Rapid Carbonation for Calcite from a Solid-Liquid-Gas System with an Imidazolium-Based Ionic Liquid
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Temperature


2.2. Effect of Pressure
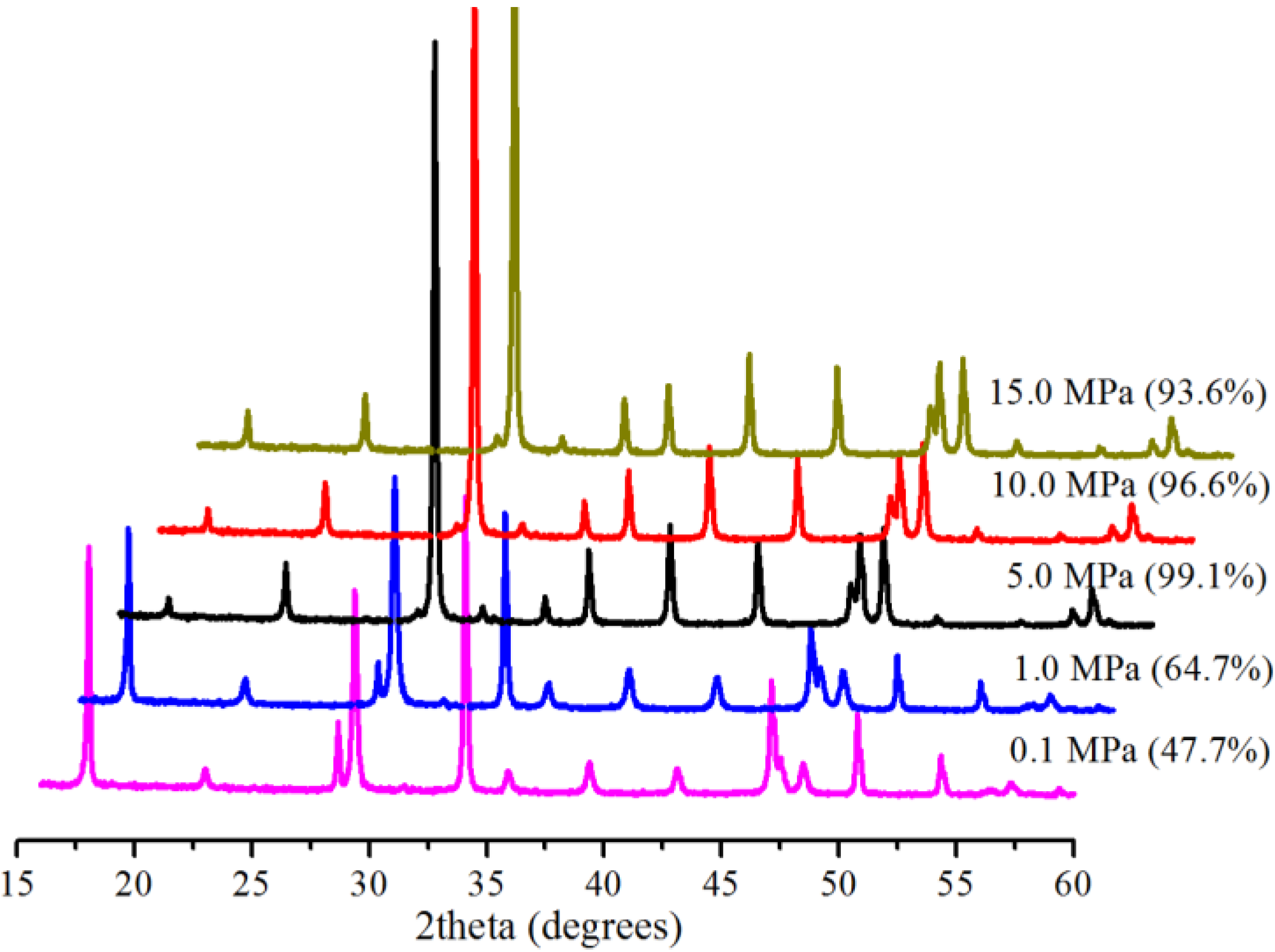
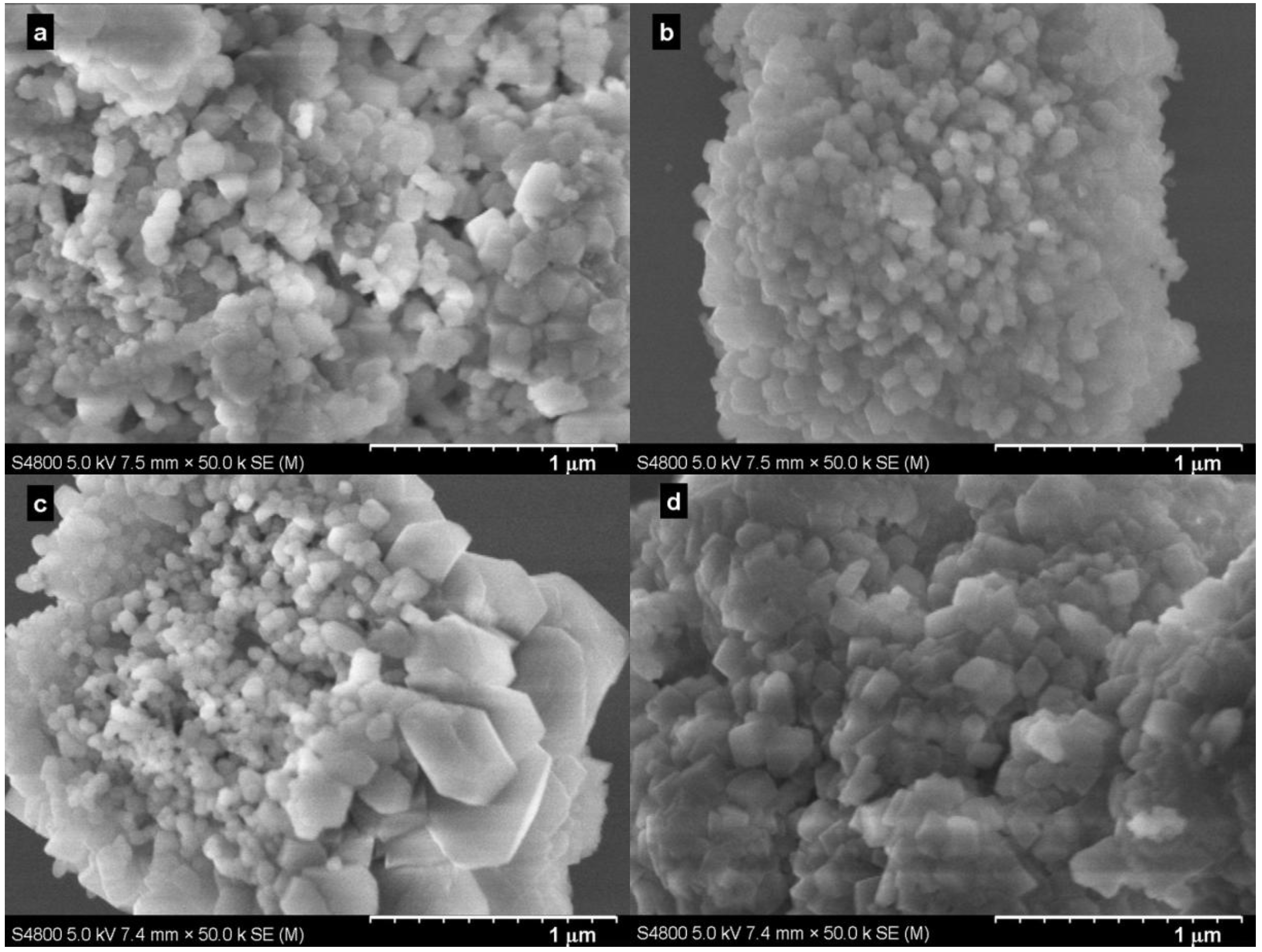
2.3. Effect of Reaction Time
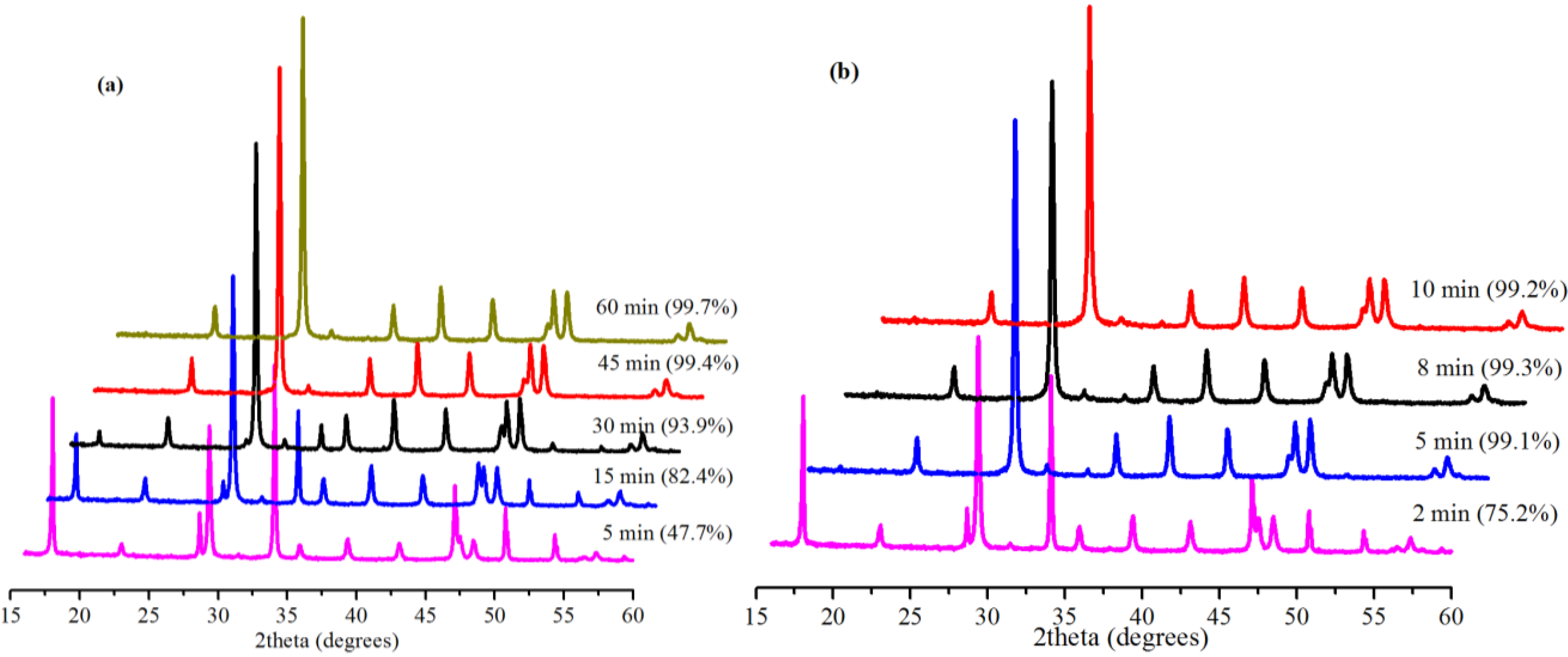
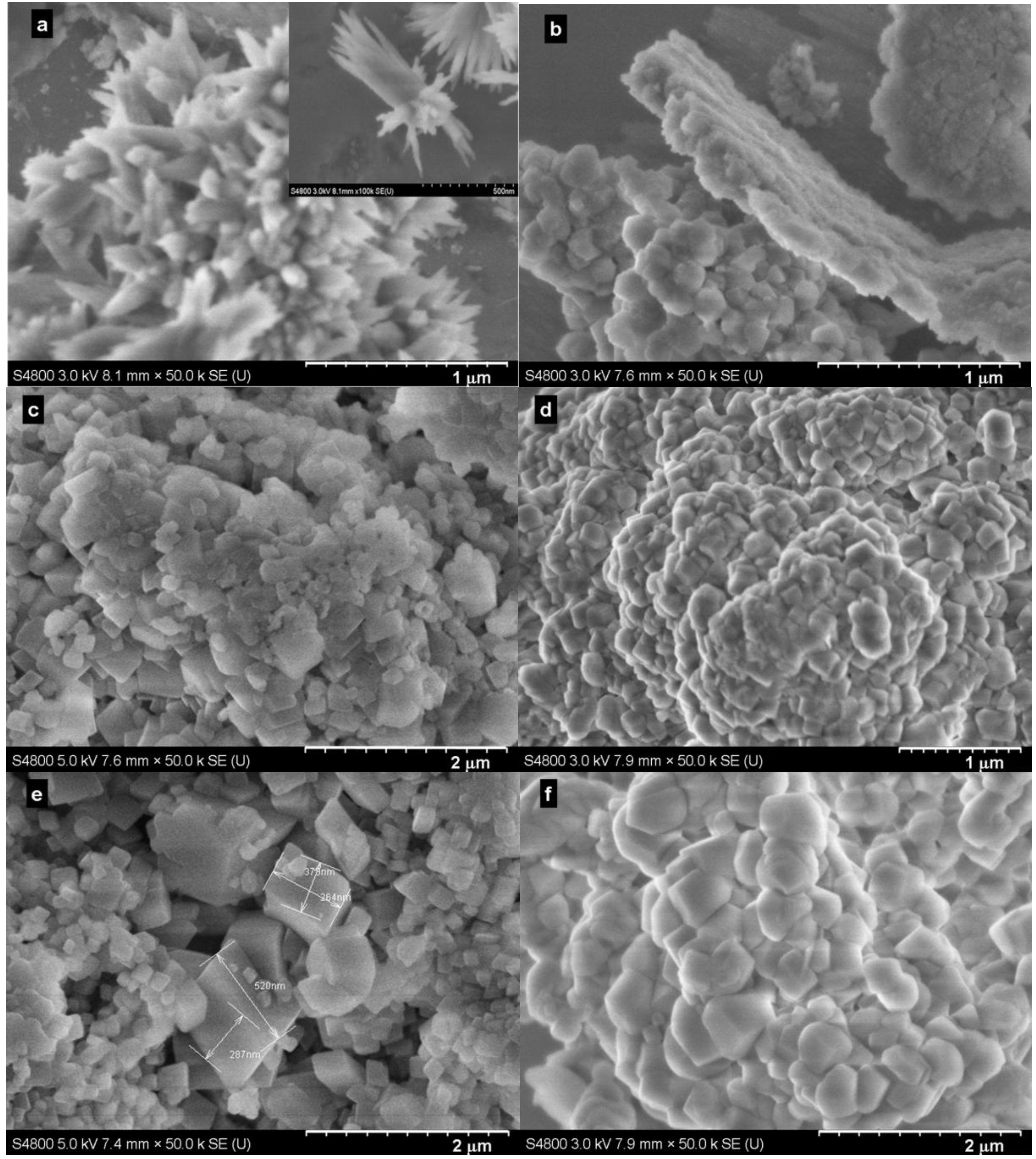
2.4. Effect of Amount of Water

2.5. Effect of Amount of Bmim[Br]
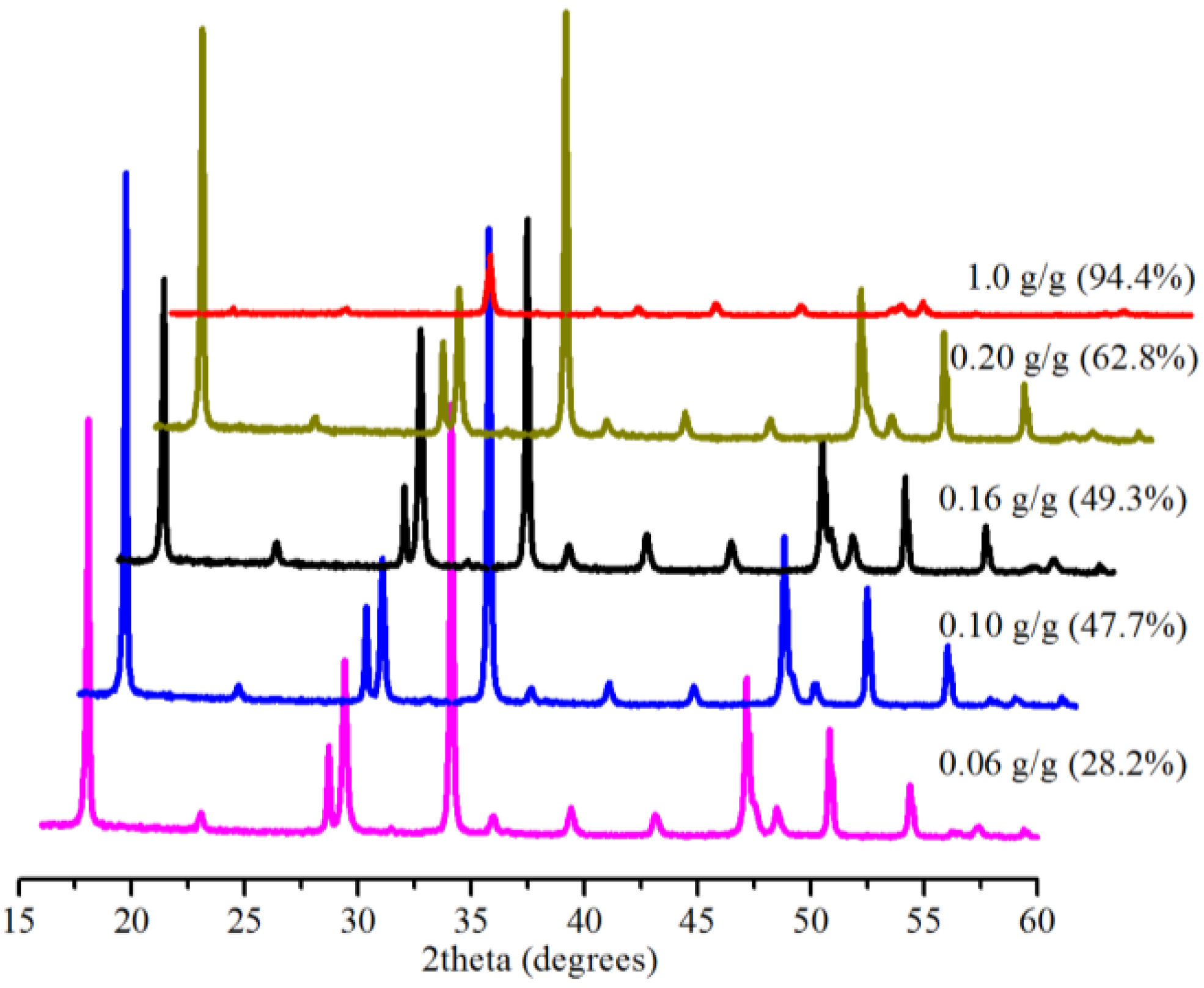
2.6. Significance of Bmim[Br] and the Mechanism
2.7. Brunner-Emmet-Teller (BET) Surface Area and Thermogravimetric Analysis (TGA)
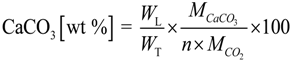
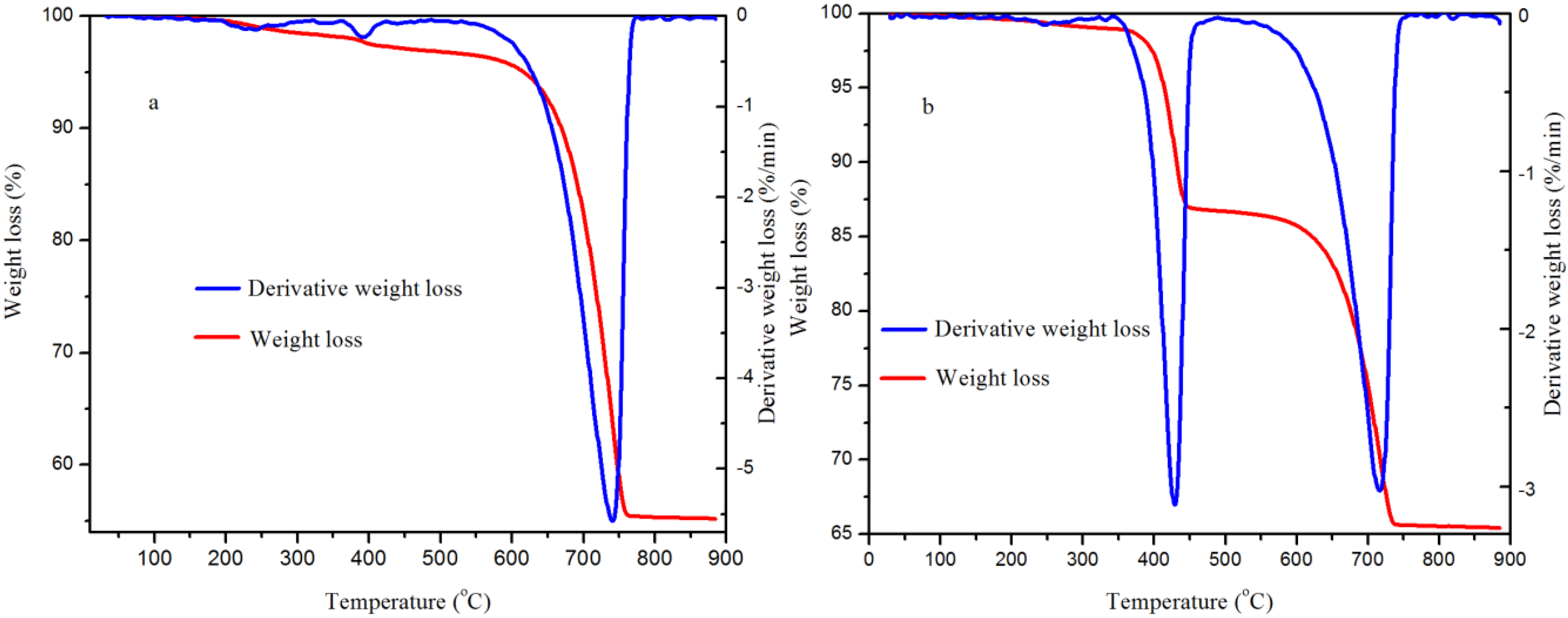
| Sample | Rietveld (wt %) | TGA (wt %) | Difference (wt %) |
|---|---|---|---|
| 5.0 MPa | 99.1 | 96.2 | 2.9 |
| 0.1 MPa | 47.7 | 49.7 | 2.0 |
3. Experimental Section
3.1. Materials
3.2. Procedure

