Geminal Brønsted Acid Ionic Liquids as Catalysts for the Mannich Reaction in Water
Abstract
:1. Introduction
2. Results and Discussion
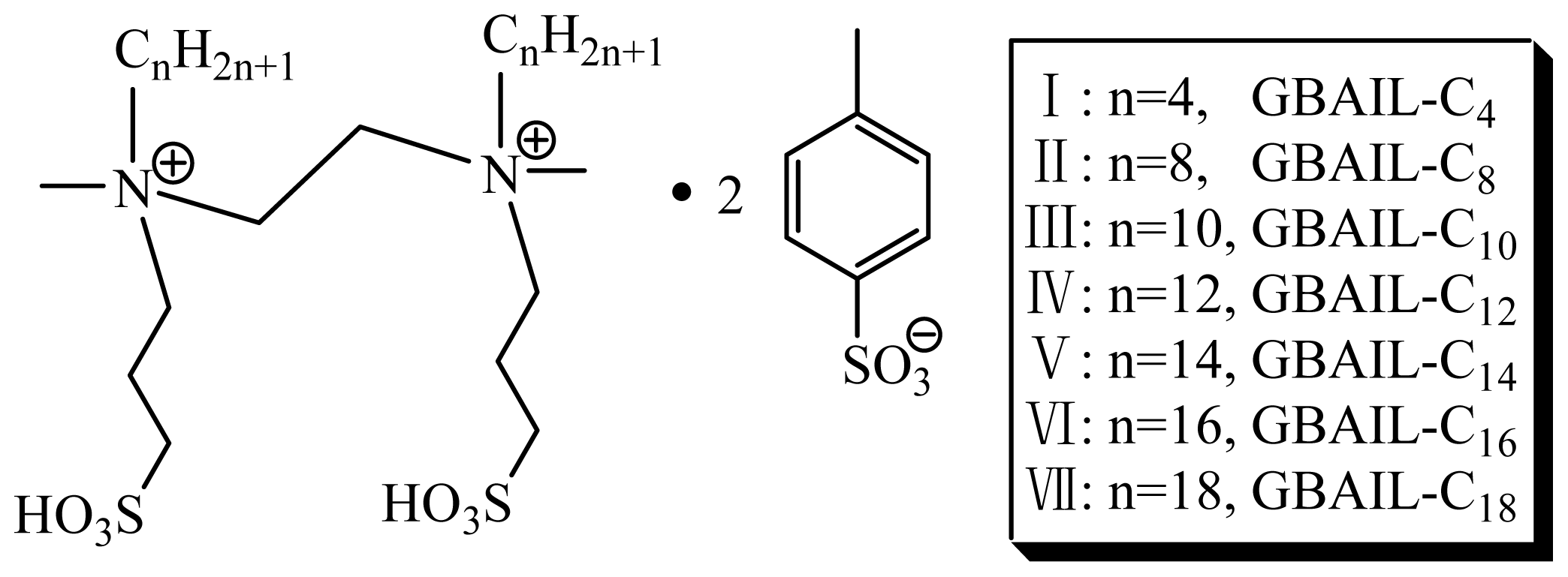
2.1. Catalyst Screening for the Mannich Reaction
2.2. Reaction Conditions of the Mannich Reaction
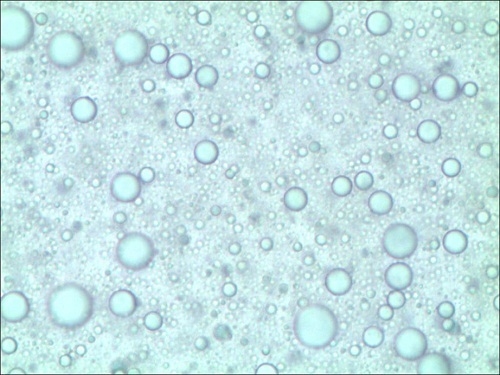
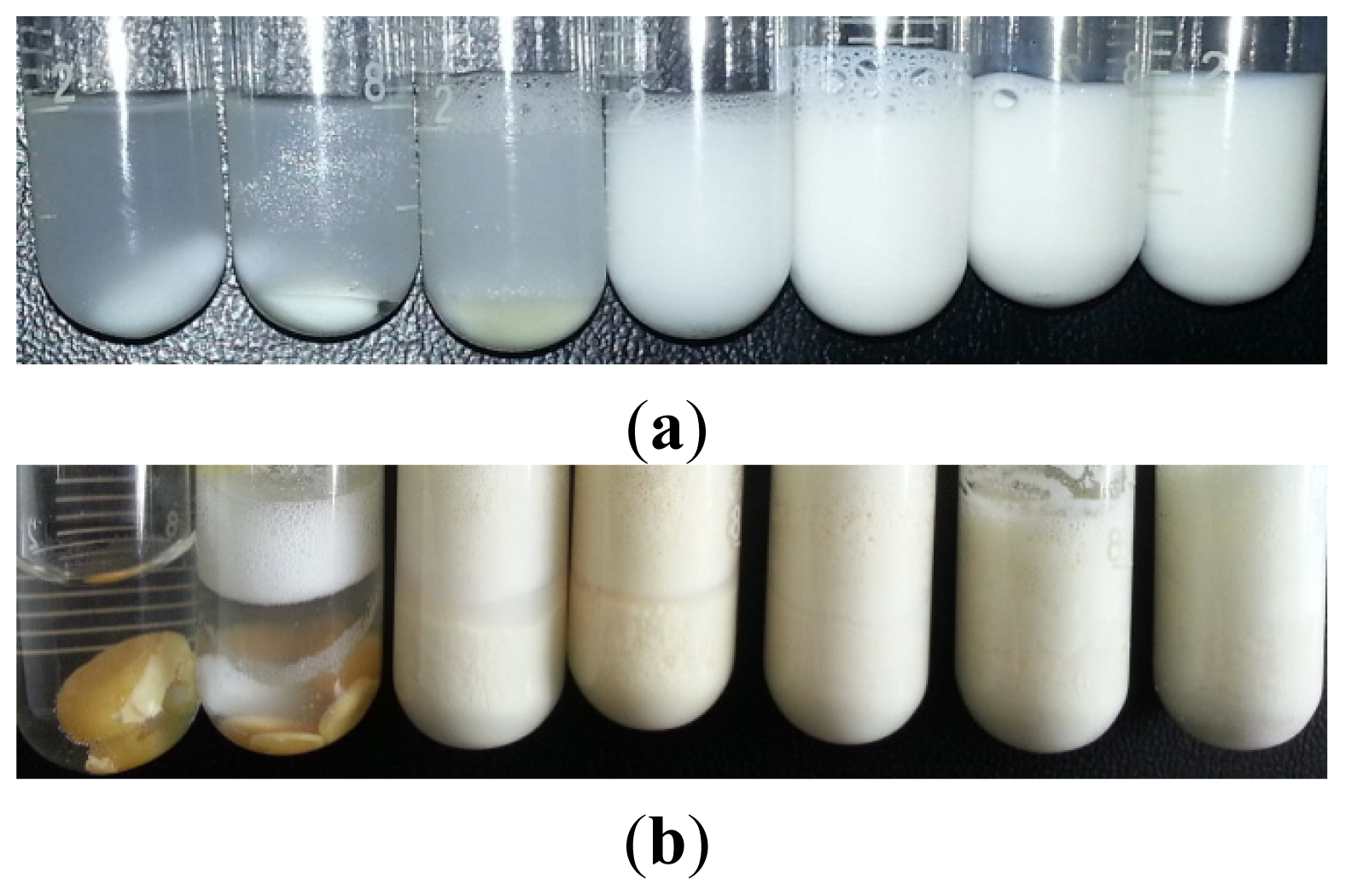
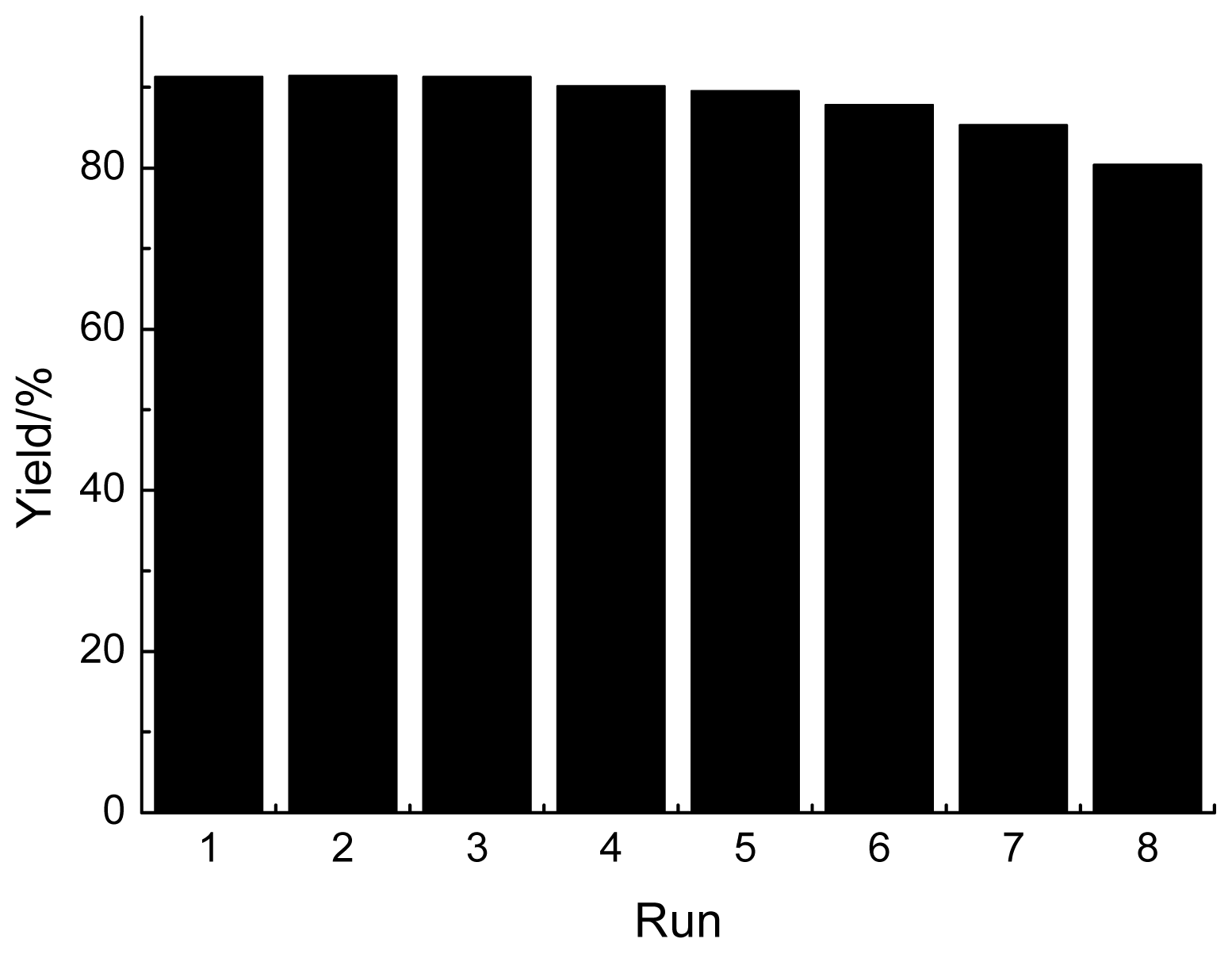
2.3. Reusability of GBAIL-C14 in the Mannich Reaction
2.4. Catalytic Performance of GBAIL-C14 in Mannich Reactions in Water
3. Experimental Section
3.1. General
3.2. Synthesis of GBAILs
3.2.1. Synthesis of Geminal Sulfobetaine-Type Zwitterion
3.2.2. GBAIL Preparation
3.3. General Procedure for Mannich Reactions Catalyzed by GBAILs
4. Conclusions
Supplementary Information
ijms-15-08656-s001.pdfAcknowledgments
Conflicts of Interest
- Author ContributionsTao Chang conceived and designed the study. Leqin He performed the synthesis and isolation. Shenjun Qin performed identification and data analysis. Leqin He wrote the paper. Jiquan Zhao and Tao Chang reviewed and edited the manuscript. Yuzhuang Sun gave useful suggestions. All authors read and approved the manuscript.
