Cyclodextrin-Complexed Ocimum basilicum Leaves Essential Oil Increases Fos Protein Expression in the Central Nervous System and Produce an Antihyperalgesic Effect in Animal Models for Fibromyalgia
Abstract
:1. Introduction
2. Results and Discussion
2.1. GC-MS and GC-FID Analysis
| Peak | RT (min) | Compound | GC-MS (%) | RRI exp. b | IRR c |
|---|---|---|---|---|---|
| 1 | 7.517 | α-pinene | 0.15 | 932 | 932 |
| 2 | 8.825 | sabinene | 0.14 | 971 | 969 |
| 3 | 8.992 | β-pinene | 0.51 | 977 | 975 |
| 4 | 10.942 | 1.8-cineole | 6.12 | 1032 | 1026 |
| 5 | 13.542 | linalool | 68.96 | 1102 | 1095 |
| 6 | 17.033 | α-terpineol | 0.72 | 1194 | 1186 |
| 7 | 19.083 | geraniol | 13.09 | 1250 | 1249 |
| 8 | 20.317 | isobornyl acetate | 0.38 | 1284 | 1283 |
| 9 | 23.592 | acetategeranyl | 2.83 | 1377 | 1379 |
| 10 | 24.017 | β-elemene | 0.35 | 1389 | 1389 |
| 11 | 25.058 | (E)-caryophyllene | 0.27 | 1420 | 1417 |
| 12 | 25.483 | trans-α-bergamotene | 2.25 | 1433 | 1432 |
| 13 | 27.100 | amorpha-4,7(11)-diene | 0.83 | 1482 | 1479 |
| 14 | 27.767 | α-bulnesene | 0.20 | 1502 | 1509 |
| 15 | 28.125 | γ-cadinene | 0.86 | 1513 | 1513 |
| 16 | 31.333 | NI a | 0.27 | 1616 | NI |
| 17 | 32.133 | epi-α-cadinol | 2.07 | 1643 | 1638 |
2.2. Thermal Analyses


2.3. Mechanical Hyperalgesia Analyses
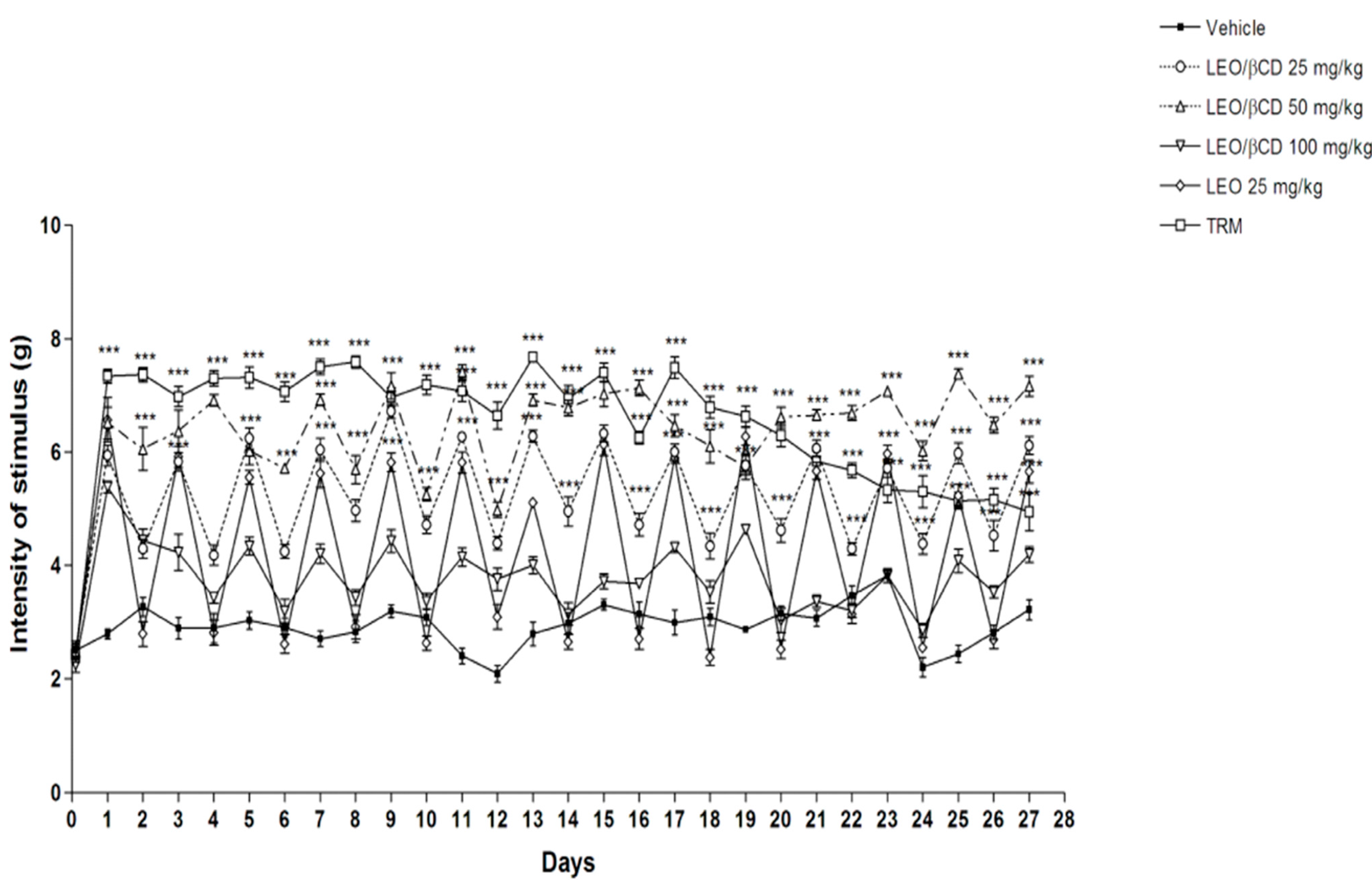
2.4. Motor Coordination and Grip Strength Performance Analyses


2.5. Immunofluorescence Analyses

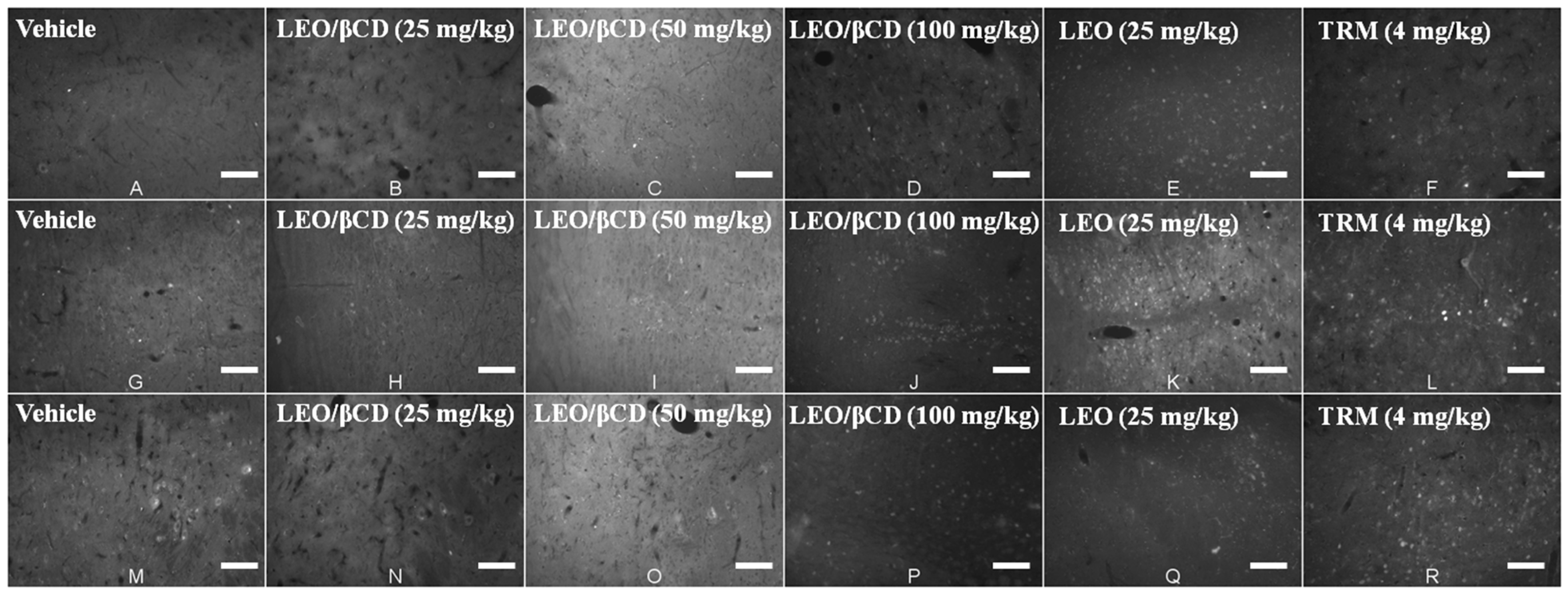
3. Experimental Section
3.1. Plant Material and Essential Oil Extraction
3.2. Preparation and Characterization of Inclusion Complexes
3.3. Animals
3.4. Drugs and Reagents
3.5. Behavioral Tests
3.5.1. Acid Saline Induced-Chronic Muscle Pain
3.5.2. Measurement of Mechanical Hyperalgesia
3.5.3. Measurement of Motor Coordination
3.5.4. Measurement of Forelimb Grip Strength
3.6. Immunofluorescence
3.7. Acquisition and Analyses of Images
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wolff, S. Is radiation all bad? The search for adaptation. Radiat. Res. 1992, 131, 117–123. [Google Scholar] [CrossRef]
- Crofford, L.J.; Appleton, B.E. Complementary and alternative therapies for fibromyalgia. Curr. Rheumatol. Rep. 2001, 3, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Sumpton, J.E.; Moulin, D.E. Fibromyalgia: Presentation and management with a focus on pharmacological treatment. Pain Res. Manag. J. Can. Pain Soc. 2008, 13, 477–483. [Google Scholar]