3.3. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gomez-Villalba, L.S.; Lopez-Arce, P.; de Buergo, M.A.; Fort, R. Atomic defects and their relationship to aragonite-calcite transformation in portlandite nanocrystal carbonation. Cryst. Growth Des. 2012, 12, 4844–4852. [Google Scholar] [CrossRef]
- Knez, S.; Klinar, D.; Golob, J. Stabilization of PCC dispersions prepared directly in the mother-liquid after synthesis through the carbonation of (hydrated) lime. Chem. Eng. Sci. 2006, 61, 5867–5880. [Google Scholar] [CrossRef]
- Montes-Hernandez, G.; Daval, D.; Chiriac, R.; Renard, F. Growth of nanosized calcite through gas-solid carbonation of nanosized portlandite under anisobaric conditions. Cryst. Growth Des. 2010, 10, 4823–4830. [Google Scholar] [CrossRef]
- Ibrahim, A.R.; Zhang, X.L.; Hong, Y.Z.; Wang, H.T.; Li, J. Instantaneous solid-liquid-gas carbonation of Ca(OH)2 and chameleonic phase transformation in CO2-expanded solution. Cryst. Growth Des. 2014, 14, 2733–2741. [Google Scholar] [CrossRef]
- Domingo, C.; Loste, E.; Gomez-Morales, J.; Garcia-Carmona, J.; Fraile, J. Calcite precipitation by a high-pressure CO2 carbonation route. J. Supercrit. Fluids 2006, 36, 202–215. [Google Scholar] [CrossRef]
- Ciullo, P.A. Industrial Minerals and Their Uses: A Handbook and Formulary; Noyes Publications: Westwood, NJ, USA, 1996; pp. 35–37. [Google Scholar]
- Montes-Hernandez, G.; Renard, F.; Geoffroy, N.; Charlet, G.; Pironon, J. Calcite precipitation from CO2–H2O–Ca(OH)2 slurry under high pressure of CO2. J. Cryst. Growth 2007, 308, 228–236. [Google Scholar] [CrossRef]
- Harja, M.; Cimpeanu, C.; Bucur, R.D. The influence of hydrodynamic conditions on the synthesis of ultra-thin calcium carbonate. J. Food Agric. Environ. 2012, 10, 1191–1195. [Google Scholar]
- Juvekar, V.A.; Sharma, M.M. Absorption of CO2 in a suspension of lime. Chem. Eng. Sci. 1973, 28, 825–837. [Google Scholar] [CrossRef]
- Sada, E.; Kumazawa, H.; Lee, C.; Fujiwara, N. Gas-liquid mass transfer characteristics in a bubble column with suspended sparingly soluble fine particles. Ind. Eng. Process Des. Dev. 1985, 24, 255–261. [Google Scholar] [CrossRef]