References
- Michael, A.; Bernhard, W.; Nikolaus, R. Modern variants of the mannich reaction. Angew. Chem. Int. Ed 1998, 37, 1044–1070. [Google Scholar]
- Nuno, R.C.; Francesco, M.; Pedro, M.S.D.C.; Pedro, M.P.G. Boronic acids and esters in the petasis-borono mannich multicomponent reaction. Chem. Rev 2010, 110, 6169–6193. [Google Scholar]
- Subramaniapillai, S.G. Mannich reaction: A versatile and convenient approach to bioactive skeletons. J. Chem. Sci 2013, 125, 467–482. [Google Scholar]
- List, B. The direct catalytic asymmetric three-component Mannich reaction. J. Am. Chem. Soc 2000, 122, 9336–9337. [Google Scholar]
- Bekircan, O.; Bektas, H. Synthesis of schiff and mannich bases of isatin derivatives with 4-amino-4,5-dihydro-1H-1,2,4-triazole-5-ones. Molecules 2008, 13, 2126–2135. [Google Scholar]
- Blatt, A.H.; Gross, N. The addition of ketones to schiff bases. J. Org. Chem 1964, 29, 3306–3311. [Google Scholar]
- List, B.; Pojarliev, P.; Biller, W.T.; Martin, H.J. The proline-catalyzed direct asymmetric three-component mannich reaction: Scope, optimization, and application to the highly enantioselective synthesis of 1,2-amino alcohols. J. Am. Chem. Soc 2002, 124, 827–833. [Google Scholar]
- Manabe, K.; Mori, Y.; Kobayashi, S. Three-component carbon-carbon bond-forming reactions catalyzed by a Brønsted acid-surfactant-combined catalyst in water. Tetrahedron 2001, 57, 2537–2544. [Google Scholar]
- Kobayashi, S.; Hamada, T.; Manabe, K. The catalytic asymmetric Mannich-type reactions in aqueous Media. J. Am. Chem. Soc 2002, 124, 5640–5641. [Google Scholar]
- Azizi, N.; Torkiyan, L.; Saidi, M.R. Highly efficient one-pot three-component mannich reaction in water catalyzed by heteropoly acids. Org. Lett 2006, 8, 2079–2082. [Google Scholar]
- Iimura, S.; Nobutou, D.; Manabe, K.; Kobayashi, S. Mannich-type reactions in water using a hydrophobic polymer-supported sulfonic acid catalyst. Chem. Commun 2003, 1644–1645. [Google Scholar]
- Nitabaru, T.; Kumagai, N.; Shibasaki, M. Catalytic asymmetric nitro-mannich reactions with a Yb/K heterobimetallic catalyst. Molecules 2010, 15, 1280–1290. [Google Scholar]
