Gamma Knife Treatment of Brainstem Metastases
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
| Characteristic | Renal/Melanoma | SC Lung | Other Lung | Breast | Other/Unknown | Total |
|---|---|---|---|---|---|---|
| Patient Number | n = 7 | n = 4 | n = 17 | n = 10 | n = 3 | n = 41 |
| Age at Diagnosis Mean/Median (Range) (years) | 69.3/73.0 (47–90) | 60.0/60.5 (52–67) | 59.1/59.0 (38–82) | 56.2/52.5 (38–96) | 64.7/66.0 (58–70) | 60.6/59.0 (38–96) |
| <60 | 2 | 2 | 10 | 8 | 1 | 23 |
| ≥60 | 5 | 2 | 7 | 2 | 2 | 18 |
| Gender | ||||||
| Female | 2 | 2 | 9 | 10 | 1 | 24 |
| Male | 5 | 2 | 8 | 0 | 2 | 17 |
| Prior WBRT | ||||||
| Yes | 1 | 4 | 6 | 6 | 2 | 19 |
| No | 6 | 0 | 10 | 3 | 1 | 20 |
| Unknown | 0 | 0 | 1 | 1 | 0 | 2 |
| Lesion Number | ||||||
| 1 | 6 | 3 | 11 | 7 | 3 | 30 |
| >1 | 1 | 1 | 6 | 3 | 0 | 11 |
| Karnofsky Performance Score (KPS) | ||||||
| ≤70 | 3 | 0 | 10 | 4 | 3 | 20 |
| ≥80 | 4 | 4 | 7 | 6 | 0 | 21 |
| Gamma Knife (GK) Dose (Gy) | ||||||
| <16 | 1 | 1 | 1 | 2 | 0 | 5 |
| ≥16 | 6 | 3 | 16 | 8 | 3 | 36 |
| Tumor Volume (cc) | ||||||
| <0.5 | 2 | 2 | 7 | 7 | 1 | 19 |
| ≥1.0 | 3 | 1 | 1 | 0 | 1 | 6 |
| Unknown | 2 | 1 | 2 | 10 | 1 | 16 |

2.2. Discussion
| Characteristic | Median Survival | Hazard Ratio | |||
|---|---|---|---|---|---|
