Production of Structured Phosphatidylcholine with High Content of DHA/EPA by Immobilized Phospholipase A1-Catalyzed Transesterification
Abstract
:1. Introduction
| Process | Incorporation (%) | Reaction Substrate | Enzyme Load | System | Reference |
|---|---|---|---|---|---|
| Transesterification | 12.3 | Ethyl esters a/PL | 10% Lipozyme RM IM | Hexane | [8] |
| Acidolysis | 43 | FFA b/PC | 15% Immobilized PLA1 | Solvent-free | [9] |
| Acidolysis | 35 | FFA c/PC | 10% Immobilized PLA1 | Solvent-free | [10] |
| Acidolysis | 28 | FFA b/PC | 10% Liquid PLA1 | Solvent-free | [11] |
| Acidolysis | 20 | FFA d/PC | 30% Immobilized PLA2 | Solvent-free | [12] |
| Acidolysis | 18.9 | FFA e/PL | 20% Lipozyme TL IM | Solvent-free | [7] |
2. Results and Discussion
2.1. Immobilization of Phospholipase A1 (PLA1)
2.1.1. Effect of Support/PLA1 Ratio


2.1.2. Effect of pH
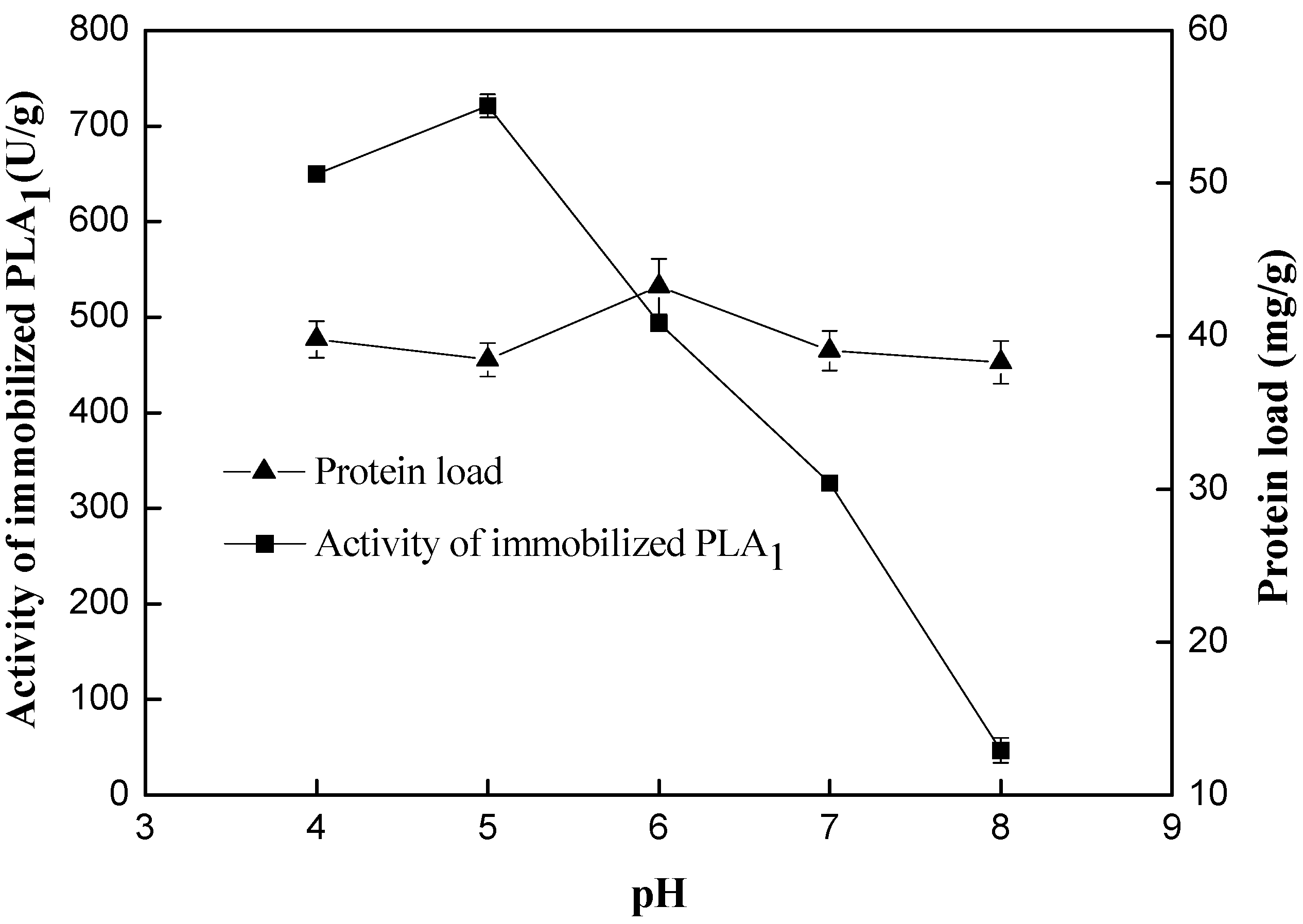
2.1.3. Catalyzing the Transesterification of PC and Docosahexaenoic Acid (DHA)/Eicosapentaenoic Acid (EPA)-Rich Ethyl Esters by Free PLA1 and Immobilized PLA1
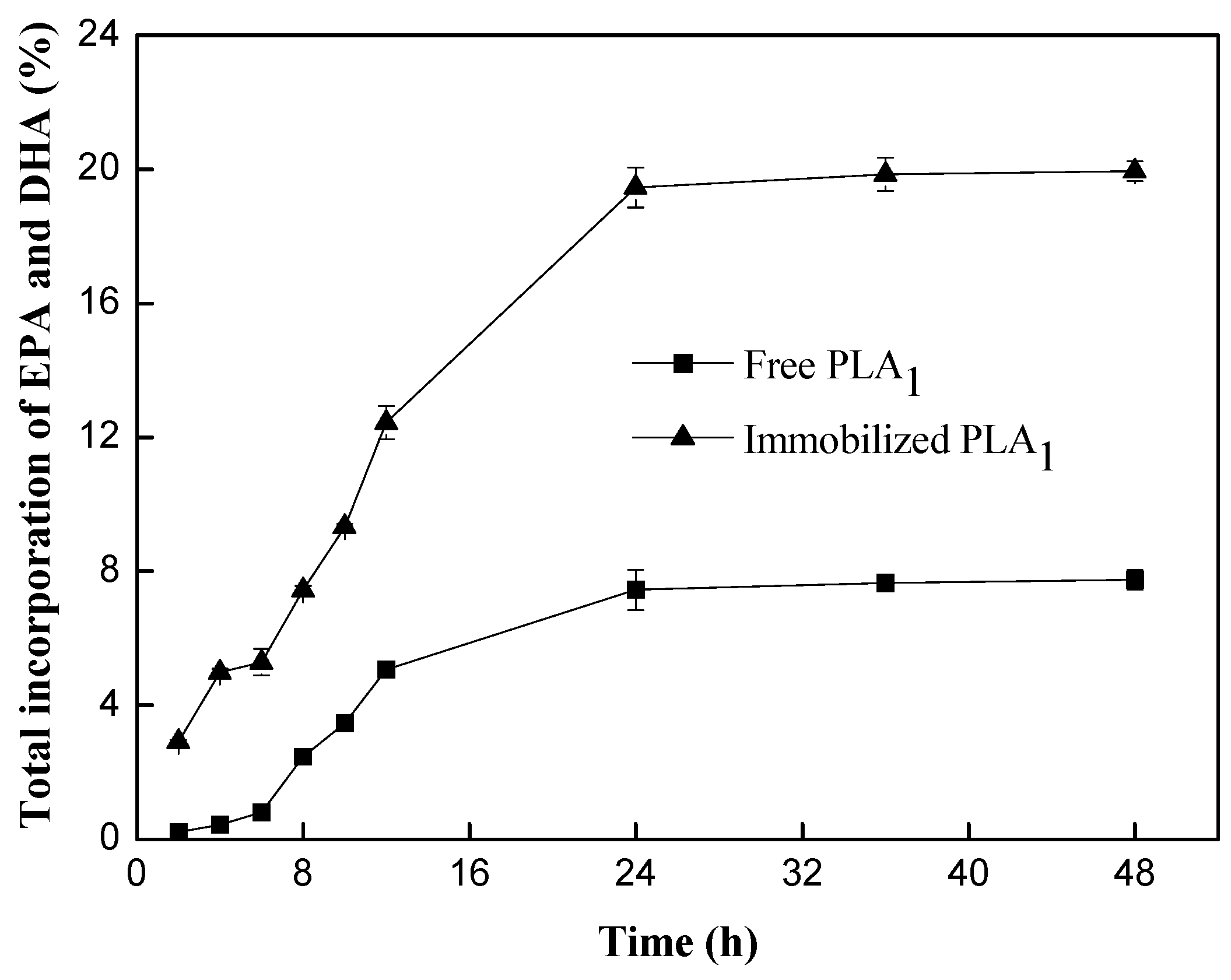
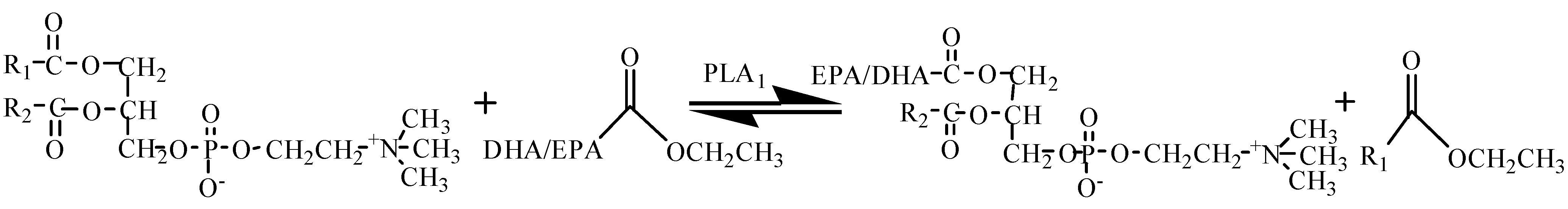