- Xiang, L.; Xiang, Y.; Wen, Y.; Wei, F. Formation of CaCO3 nanoparticles in the presence of terpineol. Mater. Lett. 2004, 58, 959–965. [Google Scholar]
- Yagi, H.; Iwazawa, A.; Sonobe, R.; Matsubara, T.; Hikita, H. Crystallization of calcium carbonate accompanying chemical absorption. Ind. Eng. Chem. Fundam. 1984, 23, 153–158. [Google Scholar] [CrossRef]
- Meldrum, F.C.; Hyde, S.T. Morphological influence of magnesium and organic additives on the precipitation of calcite. J. Cryst. Growth 2001, 231, 544–558. [Google Scholar] [CrossRef]
- Qi, L.; Li, J.; Ma, J. Biomimetic morphogenesis of calcium carbonate in mixed solutions of surfactants and double-hydrophilic block copolymers. Adv. Mater. 2002, 14, 300–303. [Google Scholar] [CrossRef]
- Colfen, H.; Qi, L. A systematic examination of the morphogenesis of calcium carbonate in the presence of a double-hydrophilic block copolymer. Chem. A Eur. J. 2001, 7, 106–116. [Google Scholar] [CrossRef]
- Rudloff, J.; Antonietti, M.; Colfen, H.; Pretula, J.; Kaluzynski, K.; Penczek, S. Double-hydrophilic block copolymers with monophosphate ester moieties as crystal growth modifiers of CaCO3. Macromol. Chem. Phys. 2002, 203, 627–635. [Google Scholar]
- Mann, S.; Heywood, B.R.; Rajam, S.; Birchall, J.D. Controlled crystallisation of CaCO3 under stearic acid monolayers. Nature 1988, 334, 692–965. [Google Scholar] [CrossRef]
- Naka, K.; Huang, S.C.; Chujo, Y. Formation of stable vaterite with poly(acrylic acid) by the delayed addition method. Langmuir 2006, 22, 7760–7767. [Google Scholar]
- Aki, S.N.V.K.; Mellein, B.R.; Saurer, E.M.; Brennecke, J.F. High-pressure phase behavior of carbon dioxide with imidazolium-based ionic liquids. J. Phys. Chem. B 2004, 108, 20355–20365. [Google Scholar] [CrossRef]
- Anthony, J.L.; Maginn, E.J.; Brennecke, J.F. Solution thermodynamics of imidazolium-based ionic liquids and water. J. Phys. Chem. B 2001, 105, 10942–10949. [Google Scholar] [CrossRef]