| n | 95% CI | Estimate | 95% CI | p Value ** | |
| Histology | |||||
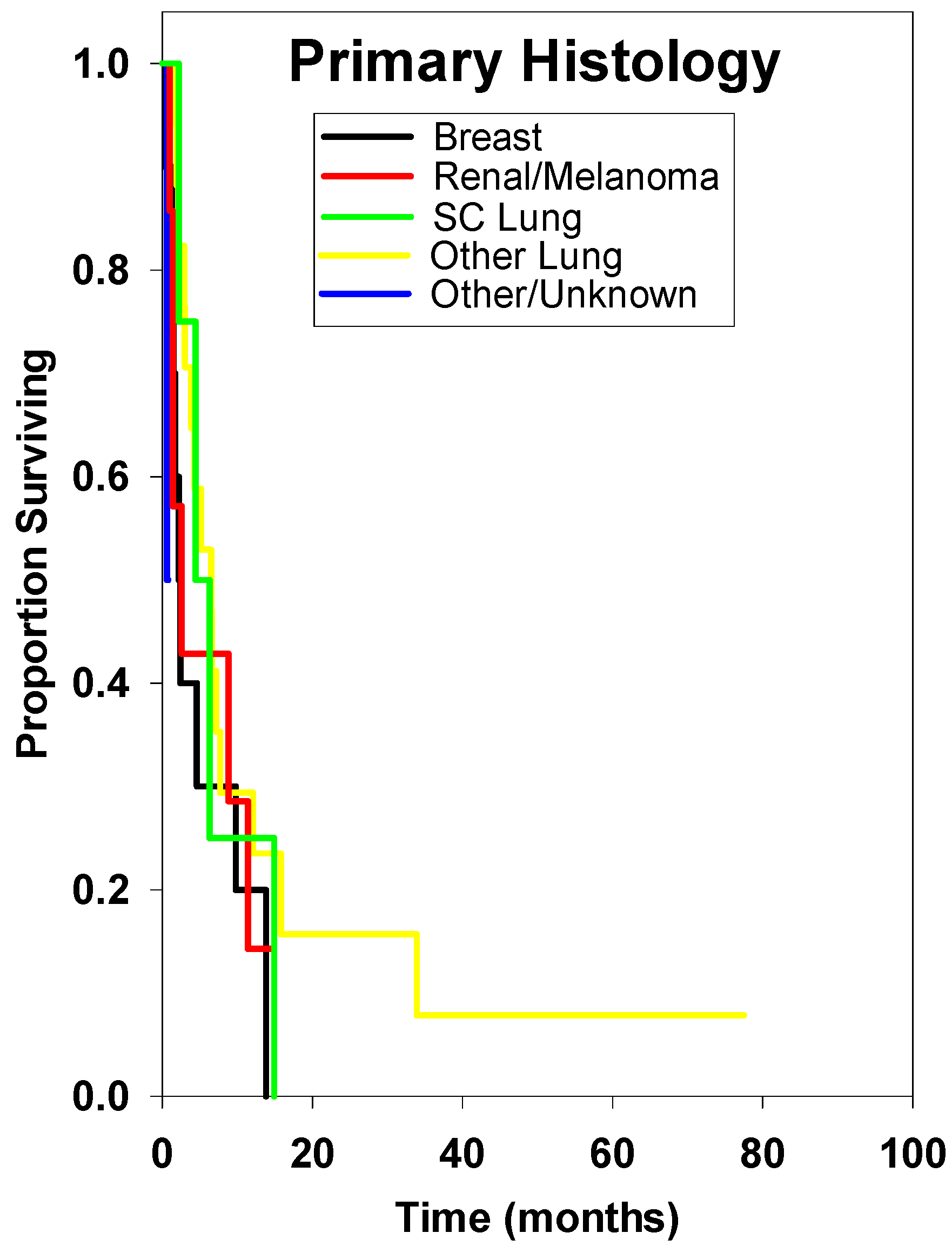
| Breast * | 10 | 2.40 ± 1.06 | Reference | ||
| SC Lung | 4 | 4.40 ± 4.02 | 0.57 | 0.10–2.33 | 0.546 |
| Other Lung | 17 | 6.50 ± 3.10 | 0.57 | 0.23–1.54 | 0.235 |
| Renal/Melanoma | 7 | 2.60 ± 3.08 | 0.88 | 0.25–2.78 | 0.999 |
| Other/Unknown | 3 | 0.60 ± 0.96 | 8.33 | 0.44–504.80 | 0.097 |
| Age at Diagnosis (years) | |||||
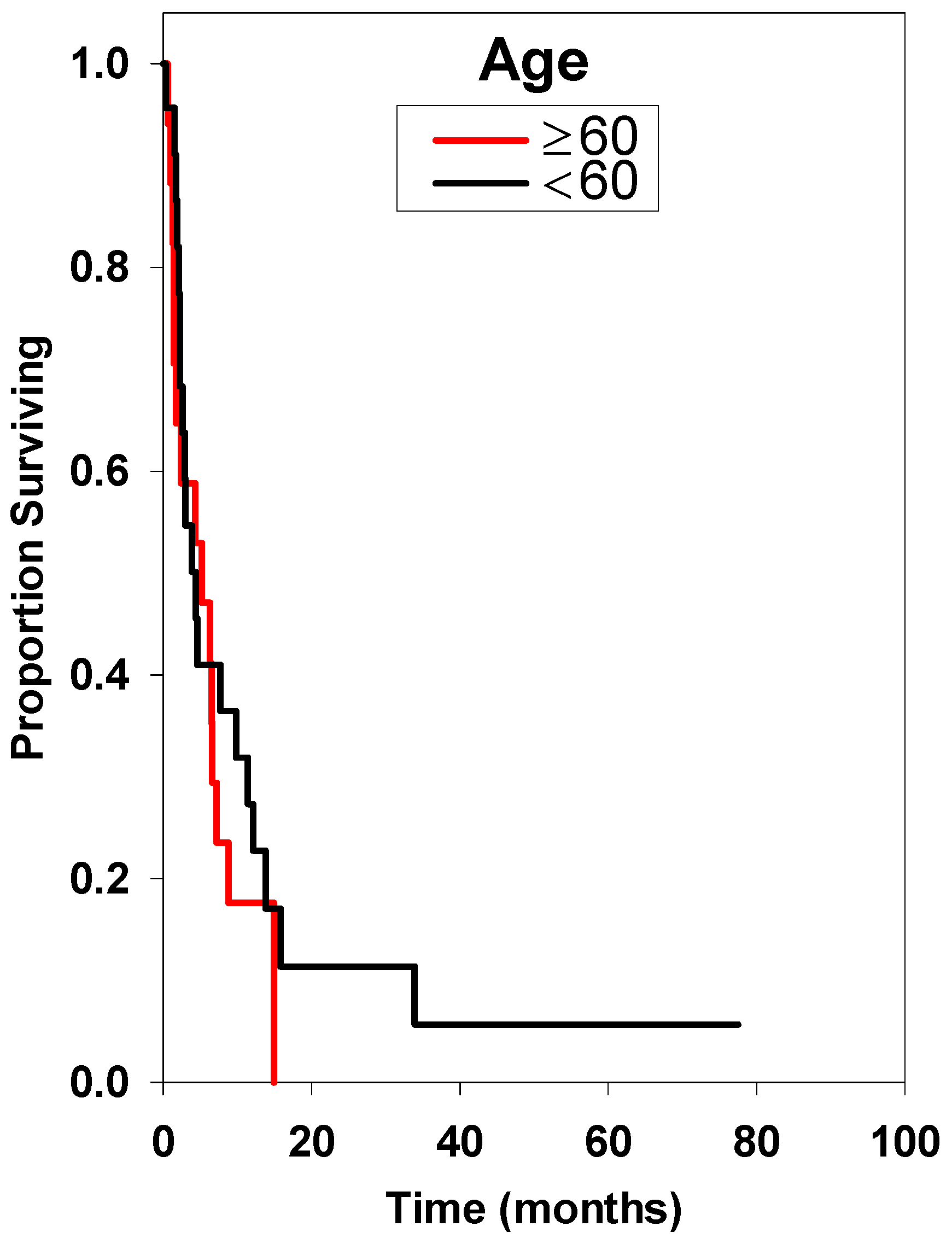
| <60 * | 23 | 4.30 ± 5.82 | Reference | ||
| ≥60 | 18 | 4.40 ± 1.94 | 1.37 | 0.65–2.88 | 0.379 |
| KPS | |||||
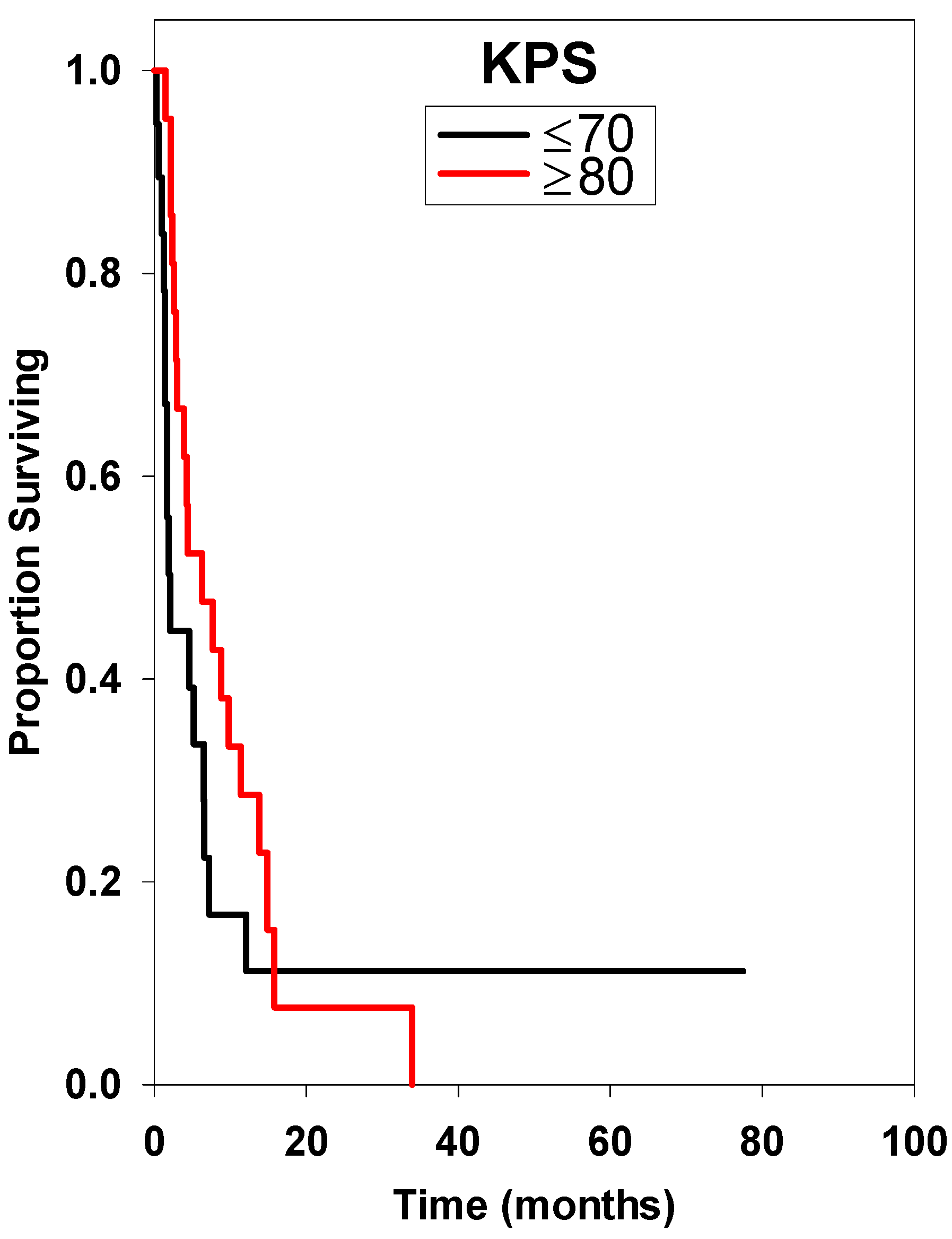
| ≤70 * | 20 | 1.90 ± 0.74 | Reference | ||
| ≥80 | 21 | 6.30 ± 5.08 | 0.60 | 0.29–1.23 | 0.155 |
| Lesion Number | |||||
| 1 * | 30 | 4.40 ± 2.90 | Reference | ||
| >1 | 11 | 3.90 ± 10.79 | 0.72 | 0.30–1.59 | 0.468 |
| Gamma Knife (GK) Dose (Gy) | |||||
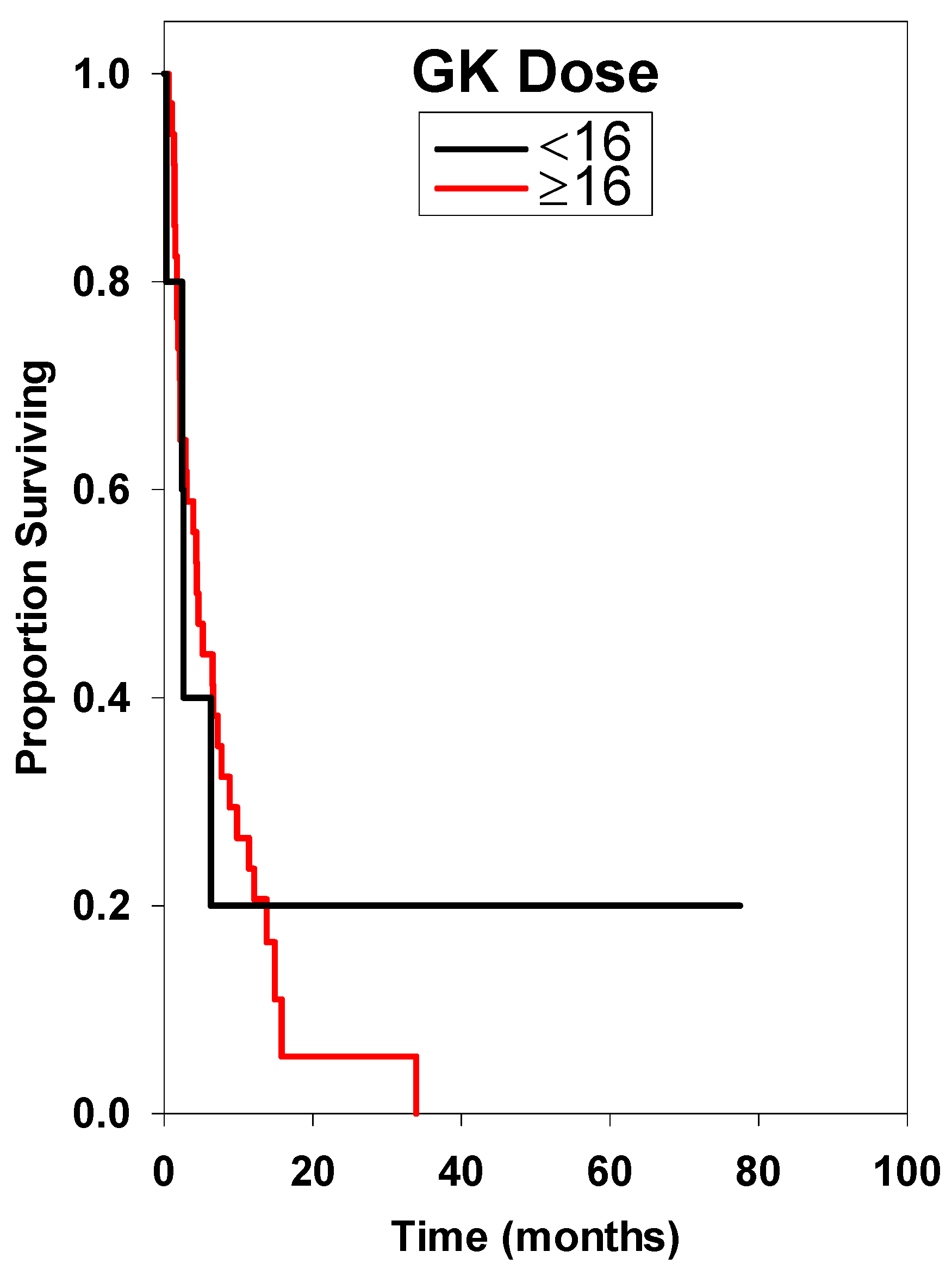
| <16 * | 5 | 2.60 ± 0.43 | Reference | ||
| ≥16 | 36 | 4.40 ± 2.55 | 1.22 | 0.41–4.90 | 0.999 |
| Prior WBRT | |||||
| Yes * | 19 | 3.90 ± 2.05 | Reference | ||
| No | 20 | 6.50 ± 6.57 | 0.55 | 0.25–1.194 | 0.101 |
| Unknown | 2 | Incalculable | 0.87 | 0.09–3.92 | 0.999 |
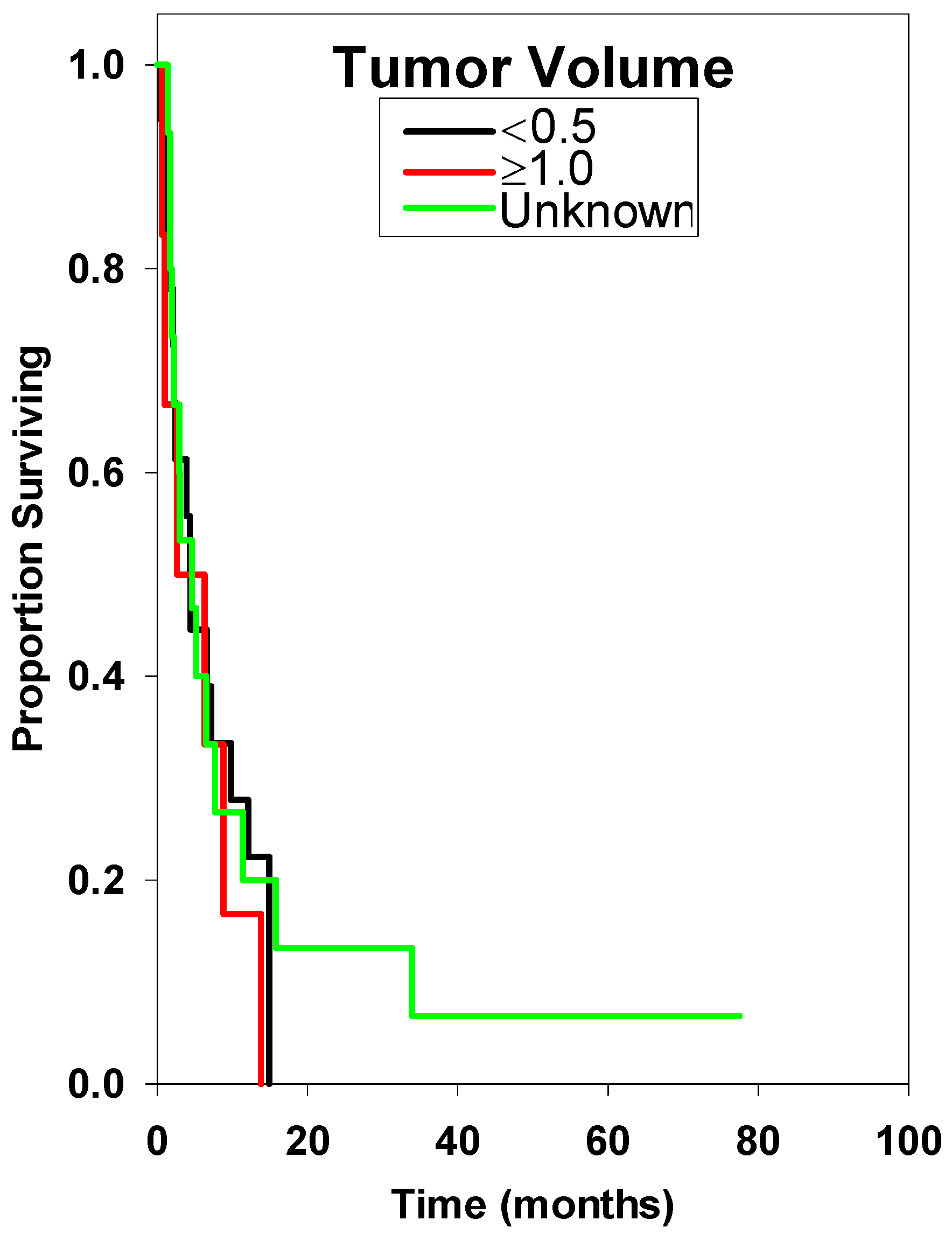
| Tumor Volume (cc) | |||||
| <0.5 * | 19 | 4.40 ± 1.03 | Reference | ||