2.2. Transesterification of PC with DHA/EPA-Rich Ethyl Esters by Immobilized PLA1
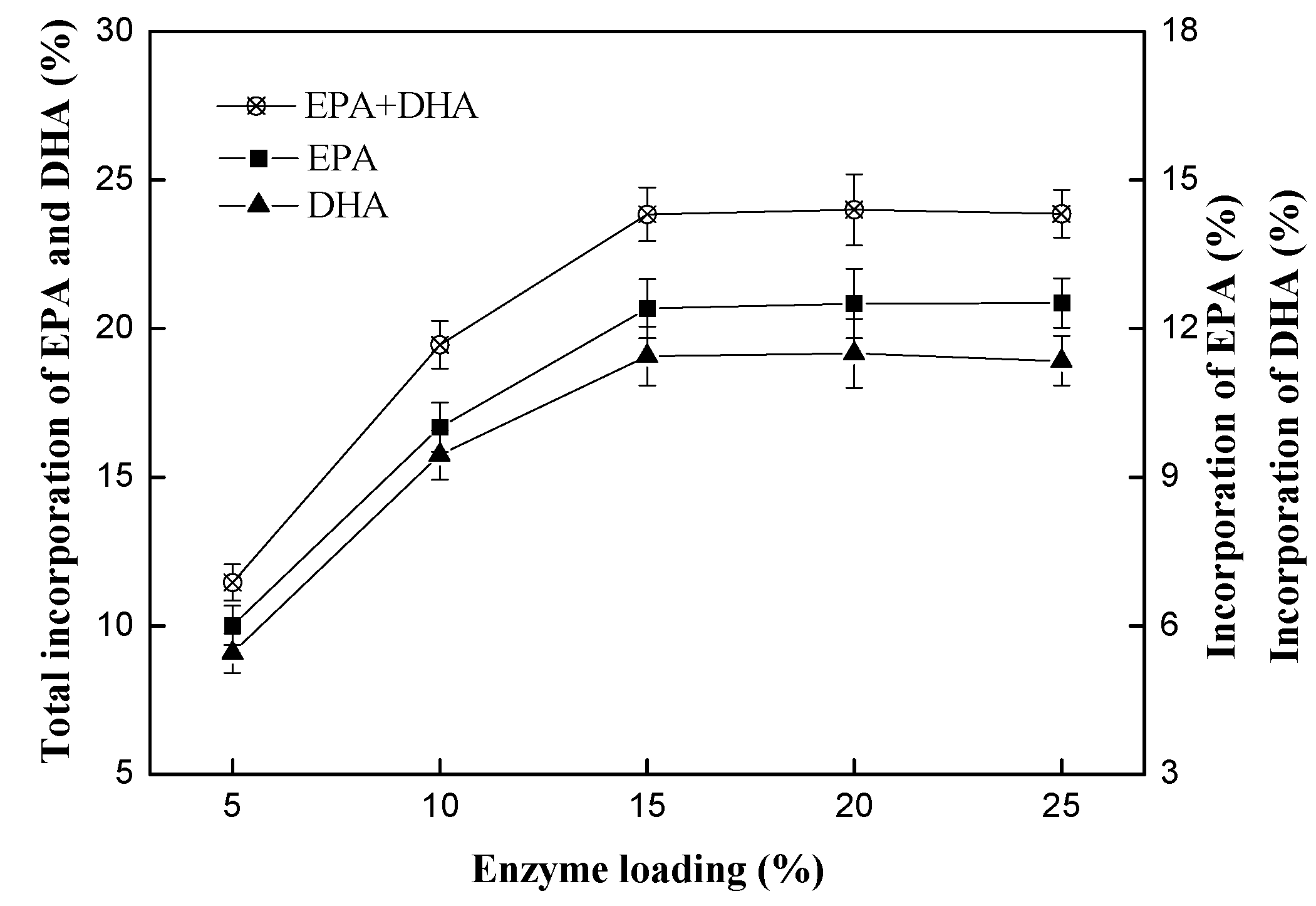
2.2.1. Effect of Enzyme Loading
2.2.2. Effect of Mass Ratio of PC to DHA/EPA-Rich Ethyl Esters
2.2.3. Effect of Temperature
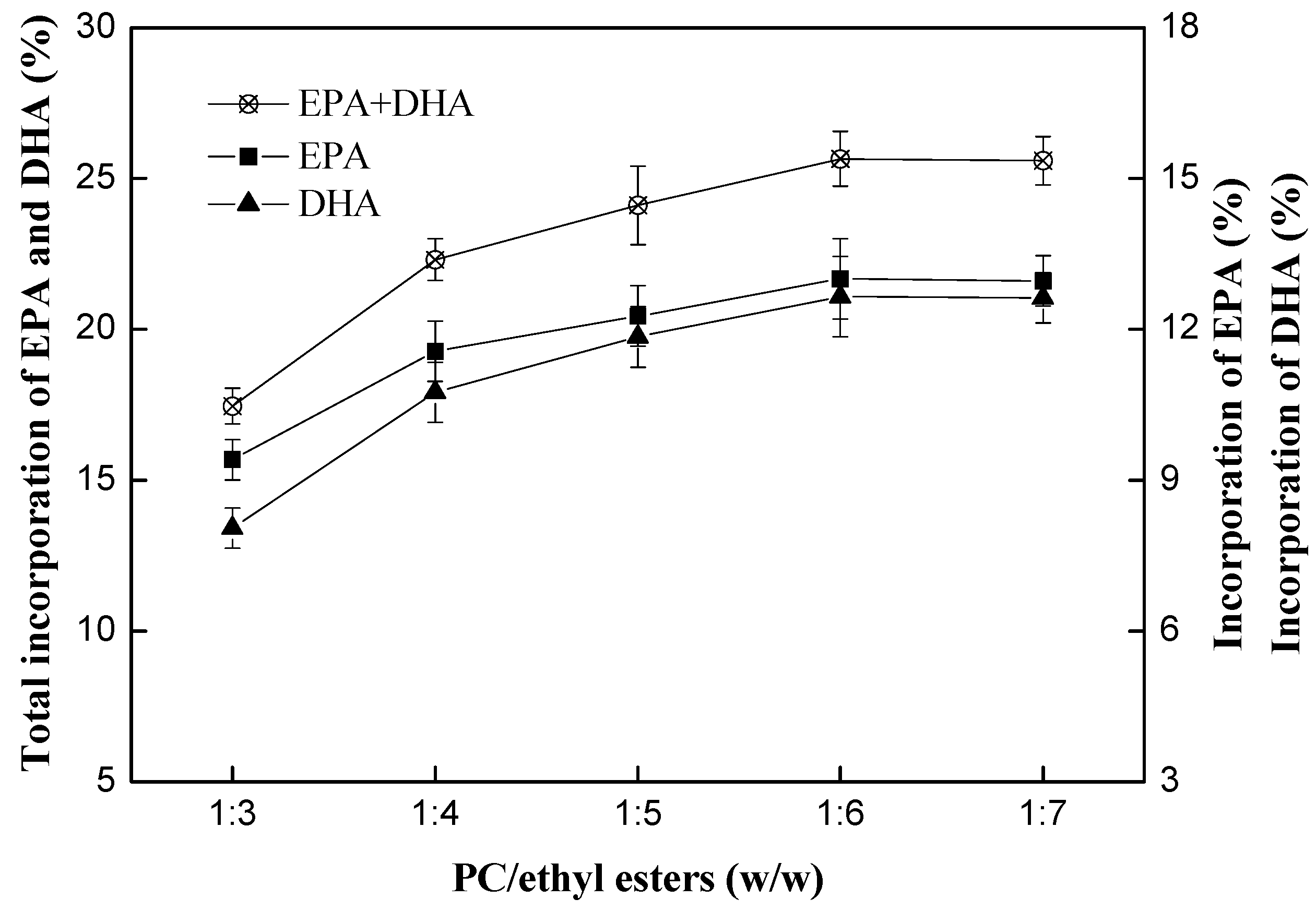
| Fatty Acid | DHA/EPA-Rich Ethyl Esters | Structured PC f | ||||
|---|---|---|---|---|---|---|
| 40 °C | 45 °C | 50 °C | 55 °C | 60 °C | ||
| EPA (%) | 38.1 ± 0.24 | 7.3 ± 0.28 | 10.1 ± 0.36 | 13.1 ± 0.39 | 14.0 ± 0.48 | 13.9 ± 0.58 |
| DHA (%) | 45.5 ± 0.31 | 6.1 ± 0.17 | 9.1 ± 0.29 | 12.6 ± 0.28 | 13.8 ± 0.39 | 14.2 ± 0.49 |
| EPA + DHA (%) | 83.6 ± 0.38 | 13.4 ± 0.42 | 19.2 ± 0.64 | 25.7 ± 0.58 | 27.8 ± 0.65 | 28.1 ± 0.71 |
| Ratio of EPA to DHA | 0.84 ± 0.01 | 1.20 ± 0.03 | 1.11 ± 0.03 | 1.04 ± 0.03 | 1.01 ± 0.04 | 0.98 ± 0.04 |
2.2.4. Effect of Water Dosage
| Samples | Fatty Acids Profiles (%) | Ratio of EPA to DHA | ||
|---|---|---|---|---|
| EPA | DHA | DHA + EPA | ||
| DHA/EPA-rich ethyl esters | 38.1 ± 0.24 | 45.5 ± 0.31 | 83.6 ± 0.38 | 0.84 ± 0.01 |
| Structured PC g–0.5% h | 10.7 ± 0.29 | 9.1 ± 0.22 | 19.8 ± 0.45 | 1.18 ± 0.02 |
| Structured PC–0.75% | 12.7 ± 0.37 | 12.5 ± 0.32 | 25.2 ± 0.62 | 1.02 ± 0.01 |