- Cadena, C.; Anthony, J.L.; Shah, J.K.; Morrow, T.I.; Brennecke, J.F.; Maginn, E.J. Why is CO2 so soluble in imidazolium-based ionic liquids? J. Am. Chem. Soc. 2004, 126, 5300–5308. [Google Scholar] [CrossRef]
- Sudha, S.Y.; Khanna, A. Evaluation of CO2-ionic liquid systems through molecular modeling. World Acad. Sci. Eng. Technol. 2009, 57, 539–542. [Google Scholar]
- Ibrahim, A.-R.; Vuningoma, J.B.; Hu, X.H.; Gong, Y.; Dan, H.; Hong, Y.Z.; Wang, H.T.; Li, J. High-pressure gas-solid carbonation route coupled with a solid ionic liquid for rapid synthesis of rhombohedral calcite. J. Supercrit. Fluids 2012, 72, 78–83. [Google Scholar] [CrossRef]
- Ibrahim, A.-R.; Gong, Y.; Hu, X.H.; Hong, Y.Z.; Su, Y.Z.; Wang, H.T.; Li, J. Solid-gas carbonation coupled with solid ionic liquid for synthesis of CaCO3: Performance, polymorphic control and self-catalytic kinetics. Ind. Eng. Chem. Res. 2013, 52, 9515–9524. [Google Scholar] [CrossRef]