| ≥1.0 | 6 | 2.60 ± 6.36 | 1.45 | 0.45–4.00 | 0.428 |
| Unknown | 16 | 3.00 ± 3.33 | 1.00 | 0.43–2.27 | 0.999 |
| Characteristic | Hazard Ratio | ||
|---|---|---|---|
| Estimate | 95% CI | p Value ** | |
| Histology | |||
| Breast * | Reference | ||
| Small Cell Lung | 0.51 | 0.10–2.69 | 0.427 |
| Other Lung | 0.54 | 0.19–1.53 | 0.242 |
| Renal/Melanoma | 2.10 | 0.50–8.82 | 0.310 |
| Other/Unknown | 5.31 | 0.30–4.03 | 0.255 |
| Age at Diagnosis (years) | |||
| <60 * | Reference | ||
| ≥60 | 1.39 | 0.55–3.53 | 0.493 |
| KPS | |||
| <70 * | Reference | ||
| ≥80 | 0.31 | 0.12–0.82 | 0.019 |
| Lesion Number | |||
| 1 * | Reference | ||
| >1 | 1.43 | 0.50–4.12 | 0.507 |
| Gamma Knife (GK) Dose (Gy) | |||
| <16 * | Reference | ||
| ≥16 | 1.30 | 0.38–4.45 | 0.676 |
| Prior WBRT | |||
| Yes * | Reference | ||
| No | 0.28 | 0.10–0.81 | 0.019 |
| Unknown | 0.38 | 0.05–2.82 | 0.341 |


3. Experimental Section
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tosoni, A.; Ermani, M.; Brandes, A.A. The pathogenesis and treatment of brain metastases: A comprehensive review. Crit. Rev. Oncol. Hematol. 2004, 52, 199–215. [Google Scholar] [CrossRef]
- Yoo, T.W.; Park, E.S.; Kwon do, H.; Kim, C.J. Gamma Knife radiosurgery for brainstem metastasis. J. Korean Neurosurg. Soc. 2011, 50, 299–303. [Google Scholar] [CrossRef]
