSC or NAC for 4 h, then stimulated with H2O2 (200 μM) for 2 or 12 h; the ΔΨm (A) and apoptosis (B) were measured by flow cytometry, respectively. Data shown are means ± SEM, & p < 0.05 compared with unstimulated cells, * p < 0.05 compared with H2O2-stimulated cells. Data were from at least three independent experiments, each performed in duplicate.
Figure 7.
DSC inhibited H2O2-induced mitochondrial membrane potential (ΔΨm) loss and apoptosis in HUVEC. HUVEC were incubated with indicated concentrations of DSC or NAC for 4 h, then stimulated with H2O2 (200 μM) for 2 or 12 h; the ΔΨm (A) and apoptosis (B) were measured by flow cytometry, respectively. Data shown are means ± SEM, & p < 0.05 compared with unstimulated cells, * p < 0.05 compared with H2O2-stimulated cells. Data were from at least three independent experiments, each performed in duplicate.
2.8. Discussion
Danshensu, a hydrophilic bioactive component of Danshen, attracts considerable interest due to its salubrious biological activities. However, the chemical structure of Danshensu contains unstable phenolic hydroxyl groups, which limits its further therapeutic development for clinical use [
11]. Recently, we reported that the Danshensu-cysteine conjugate, DSC, is found to have an improved antioxidant and anti-apoptotic activity in
vitro [
6,
12]. The present study provides the first evidence that the two diastereoisomers (
2 and
3) of DSC are synthesized asymmetrically in excellent stereoselectivities and high yields using a chemical approach based on asymmetric hydrogenation [
14]. As the presence of the active hydroxyl group contributes to the instability of Danshensu, we used the asymmetric synthesis to obtain Danshensu derivatives, with an acetyl analogue of Danshensu to eliminate the unstable hydroxyl group, which showed a much better stability in our previous study [
6,
12]. The cytoprotective property and the improved stability make the novel Danshensu-cysteine conjugates promising as anti-apoptotic reagents.
Recently, we demonstrated that DSC protected SH-SY5Y cells against H
2O
2-induced cellular injury, at least in part, due to its strong anti-oxidative and anti-apoptotic properties [
6]. Therefore, the potential anti-oxidative and anti-apoptotic activity of the diastereoisomers of DSC was firstly evaluated in H
2O
2-stimulated SH-SY5Y cells. Consistent with previous studies [
6,
16], H
2O
2 stimulation significantly induces cellular damage and decreases intercellular anti-oxidative capacity. However, DSC and its diastereoisomers (all at 50 μM) significantly attenuate H
2O
2-induced cellular injury in SH-SY5Y cells, as evidenced by increase of cell viability and decrease of cellular morphological changes and nuclear condensation. However, the three compounds pretreatment also markedly increase SOD and GSH activity as well as GPx expression, and decrease LDH release and MDA production in H
2O
2-stimulated SH-SY5Y cells. But, the cyto-protective activity is not significantly different among these stereoisomers. These results suggest that DSC and its two diastereoisomers have similar protective effects on the anti-oxidative activity against H
2O
2-induced cellular damage. Our findings indicate that the Danshensu-cysteine conjugates exert their cytoprotective effects against oxidative insults through modulation of intercellular anti-oxidative capacities.