- Nascimento, S.S.; DeSantana, J.M.; Nampo, F.K.; Ribeiro, E.A.N.; da Silva, D.L.; Araújo-Júnior, J.X.; da Silva Almeida, J.R.G.; Bonjardim, L.R.; de Souza Araújo, A.A.; Quintans-Júnior, L.J. Efficacy and safety of medicinal plants or related natural products for fibromyalgia: A systematic review. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Quintans, J.S.S.; Antoniolli, A.R.; Almeida, J.R.; Santana-Filho, V.J.; Quintans-Júnior, L.J. Natural products evaluated in neuropathic pain models-A systematic review. Basic Clin. Pharmacol. Toxicol. 2014, 114, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.-H.; Vederas, J.C. Drug discovery and natural products: End of an era or an endless frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Quintans-Júnior, L.J.; Souza, T.; Leite, B.; Lessa, N.; Bonjardim, L.; Santos, M.; Alves, P.; Blank, A.; Antoniolli, A. Phythochemical screening and anticonvulsant activity of Cymbopogon winterianus Jowitt (Poaceae) leaf essential oil in rodents. Phytomedicine 2008, 15, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.R.; Moreira, F.V.; Fraga, B.P.; Souza, D.P.; Bonjardim, L.R.; Quintans-Júnior, L.J. Cardiovascular effects of monoterpenes: A review. Rev. Bras. Farmacogn. 2011, 21, 764–771. [Google Scholar] [CrossRef]
- Brito, R.G.; Santos, P.L.; Prado, D.S.; Santana, M.T.; Araújo, A.A.; Bonjardim, L.R.; Santos, M.R.V.; Lucca Júnior, W.; Oliveira, A.P.; Quintans-Júnior, L.J. Citronellol reduces orofacial nociceptive behaviour in mice-Evidence of involvement of retrosplenial cortex and periaqueductal grey areas. Basic Clin. Pharmacol. Toxicol. 2013, 112, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.G.; Quintans, J.S.S.; Quintans-Júnior, L.J. Monoterpenes with analgesic activity—A systematic review. Phytother. Res. 2013, 27, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.G.; Serafini, M.R.; Quintans-Júnior, L.J. Terpenes and derivatives as a new perspective for pain treatment: A patent review. Expert Opin. Ther. Pat. 2014, 24, 243–265. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.S.; Porto, L.A.; Estevam, C.S.; Siqueira, R.S.; Alves, P.B.; Niculau, E.S.; Blank, A.F.; Almeida, R.N.; Marchioro, M.; Quintans-Júnior, L.J. Phytochemical screening and anticonvulsant property of Ocimum basilicum leaf essential oil. Bol. Latinoam. Caribe Plant. Med. Aromat. 2009, 8, 195–202. [Google Scholar]
- Venâncio, A.M.; Marchioro, M.; Estavam, C.S.; Melo, M.S.; Santana, M.T.; Onofre, A.S.; Guimarães, A.G.; Oliveira, M.G.; Alves, P.B.; Pimentel, H.C. Ocimum basilicum leaf essential oil and (−)-linalool reduce orofacial nociception in rodents: A behavioral and electrophysiological approach. Rev. Bras. Farmacogn. 2011, 21, 1043–1051. [Google Scholar] [CrossRef]