| Structured PC–1.0% | 14.0 ± 0.28 | 13.9 ± 0.47 | 27.9 ± 0.51 | 1.01 ± 0.01 |
| Structured PC–1.25% | 15.4 ± 0.49 | 15.3 ± 0.37 | 30.7 ± 0.55 | 1.01± 0.03 |
| Structured PC–1.5% | 13.6 ± 0.39 | 13.9 ± 0.61 | 27.9 ± 0.63 | 0.98 ±0.03 |
2.3. Analyzing the Composition of Reaction Product
| Phospholipids | Content (%) | |
|---|---|---|
| Original PC | Structured PC i | |
| PC | 97.7 ± 0.45 | 16.5 ± 0.65 |
| 1-LPC | 0.4 ± 0.12 | 26.3 ± 0.58 |
| 2-LPC | 1.9 ± 0.22 | 31.4 ± 0.89 |
| GPC | – | 25.8 ± 0.72 |
2.4. Evaluation of Enzyme Reusability
| Substrate | Structured PC j | ||||
|---|---|---|---|---|---|
| Fatty Acid | DHA/EPA-Rich Ethyl Esters | PC k | LPC k | Total PC l | Total LPC m |
| C14:0 | 0.2 ± 0.04 | 1.1 ± 0.05 | 1.0 ± 0.04 | 0.5 ± 0.01 | 0.4 ± 0.03 |
| C16:0 | 1.1 ± 0.03 | 13.8 ± 0.13 | 13.3 ± 0.15 | 7.1 ± 0.25 | 8.0 ± 0.22 |
| C16:1 | 0.2 ± 0.02 | 0.3 ± 0.01 | 0.2 ± 0.04 | 0.3 ± 0.04 | 0.2 ± 0.03 |
| C18:0 | 0.6 ± 0.06 | 3.6 ± 0.08 | 3.7 ± 0.10 | 2.1 ± 0.14 | 2.4 ± 0.08 |
| C18:1 | 2.2 ± 0.08 | 10.6 ± 0.18 | 11.1 ± 0.25 | 8.4 ± 0.23 | 8.6 ± 0.12 |
| C18:2 | 0.6 ± 0.03 | 64.8 ± 0.32 | 65.2 ± 0.27 | 43.1 ± 0.35 | 42.9 ± 0.31 |