- Yen, C.P.; Sheehan, J.; Patterson, G.; Steiner, L. Gamma Knife surgery for metastatic brainstem tumors. J. Neurosurg. 2006, 105, 213–219. [Google Scholar] [CrossRef]
- Peterson, H.E.; Larson, E.W.; Fairbanks, R.K.; Lamoreaux, W.T.; Mackay, A.R.; Call, J.A.; Demakas, J.J.; Cooke, B.S.; Lee, C.M. Gamma Knife radiosurgery treatment for metastatic melanoma of the trigeminal nerve and brainstem: A case report and a review of the literature. Case Rep. Neurol. Med. 2013, 2013, 256962. [Google Scholar]
- Fuentes, S.; Delsanti, C.; Metellus, P.; Peragut, J.C.; Grisoli, F.; Regis, J. Brainstem metastases: Management using Gamma Knife radiosurgery. Neurosurgery 2006, 58, 37–42; discussion 37–42. [Google Scholar] [CrossRef]
- Huang, C.F.; Kondziolka, D.; Flickinger, J.C.; Lunsford, L.D. Stereotactic radiosurgery for brainstem metastases. J. Neurosurg. 1999, 91, 563–568. [Google Scholar] [CrossRef]
- Hussain, A.; Brown, P.D.; Stafford, S.L.; Pollock, B.E. Stereotactic radiosurgery for brainstem metastases: Survival, tumor control, and patient outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 521–524. [Google Scholar] [CrossRef]
- Kased, N.; Huang, K.; Nakamura, J.L.; Sahgal, A.; Larson, D.A.; McDermott, M.W.; Sneed, P.K. Gamma Knife radiosurgery for brainstem metastases: The UCSF experience. J. Neuro-Oncol. 2008, 86, 195–205. [Google Scholar] [CrossRef]