- Quintans‐Júnior, L.J.; Barreto, R.S.; Menezes, P.P.; Almeida, J.R.; Viana, A.F.S.; Oliveira, R.; Oliveira, A.P.; Gelain, D.P.; Lucca Júnior, W.; Araújo, A.A. β-Cyclodextrin-complexed (−)-linalool produces antinociceptive effect superior to that of (−)-linalool in experimental pain protocols. Basic Clin. Pharmacol. Toxicol. 2013, 113, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Mazutti, M.; Beledelli, B.; Mossi, A.J.; Cansian, R.L.; Dariva, C.; de Oliveira, J.V.; Paroul, N. Caracterização quimica de extratos de Ocimum basilicum L. obtidos através de extração com CO2 a altas pressões. Quim. Nova 2006, 29, 1198–1202. [Google Scholar]
- Salústio, P.J.; Pontes, P.; Conduto, C.; Sanches, I.; Carvalho, C.; Arrais, J.; Marques, H.M.C. Advanced technologies for oral controlled release: Cyclodextrins for oral controlled release. AAPS Pharm. 2011, 12, 1276–1292. [Google Scholar] [CrossRef] [Green Version]
- Quintans, J.S.S.; Menezes, P.P.; Santos, M.R.V.; Bonjardim, L.R.; Almeida, J.R.G.S.; Gelain, D.P.; Araújo, A.A.S.; Quintans-Júnior, L.J. Improvement of p-cymene antinociceptive and anti-inflammatory effects by inclusion in β-cyclodextrin. Phytom 2013, 20, 436–440. [Google Scholar] [CrossRef]
- Siqueira-Lima, P.S.; Araújo, A.A.; Lucchese, A.M.; Quintans, J.S.S.; Menezes, P.P.; Alves, P.B.; Lucca Júnior, W.; Santos, M.R.; Bonjardim, L.R.; Quintans-Júnior, L.J. β-Cyclodextrin complex containing lippia grata leaf essential oil reduces orofacial nociception in mice-evidence of possible involvement of descending inhibitory pain modulation pathway. Basic Clin. Pharmacol. Toxicol. 2014, 114, 188–196. [Google Scholar] [CrossRef] [PubMed]
- López-Nicolás, J.M.; Rodríguez-Bonilla, P.; García-Carmona, F. Cyclodextrins and antioxidants. Crit. Rev. Food Sci. Nutr. 2012, 54, 251–276. [Google Scholar] [CrossRef]
- Menezes, P.P.; Serafini, M.R.; Santana, B.V.; Nunes, R.S.; Quintans, L.J., Jr.; Silva, G.F.; Medeiros, I.A.; Marchioro, M.; Fraga, B.P.; Santos, M.R. Solid-state β-cyclodextrin complexes containing geraniol. Thermochim. Acta 2012, 548, 45–50. [Google Scholar] [CrossRef]
- Nascimento, S.S.; Camargo, E.A.; DeSantana, J.M.; Araújo, A.A.; Menezes, P.P.; Lucca-Júnior, W.; Albuquerque-Júnior, R.L.; Bonjardim, L.R.; Quintans-Júnior, L.J. Linalool and linalool complexed in β-cyclodextrin produce anti-hyperalgesic activity and increase Fos protein expression in animal model for fibromyalgia. Naunyn-Schmiedeberg Arch. Pharmacol. 2014, 387, 935–942. [Google Scholar] [CrossRef]