| C18:3 | 1.4 ± 0.08 | 5.8 ± 0.07 | 5.5 ± 0.11 | 3.8 ± 0.16 | 3.6 ± 0.08 |
| C22:5 | 6.4 ± 0.12 | – | – | 2.5 ± 0.18 | 2.3 ± 0.14 |
| EPA | 38.1 ± 0.24 | – | – | 15.6 ± 0.25 | 15.2 ± 0.22 |
| DHA | 45.5 ± 0.31 | – | – | 15.2 ± 0.22 | 15.1 ± 0.19 |
| EPA + DHA | 83.6 ± 0.32 | – | – | 30.8 ± 0.19 | 30.3 ± 0.23 |
| Others | 3.7 ± 0.11 | – | – | 1.4 ± 0.10 | 1.3 ± 0.09 |
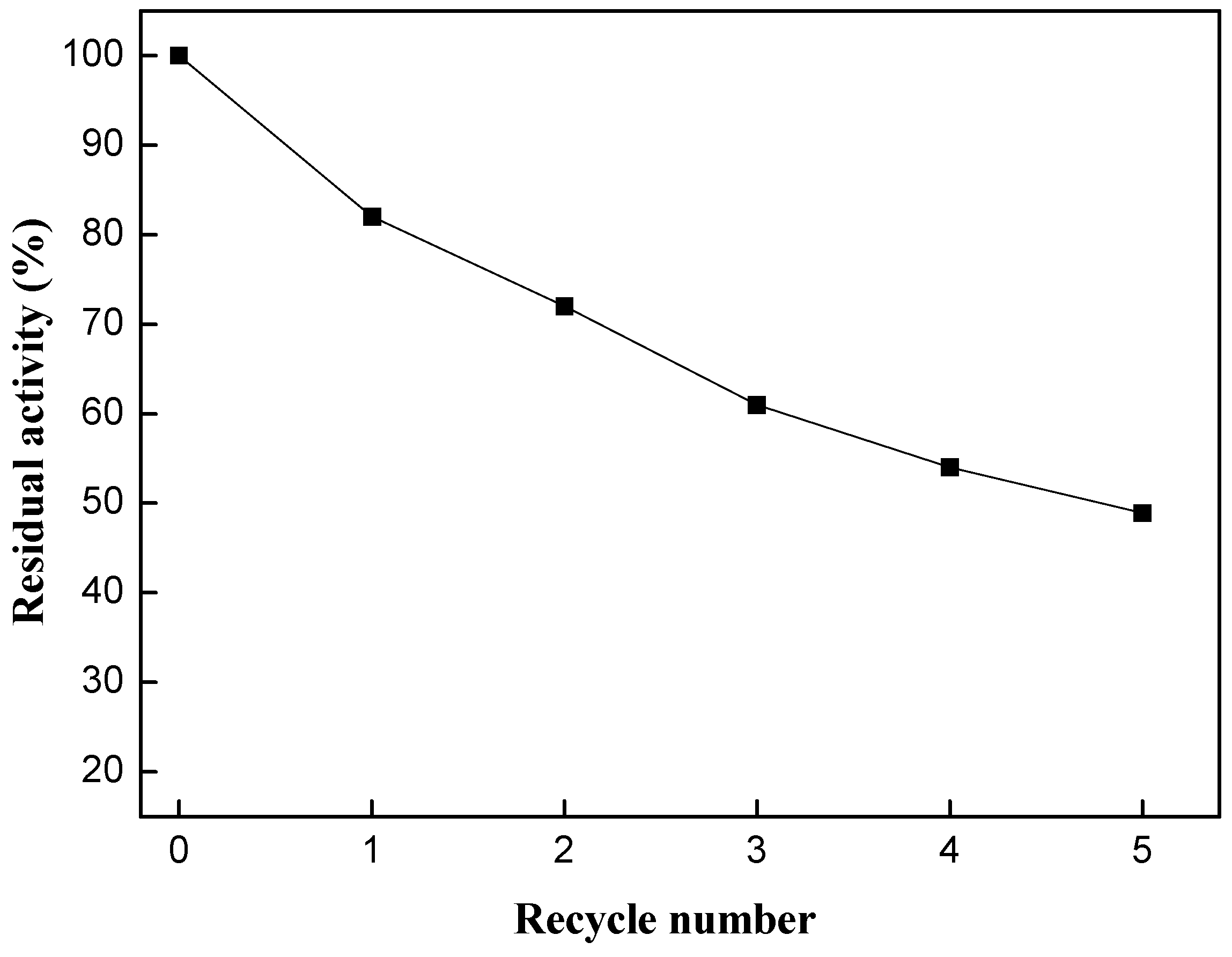
3. Experimental Section
3.1. Materials
3.2. Immobilization of Phospholipase A1
3.3. Phospholipase A1-Catalyzed Transesterification of PC to DHA/EPA-Rich Ethyl Esters
3.4. Evaluation of Enzyme Reusability
3.5. Analyzing Activity of Immobilized PLA1
3.6. Analyzing Moisture Content of Immobilized PLA1
3.7. Analyzing FA Composition by Gas Chromatography (GC)
3.8. Analyzing Structured PC Composition in Reaction Products by 31P NMR
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Guo, Z.; Vikbjerg, A.F.; Xu, X. Enzymatic modification of phospholipids for functional applications and human nutrition. Biotech. Adv. 2005, 23, 203–259. [Google Scholar]