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev 1999, 99, 2071–2083. [Google Scholar]
- Chaturvedi, D. Recent developments on task specific ionic liquids. Curr. Org. Chem 2011, 15, 1236–1248. [Google Scholar]
- Stepnowski, P. Application of chromatographic and electrophoretic methods for the analysis of imidazolium and pyridinium cations as used in ionic liquids. Int. J. Mol. Sci 2006, 7, 497–509. [Google Scholar]
- Pârvulescu, V.I.; Hardacre, C. Catalysis in ionic liquids. Chem. Rev 2007, 107, 2615–2665. [Google Scholar]
- Hallett, J.P.; Welton, T. Room-temperature ionic liquids: Solvents for synthesis and catalysis. Chem. Rev 2011, 111, 3508–3576. [Google Scholar]
- Yang, X.; Wang, M.; Varma, R.; Li, C. Ruthenium-catalyzed tandem olefin migration—Aldol- and mannich-type reactions in ionic liquid. J. Mol. Catal. A 2004, 214, 147–154. [Google Scholar]
- Akiyama, T.; Suzuki, A.; Fuchibe, K. Mannich-type reaction promoted by an ionic liquid. Synlett 2005, 6, 1024–1026. [Google Scholar]
- Yong, F.F.; Teo, Y.C. Recyclable siloxy serine organocatalyst for the direct asymmetric Mannich reaction in ionic liquids. Synth. Commun 2011, 41, 1293–1300. [Google Scholar]
- Li, J.; Peng, Y.; Song, G. Mannich reaction catalyzed by carboxyl-functionalized ionic liquid in aqueous media. Catal. Lett 2005, 102, 159–162. [Google Scholar]
- Zheng, X.; Qian, Y.B.; Wang, Y. 2-Pyrrolidinecarboxylic acid ionic liquid as a highly efficient organocatalyst for the asymmetric one-pot Mannich reaction. Eur. J. Org. Chem 2010, 2010, 515–522. [Google Scholar]
- Zhao, G.; Jiang, T.; Gao, H.; Han, B.; Huang, J.; Sun, D. Mannich reaction using acidic ionic liquids as catalysts and solvents. Green Chem 2004, 6, 75–77. [Google Scholar]
- Sahoo, S.; Joseph, T.; Halligudi, S.B. Mannich reaction in brønsted acidic ionic liquid: A facile synthesis of β-amino carbonyl compounds. J. Mol. Catal. A 2006, 244, 179–182. [Google Scholar]