- Marques, H.M.C. A review on cyclodextrin encapsulation of essential oils and volatiles. Flavour Fragr. J. 2010, 25, 313–326. [Google Scholar] [CrossRef]
- Serafini, M.; Menezes, P.; Costa, L.; Lima, C.; Quintans-Júnior, L.J.; Cardoso, J.; Matos, J.; Soares-Sobrinho, J.; Grangeiro, S.; Nunes, P. Interaction of p-cymene with β-cyclodextrin. J. Therm. Anal. Calorim. 2012, 109, 951–955. [Google Scholar] [CrossRef]
- DeSantana, J.M.; da Cruz, K.; Sluka, K.A. Animal models of fibromyalgia. Arthritis Res. Ther. 2013, 15. [Google Scholar] [CrossRef]
- Sluka, K.; Kalra, A.; Moore, S. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve 2001, 24, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Sluka, K.; Rohlwing, J.; Bussey, R.; Eikenberry, S.; Wilken, J. Chronic muscle pain induced by repeated acid injection is reversed by spinally administered μ- and δ-, but not κ-, opioid receptor agonists. J. Pharmacol. Exp. Ther. 2002, 302, 1146–1150. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4rd ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; p. 387. [Google Scholar]
- Hădărugă, D.I.; Hadaruga, N.G.; Bandur, G.N.; Isengard, H.D. Water content of flavonoid/cyclodextrin nanoparticles: Relationship with the structural descriptors of biologically active compounds. Food Chem. 2012, 132, 1651–1659. [Google Scholar] [CrossRef]
- Marreto, R.N.; Almeida, E.E.; Alves, P.B.; Niculau, E.S.; Nunes, R.S.; Matos, C.R.; Araújo, A.A. Thermal analysis and gas chromatography coupled mass spectrometry analyses of hydroxypropyl-β-cyclodextrin inclusion complex containing Lippi agracilis essential oil. Thermochim. Acta 2008, 475, 53–58. [Google Scholar] [CrossRef]
- Stebbing, A. Hormesis—The stimulation of growth by low levels of inhibitors. Sci. Total Environ. 1982, 22, 213–234. [Google Scholar] [CrossRef] [PubMed]
- Sakurada, T.; Mizoguchi, H.; Kuwahata, H.; Katsuyama, S.; Komatsu, T.; Morrone, L.A.; Corasaniti, M.T.; Bagetta, G.; Sakurada, S. Intraplantar injection of bergamot essential oil induces peripheral antinociception mediated by opioid mechanism. Pharmacol. Biochem. Behav. 2011, 97, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Batista, P.A.; Werner, M.F.P.; Oliveira, E.C.; Burgos, L.; Pereira, P.; Brum, L.F.S.; Santos, A.R.S. Evidence for the involvement of ionotropic glutamatergic receptors on the antinociceptive effect of (−)-linalool in mice. Neurosci. Lett. 2008, 440, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Bendtsen, L.; Nørregaard, J.; Jensen, R.; Olesenm, J. Evidence of qualitatively altered nociception in patients with fibromyalgia. Arthritis Rheumatol. 1997, 40, 98–102. [Google Scholar] [CrossRef]