- Chakraborty, K.; Paul Raj, P. Selective enrichment of n-3 polyunsaturated fatty acids with C18–C20 acyl chain length from sardine oil using Pseudomonas fluorescens MTCC 2421 lipase. Food Chem. 2009, 114, 142–150. [Google Scholar]
- Larsson, S.C.; Kumlin, M.; Ingelman-Sundberg, M.; Wolk, A. Dietary long-chain n-3 fatty acids for the prevention of cancer: A review of potential mechanisms. Am. J. Clin. Nutr. 2003, 79, 935–945. [Google Scholar]
- Ward, O.P.; Singh, A. Omega-3/6 fatty acids: Alternative sources of production. Process Biochem. 2005, 40, 3627–3652. [Google Scholar] [CrossRef]
- Galli, C.; Sirtori, C.R.; Mosconi, C.; Medini, L.; Gianfranceschi, G.; Vaccarino, V.; Scolastico, C. Prolonged retention of doubly labeled phosphatidylcholine in human plasma and erythrocytes after oral administration. Lipids 1992, 27, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Cansell, M.; Nacka, F.; Combe, N. Marine lipid-based liposomes increase in vivo FA bioavailability. Lipids 2003, 38, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Xu, X.; Mu, H.; Høy, C.E.; Adler-Nissen, J. Production of phospholipids by lipase-catalyzed acidolysis: Optimization using response surface methodology. Enzym. Microb. Technol. 2002, 31, 523–532. [Google Scholar] [CrossRef]