- Dong, F.; Zhenghao, F.; Zuliang, L. Functionalized ionic liquid as the recyclable catalyst for mannich-type reaction in aqueous media. Catal. Commun 2009, 10, 1267–1270. [Google Scholar]
- Manabe, K.; Kobayashi, S. Mannich-type reactions of aldehydes, amines, and ketones in a colloidal dispersion system created by a brønsted acid-surfactant-combined catalyst in Water. Org. Lett 1999, 1, 1965–1967. [Google Scholar]
- Fang, D.; Luo, J.; Zhou, X.; Lui, Z. Mannich reaction in water using acidic ionic liquid as recoverable and reusable catalyst. Catal. Lett 2007, 116, 76–80. [Google Scholar]
- Shimizu, S.; Shimada, N.; Sasaki, Y. Mannich-type reactions in water using anionic water-soluble calixarenes as recoverable and reusable catalysts. Green Chem 2006, 8, 608–614. [Google Scholar]
- Chang, T.; He, L.; Bian, L.; Han, H.; Yuan, M.; Gao, X. Brønsted acid-surfactant-combined catalyst for the mannich reaction in water. RSC Adv 2014, 4, 727–731. [Google Scholar]
- Wu, C.; Fu, X.; Ma, X.; Li, S.; Li, C. Threonine-surfactant organocatalysts for the highly diastereo- and enantioselective direct anti-Mannich reactions of hydroxyacetone. Tetrahedron Lett 2010, 51, 5775–5777. [Google Scholar]
- Wu, C.; Fu, X.; Li, S. Simple and inexpensive threonine-based organocatalysts for the highly diastereo- and enantioselective direct large-scale syn-aldol and anti-Mannich reactions of α-hydroxyacetone. Tetrahedron: Asymmetry 2011, 22, 1063–1073. [Google Scholar]
- Ding, Y.; Zha, M.; Zhang, J.; Wang, S. Synthesis, characterization and properties of geminal imidazolium ionic liquids. Colloids Surf. A 2007, 298, 201–205. [Google Scholar]
- Ao, M.; Xu, G.; Kang, W.; Meng, L.; Gong, H.; Zhou, T. Surface rheological behavior of gelatin/ionic liquid-type imidazolium gemini surfactant mixed systems. Soft Matter 2011, 7, 1199–1206. [Google Scholar]
- Zhang, S.; Yan, H.; Zhao, M.; Zheng, L. Aggregation behavior of gemini pyrrolidine-based ionic liquids 1,1′-(butane-1,4-diyl)bis(1-alkylpyrrolidinium) bromide ([Cnpy-4-Cnpy][Br2]) in aqueous solution. J. Colloid Interface Sci 2012, 372, 52–57. [Google Scholar]