- Kawabe, T.; Yamamoto, M.; Sato, Y.; Barfod, B.E.; Urakawa, Y.; Kasuya, H.; Mineura, K. Gamma Knife surgery for patients with brainstem metastases. J. Neurosurg. 2012, 117 (Suppl.), 23–30. [Google Scholar]
- Koyfman, S.A.; Tendulkar, R.D.; Chao, S.T.; Vogelbaum, M.A.; Barnett, G.H.; Angelov, L.; Weil, R.J.; Neyman, G.; Reddy, C.A.; Suh, J.H. Stereotactic radiosurgery for single brainstem metastases: The cleveland clinic experience. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 409–414. [Google Scholar]
- Li, Y.; Xu, D.; Zhang, Z.; Zhang, Y.; Liu, D.; Liu, X.; Wang, G.; Lin, Y. Gamma Knife surgery for brainstem metastases. J. Neurosurg. 2012, 117 (Suppl.), 13–16. [Google Scholar]
- Lorenzoni, J.G.; Devriendt, D.; Massager, N.; Desmedt, F.; Simon, S.; van Houtte, P.; Brotchi, J.; Levivier, M. Brain stem metastases treated with radiosurgery: Prognostic factors of survival and life expectancy estimation. Surg. Neurol. 2009, 71, 188–195. [Google Scholar] [CrossRef]
- Sengoz, M.; Kabalay, I.A.; Tezcanli, E.; Peker, S.; Pamir, N. Treatment of brainstem metastases with Gamma Knife radiosurgery. J. Neuro-Oncol. 2013, 113, 33–38. [Google Scholar] [CrossRef]