- Marsaoui, N.; Laplante, S.; Raies, A.; Naghmouchi, K. Incorporation of omega-3 polyunsaturated fatty acids into soybean lecithin: Effect of amines and divalent cations on transesterification by lipases. World J. Microbiol. Biotechnol. 2013. [Google Scholar] [CrossRef]
- Kim, I.H.; García, H.S.; Hill, C.G., Jr. Synthesis of structured phosphatidylcholine containing n-3 PUFA residues via acidolysis mediated by immobilized phospholipase A1. J. Am. Oil Chem. Soc. 2010, 87, 1293–1299. [Google Scholar] [CrossRef]
- García, H.S.; Kim, I.; López-Hernández, A.; Hill, C.G., Jr. Enrichment of lecithin with n-3 fatty acids by acidolysis using immobilized phospholipase A1. Grasas Aceites. 2008, 59, 368–374. [Google Scholar]
- Kim, I.H.; García, H.S.; Hill, C.G., Jr. Phospholipase A1-catalyzed synthesis of phospholipids enriched in n-3 polyunsaturated fatty acid residues. Enzym. Microb. Technol. 2007, 40, 1130–1135. [Google Scholar] [CrossRef]
- Vikbjerg, AF.; Mu, H.; Xu, X. Synthesis of structured phospholipids by immobilized phospholipase A2 catalyzed acidolysis. J. Biotechnol. 