Danshen and its bioactive ingredients have been mainly used for the treatment of cardiovascular diseases [
9,
17]. Meanwhile, our previous studies have demonstrated that Danshensu-cysteine conjugates exhibited cardiovascular-protective activity [
12,
18]. Thus we hypothesize that the Danshensu-cysteine conjugates may also exert beneficial effects in cardiovascular diseases. DSC and its diastereoisomers also exert similar protective effects on H
2O
2-induced cellular injury in SH-SHY5Y. Taking into account the clinical application and the difficulty of chemical synthesis of the Danshensu-cysteine conjugates, DSC was chosen for further research. As expected, DSC significantly attenuates H
2O
2-induced cellular injury and redox imbalance in endothelial cells, which is one of the major causes of cell and tissue injury and implicated in the pathological process of cardiovascular disease [
11,
19]. H
2O
2 can induce ΔΨ
m disruption and release of cytochrome C from mitochondria into the cytoplasm, and therefore initiate apoptosis by activating caspase-3 [
16,
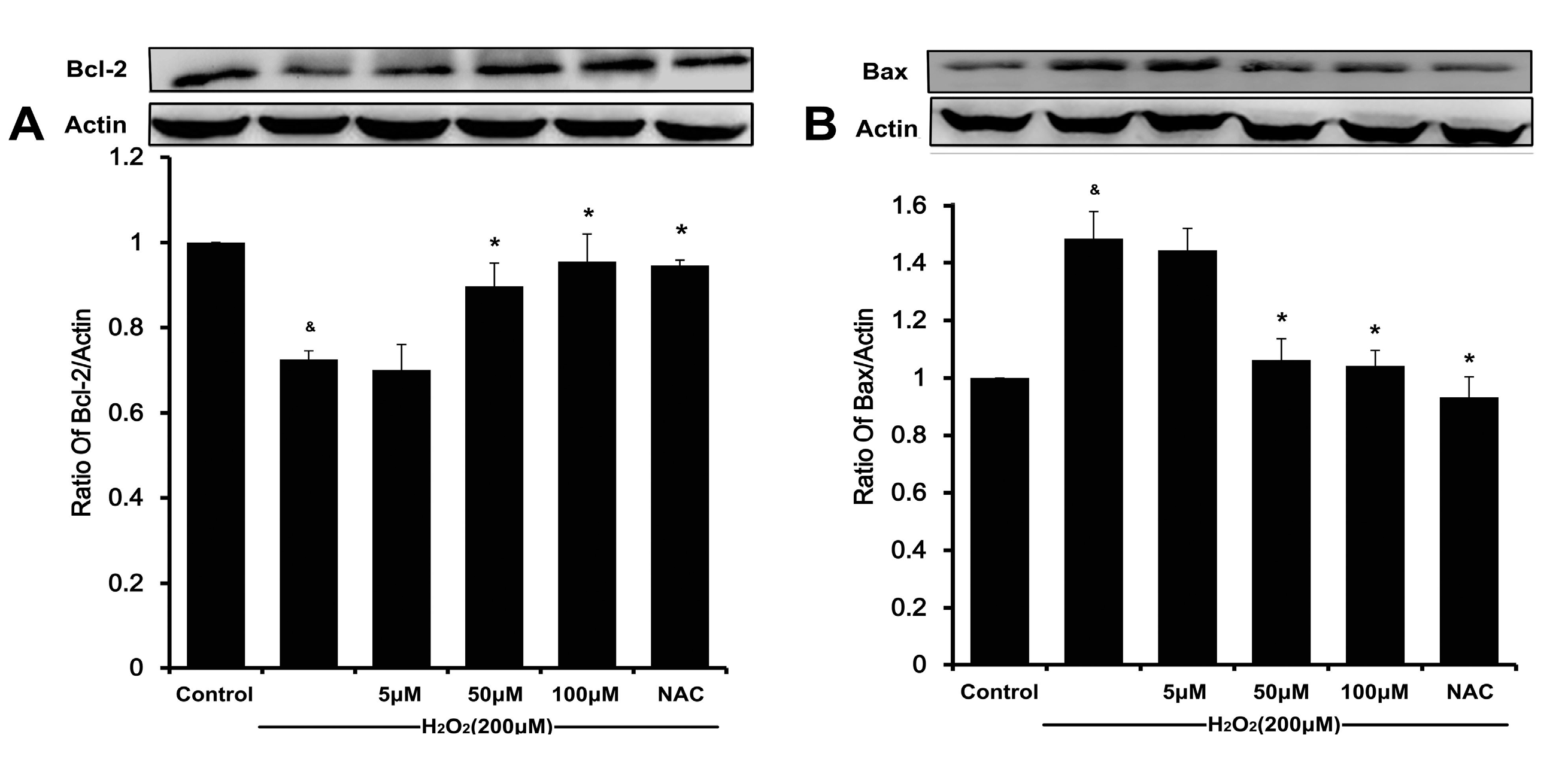
20]. Meanwhile, a decreasing Bcl-2/Bax ratio results in mitochondrial dysfunction, which ultimately triggers the activation of caspase cascades and results in apoptosis [
6,
21]. In our present study, DSC concentration-dependently reverses H
2O
2-mediated loss of ΔΨ
m and decreases the ratio of Bcl-2/Bax in HUVEC. The subsequent disruption of ΔΨ
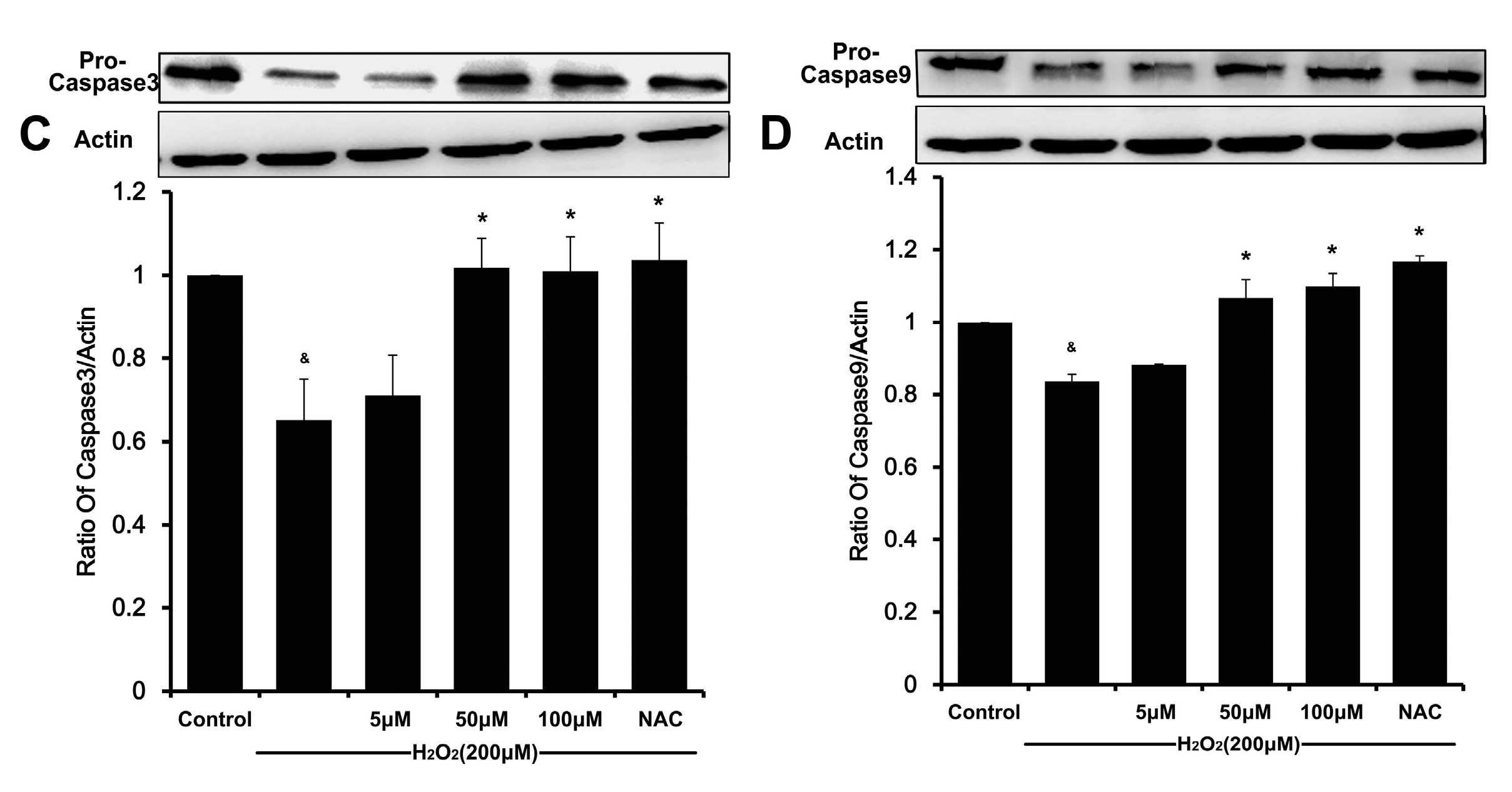
m causes activating caspase-9, which in turn activates caspase-3 and eventually apoptosis [
22,
23]. Likewise, the degradation of caspase-9 and caspase-3 in H
2O
2-stimulated HUVEC was also attenuated by DSC in a concentration-dependent manner. Altogether, these results indicate that DSC protective action against cell death involves anti-apoptotic mechanisms.