- Shuto, T.; Fujino, H.; Asada, H.; Inomori, S.; Nagano, H. Gamma Knife radiosurgery for metastatic tumours in the brain stem. Acta Neurochir. 2003, 145, 755–760. [Google Scholar] [CrossRef]
- Jung, E.W.; Rakowski, J.T.; Delly, F.; Jagannathan, J.; Konski, A.A.; Guthikonda, M.; Kim, H.; Mittal, S. Gamma Knife radiosurgery in the management of brainstem metastases. Clin. Neurol. Neurosurg. 2013, 115, 2023–2028. [Google Scholar] [CrossRef]
- Kilburn, J.M.; Ellis, T.L.; Lovato, J.F.; Urbanic, J.J.; Daniel Bourland, J.; Munley, M.T.; Deguzman, A.F.; McMullen, K.P.; Shaw, E.G.; Tatter, S.B.; et al. Local control and toxicity outcomes in brainstem metastases treated with single fraction radiosurgery: Is there a volume threshold for toxicity? J. Neuro-Oncol. 2014, 117, 167–174. [Google Scholar] [CrossRef]
- Hatiboglu, M.A.; Chang, E.L.; Suki, D.; Sawaya, R.; Wildrick, D.M.; Weinberg, J.S. Outcomes and prognostic factors for patients with brainstem metastases undergoing stereotactic radiosurgery. Neurosurgery 2011, 69, 796–806; discussion 806. [Google Scholar] [CrossRef]
- Hong, T.; Tome, W.; Hayes, L.; Yuan, Z.; Badie, B.; Rao, R.; Mehta, M. Acute Sequelae of Stereotactic Radiosurgery; Karger: Basel, Switzerland, 2004; volume 5. [Google Scholar]