2007, 128, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.F.; Xu, Y.; Qin, X.L.; Lan, D.M.; Yang, B.; Wang, Y.H. Immobilization of lipase SMG1 and its application in synthesis of partial glycerides. Eur. J. Lipid Sci. Technol. 2014. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, Z.; Bie, X.; Lu, F.; Liu, Z. Lipase catalyzed acidolysis of lard with capric acid in organic solvent. J. Food Eng. 2007, 78, 41–46. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram 342 quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.G.; Wang, Y.H.; Yang, B.; Mainda, G.; Guo, Y. Degumming of vegetable oil by a new microbial lipase. Food Technol. Biotechnol. 2006, 44, 101–104. [Google Scholar]
- Wang, Y.H.; Mai, Q.Y.; Qin, X.L.; Yang, B.; Wang, Z.L.; Chen, H.T. Establishment of an evaluation model for human milk fat substitutes. J. Agric. Food Chem. 2010, 58, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.L.; Wang, Y.M.; Wang, Y.H.; Huang, H.H.; Yang, B. Preparation and characterization of 1,3-dioleoyl-2-palmitoylglycerol. J. Agric. Food Chem. 2011, 59, 5714–5719. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, X.; Chen, J.-F.; Yang, B.; Li, D.-M.; Wang, Y.-H.; Wang, W.