- Leal-Cardoso, J.H.; da Silva-Alves, K.S.; Ferreira-da-Silva, F.W.; dos Santos-Nascimento, T.; Joca, H.C.; de Macedo, F.H.P.; de Albuquerque-Neto, P.M.; Magalhães, P.J.C.; Lahlou, S.; Cruz, J.S. Linalool blocks excitability in peripheral nerves and voltage-dependent Na current in dissociated dorsal root ganglia neurons. Eur. J. Pharmacol. 2010, 645, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Zubrzycka, M.; Szemraj, J.; Janecka, A. Effect of tooth pulp and periaqueductal central gray stimulation on the expression of genes encoding the selected neuropeptides and opioid receptors in the mesencephalon, hypothalamus and thalamus in rats. Brain Res. 2011, 1382, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Sluka, K.; Westlund, K. Spinal cord amino acid release and content in an arthritis model: The effects of pretreatment with non-NMDA, NMDA, and NK1 receptor antagonists. Brain Res. 1993, 627, 89–103. [Google Scholar] [CrossRef] [PubMed]
- De Santana, J.M.; Sluka, K.A. Central mechanisms in the maintenance of chronic widespread noninflammatory muscle pain. Curr. Pain Headache Rep. 2008, 12, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Watkins, L.; Maier, S. Immune regulation of central nervous system functions: From sickness responses to pathological pain. J. Intern. Med. 2005, 257, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Quintans-Júnior, L.J.; Santana, M.T.; Melo, M.S.; de Sousa, D.P.; Santos, I.S.; Siqueira, R.S.; Lima, T.C.; Silveira, G.O.; Antoniolli, A.R.; Ribeiro, L.A. Antinociceptive and anti-inflammatory effects of Costus spicatus in experimental animals. Pharm. Biol. 2010, 48, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Calvino, B.; Grilo, R.M. Central pain control. Jt. Bone Spine 2006, 73, 10–16. [Google Scholar] [CrossRef]
- Dadabhoy, D.; Clauw, D.J. Fibromyalgia: Progress in diagnosis and treatment. Curr. Pain Headache Rep. 2005, 9, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Batista, J.; Almeida, R.N.; Bhattacharyya, J. Analgesic effect of Dioclea grandiflora constituents in rodents. J. Ethnopharmacol. 