- Boden, G. Radiation myelitis of the cervical spinal cord. Br. J. Radiol. 1948, 21, 464–469. [Google Scholar] [CrossRef]
- Valery, C.A.; Boskos, C.; Boisserie, G.; Lamproglou, I.; Cornu, P.; Mazeron, J.J.; Simon, J.M. Minimized doses for linear accelerator radiosurgery of brainstem metastasis. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 362–368. [Google Scholar] [CrossRef]
- Patil, C.G.; Pricola, K.; Sarmiento, J.M.; Garg, S.K.; Bryant, A.; Black, K.L. Whole brain radiation therapy (WBRT) alone vs. WBRT and radiosurgery for the treatment of brain metastases. Cochrane Database Syst. Rev. 2012, 9. CD006121. The Cochrane Library. Available online: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD006121.pub3/full (accessed on 13 January 2014).
- Aoyama, H.; Shirato, H.; Tago, M.; Nakagawa, K.; Toyoda, T.; Hatano, K.; Kenjyo, M.; Oya, N.; Hirota, S.; Shioura, H.; et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs. stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA 2006, 295, 2483–2491. [Google Scholar] [CrossRef]
- Chang, E.L.; Wefel, J.S.; Hess, K.R.; Allen, P.K.; Lang, F.F.; Kornguth, D.G.; Arbuckle, R.B.; Swint, J.M.; Shiu, A.S.; Maor, M.H.; et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009, 10, 1037–1044. [Google Scholar] [CrossRef]