- Yoshimura, T.; Ichinokawa, T.; Kaji, M.; Esumi, K. Synthesis and surface-active properties of sulfobetaine-type zwitterionic gemini surfactants. Colloids Surf. A 2006, 273, 208–212. [Google Scholar]
- He, L.; Qin, S.; Chang, T.; Sun, Y.; Gao, X. Biodiesel synthesis from the esterification of free fatty acids and alcohol catalyzed by long-chain Brønsted acid ionic liquid. Catal. Sci. Technol 2013, 3, 1102–1107. [Google Scholar]

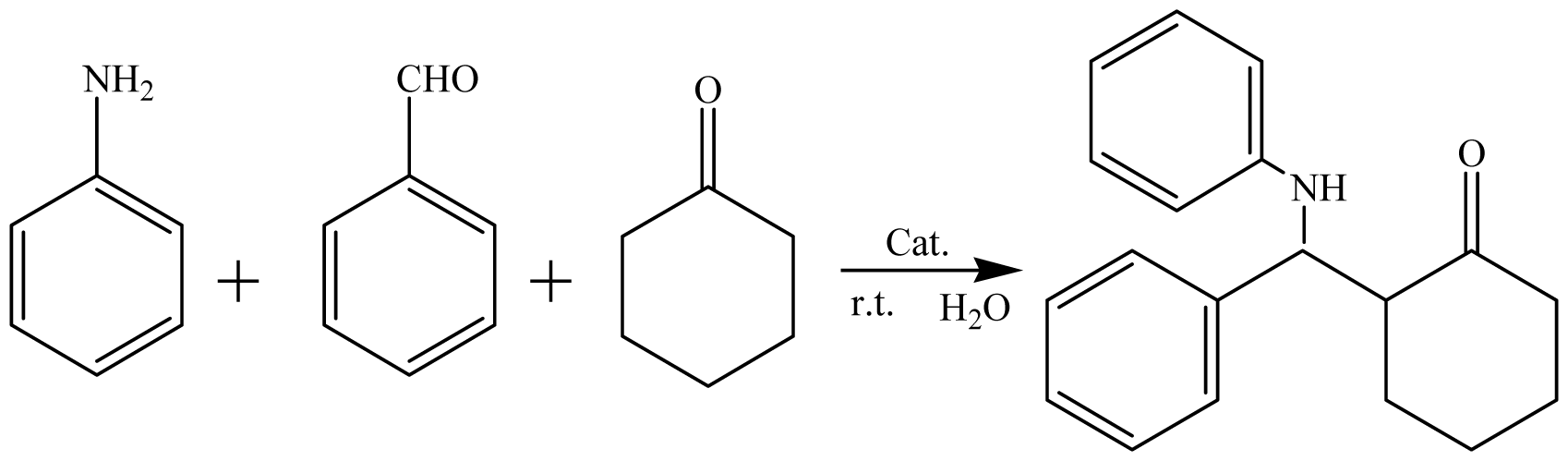 | ||
|---|---|---|
| Entry | Catalyst | Yield/% b |
| 1 | GBAIL-C4 | 64 |
| 2 | GBAIL-C8 | 70 |
| 3 | GBAIL-C10 | 76 |
| 4 | GBAIL-C12 | 82 |
| 5 | GBAIL-C14 | 88 |
| 6 | GBAIL-C16 | 87 |
| 7 | GBAIL-C18 | 85 |
| Entry | Amount of catalyst/mmol | Time/h | Yield/% b |
|---|---|---|---|
| 1 | 0.01 | 4 | 43 |
| 2 | 0.02 | 4 | 65 |
| 3 | 0.03 | 4 | 72 |
| 4 | 0.04 | 4 | 79 |
| 5 | 0.05 | 4 | 88 |
| 6 | 0.06 | 4 | 88 |
| 7 | 0.07 | 4 | 89 |
| 8 | 0.05 | 1 | 43 |
| 9 | 0.05 | 2 | 69 |
| 10 | 0.05 | 3 | 83 |
| 11 | 0.05 | 5 | 91 |
| 12 | 0.05 | 6 | 92 |
| 13 | 0.05 | 7 | 92 |
| 14 | 0.05 | 8 | 93 |
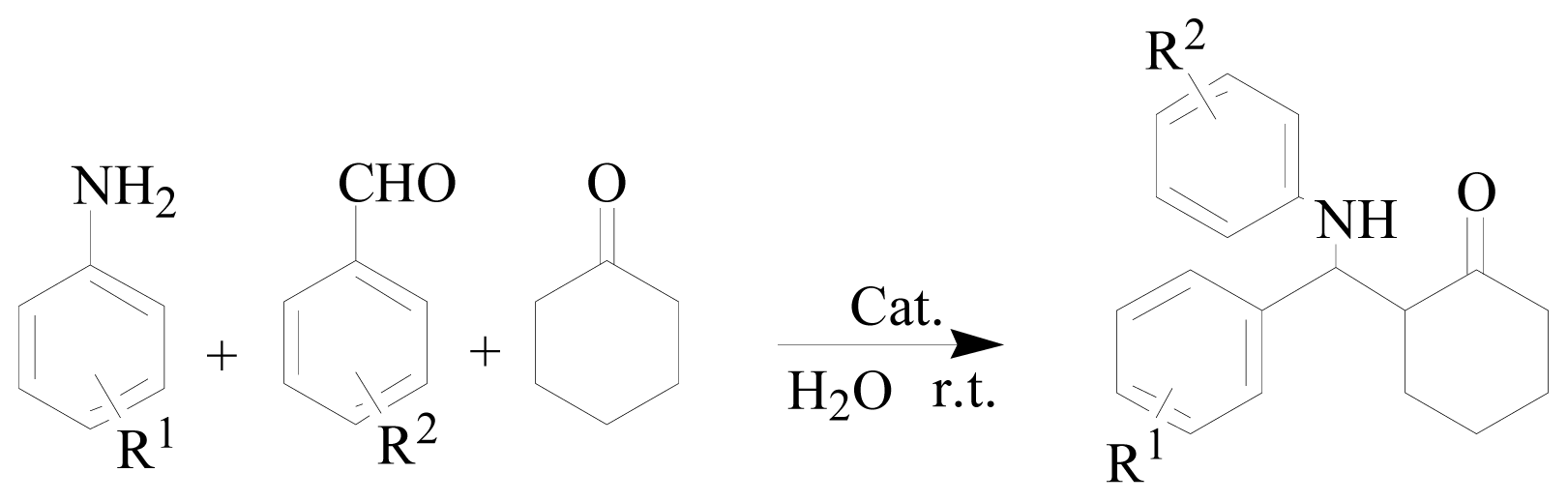 | |||
|---|---|---|---|
| Entry | R1 | R2 | Yield/% b |
| 1 | H | H | 91 |
| 2 | p-Cl | H | 94 |
| 3 | p-OMe | H | 89 |
| 4 | o-OMe | H | 85 |
| 5 | H | o-NO2 | 91 |
| 6 | H | p-NO2 | 91 |
| 7 | H | o-Cl | 92 |
| 8 | H | p-OMe | 90 |
| 9 | H | H | 87 c |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
He, L.; Qin, S.; Chang, T.; Sun, Y.; Zhao, J. Geminal Brønsted Acid Ionic Liquids as Catalysts for the Mannich Reaction in Water. Int. J. Mol. Sci. 2014, 15, 8656-8666. https://doi.org/10.3390/ijms15058656
He L, Qin S, Chang T, Sun Y, Zhao J. Geminal Brønsted Acid Ionic Liquids as Catalysts for the Mannich Reaction in Water. International Journal of Molecular Sciences. 2014; 15(5):8656-8666. https://doi.org/10.3390/ijms15058656
Chicago/Turabian StyleHe, Leqin, Shenjun Qin, Tao Chang, Yuzhuang Sun, and Jiquan Zhao. 2014. "Geminal Brønsted Acid Ionic Liquids as Catalysts for the Mannich Reaction in Water" International Journal of Molecular Sciences 15, no. 5: 8656-8666. https://doi.org/10.3390/ijms15058656