1995, 45, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Cunha, T.; Verri, W., Jr.; Vivancos, G.; Moreira, I.; Reis, S.; Parada, C.; Cunha, F.; Ferreira, S. An electronic pressure-meter nociception paw test for mice. Braz. J. Med. Biol. Res. 2004, 37, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.G.; Xavier, M.A.; de Santana, M.T.; Camargo, E.A.; Santos, C.A.; Brito, F.A.; Barreto, E.O.; Cavalcanti, S.C.; Antoniolli, A.R.; Oliveira, R.C.; et al. Carvacrol attenuates mechanical hypernociception and inflammatory response. Naunyn-Schmiedeberg Arch. Pharmacol. 2012, 385, 253–263. [Google Scholar]
- Meyer, O.; Tilson, H.; Byrd, W.; Riley, M. A method for the routine assessment of fore-and hindlimb grip strength of rats and mice. Neurobehav. Toxicol. 1979, 1, 233–236. [Google Scholar] [PubMed]
- Gama, K.B.; Quintans, J.S.S.; Antoniolli, A.R.; Quintans-Júnior, L.J.; Santana, W.A.; Branco, A.; Soares, M.B.P.; Villarreal, C.F. Evidence for the involvement of descending pain-inhibitory mechanisms in the antinociceptive effect of hecogenin acetate. J. Natl. Prod. 2013, 76, 559–563. [Google Scholar] [CrossRef]
- Burmeister, S.S.; Mangiamele, L.A.; Lebonville, C.L. Acoustic modulation of immediate early gene expression in the auditory midbrain of female túngara frogs. Brain Res. 2008, 1190, 105–114. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, S.S.; Araújo, A.A.S.; Brito, R.G.; Serafini, M.R.; Menezes, P.P.; DeSantana, J.M.; Júnior, W.L.; Alves, P.B.; Blank, A.F.; Oliveira, R.C.M.; et al. Cyclodextrin-Complexed Ocimum basilicum Leaves Essential Oil Increases Fos Protein Expression in the Central Nervous System and Produce an Antihyperalgesic Effect in Animal Models for Fibromyalgia. Int. J. Mol. Sci. 2015, 16, 547-563. https://doi.org/10.3390/ijms16010547
Nascimento SS, Araújo AAS, Brito RG, Serafini MR, Menezes PP, DeSantana JM, Júnior WL, Alves PB, Blank AF, Oliveira RCM, et al. Cyclodextrin-Complexed Ocimum basilicum Leaves Essential Oil Increases Fos Protein Expression in the Central Nervous System and Produce an Antihyperalgesic Effect in Animal Models for Fibromyalgia. International Journal of Molecular Sciences. 2015; 16(1):547-563. https://doi.org/10.3390/ijms16010547
Chicago/Turabian StyleNascimento, Simone S., Adriano A. S. Araújo, Renan G. Brito, Mairim R. Serafini, Paula P. Menezes, Josimari M. DeSantana, Waldecy Lucca Júnior, Pericles B. Alves, Arie F. Blank, Rita C. M. Oliveira, and et al. 2015. "Cyclodextrin-Complexed Ocimum basilicum Leaves Essential Oil Increases Fos Protein Expression in the Central Nervous System and Produce an Antihyperalgesic Effect in Animal Models for Fibromyalgia" International Journal of Molecular Sciences 16, no. 1: 547-563. https://doi.org/10.3390/ijms16010547