- Soffietti, R.; Kocher, M.; Abacioglu, U.M.; Villa, S.; Fauchon, F.; Baumert, B.G.; Fariselli, L.; Tzuk-Shina, T.; Kortmann, R.D.; Carrie, C.A. European organisation for research and treatment of cancer phase III trial of adjuvant whole-brain radiotherapy vs. observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: Quality-of-life results. J. Clin. Oncol. 2013, 31, 65–72. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Peterson, H.E.; Larson, E.W.; Fairbanks, R.K.; MacKay, A.R.; Lamoreaux, W.T.; Call, J.A.; Carlson, J.D.; Ling, B.C.; Demakas, J.J.; Cooke, B.S.; et al. Gamma Knife Treatment of Brainstem Metastases. Int. J. Mol. Sci. 2014, 15, 9748-9761. https://doi.org/10.3390/ijms15069748
Peterson HE, Larson EW, Fairbanks RK, MacKay AR, Lamoreaux WT, Call JA, Carlson JD, Ling BC, Demakas JJ, Cooke BS, et al. Gamma Knife Treatment of Brainstem Metastases. International Journal of Molecular Sciences. 2014; 15(6):9748-9761. https://doi.org/10.3390/ijms15069748
Chicago/Turabian StylePeterson, Halloran E., Erik W. Larson, Robert K. Fairbanks, Alexander R. MacKay, Wayne T. Lamoreaux, Jason A. Call, Jonathan D. Carlson, Benjamin C. Ling, John J. Demakas, Barton S. Cooke, and et al. 2014. "Gamma Knife Treatment of Brainstem Metastases" International Journal of Molecular Sciences 15, no. 6: 9748-9761. https://doi.org/10.3390/ijms15069748