-F. Production of Structured Phosphatidylcholine with High Content of DHA/EPA by Immobilized Phospholipase A1-Catalyzed Transesterification. Int. J. Mol. Sci. 2014, 15, 15244-15258. https://doi.org/10.3390/ijms150915244
Li X, Chen J-F, Yang B, Li D-M, Wang Y-H, Wang W-F. Production of Structured Phosphatidylcholine with High Content of DHA/EPA by Immobilized Phospholipase A1-Catalyzed Transesterification. International Journal of Molecular Sciences. 2014; 15(9):15244-15258. https://doi.org/10.3390/ijms150915244
Chicago/Turabian StyleLi, Xiang, Jia-Feng Chen, Bo Yang, Dao-Ming Li, Yong-Hua Wang, and Wei-Fei Wang. 2014. "Production of Structured Phosphatidylcholine with High Content of DHA/EPA by Immobilized Phospholipase A1-Catalyzed Transesterification" International Journal of Molecular Sciences 15, no. 9: 15244-15258. https://doi.org/10.3390/ijms150915244
APA StyleLi, X., Chen, J.-F., Yang, B., Li, D.-M., Wang, Y.-H., & Wang, W.-F. (2014). Production of Structured Phosphatidylcholine with High Content of DHA/EPA by Immobilized Phospholipase A1-Catalyzed Transesterification. International Journal of Molecular Sciences, 15(9), 15244-15258. https://doi.org/10.3390/ijms150915244