- Lopez-Periago, A.M.; Pacciani, R.; Garcia-Gonzalez, C.; Vega, L.F.; Domingo, C. A breakthrough technique for the preparation of high-yield precipitated calcium carbonate. J. Supercrit. Fluids 2010, 52, 298–305. [Google Scholar] [CrossRef]
- Chen, P.C.; Tai, C.Y.; Lee, K.C. Morphology and growth rate of calcium carbonate crystals in a gas-liquid-solid reactive crystallizer. Chem. Eng. Sci. 1997, 52, 4171–4177. [Google Scholar] [CrossRef]
- Montes-Hernandez, G.; Pommerol, A.; Renard, F.; Beck, P.; Quirico, E.; Brissaud, O. In situ kinetic measurements of gas-solid carbonation of Ca(OH)2 by using an infrared microscope coupled to a reaction cell. Chem. Eng. J. 2010, 161, 250–256. [Google Scholar]
- Beruto, D.T.; Botter, R. Liquid-like H2O adsorption layers to catalyse the Ca(OH)2/CO2 solid-gas reaction and to form a non-protective solid product layer at 20 °C. J. Eur. Ceram. Soc. 2000, 20, 497–503. [Google Scholar] [CrossRef]
- Dickinson, S.R.; Henderson, G.E.; McGrath, K.M. Controlling the kinetic versus thermodynamic crystallisation of calcium carbonate. J. Cryst. Growth 2002, 244, 369–378. [Google Scholar] [CrossRef]
- Lopez-Periago, A.M.; Pacciani, R.; Vega, L.F.; Domingo, C. Monitoring the effect of mineral precursor, fluid phase CO2-H2O composition, and stirring on CaCO3 crystallization in a supercritical-ultrasound carbonation proces. Cryst. Growth Des. 2011, 11, 5324–5332. [Google Scholar] [CrossRef]
- Kedra-Krolik, K.; Gierycz, P. Precipitation of nano-structured calcite in a controlled multiphase process. J. Cryst. Growth 2009, 311, 3674–3681. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ibrahim, A.-R.; Vuningoma, J.B.; Huang, Y.; Wang, H.; Li, J. Rapid Carbonation for Calcite from a Solid-Liquid-Gas System with an Imidazolium-Based Ionic Liquid. Int. J. Mol. Sci. 2014, 15, 11350-11363. https://doi.org/10.3390/ijms150711350
Ibrahim A-R, Vuningoma JB, Huang Y, Wang H, Li J. Rapid Carbonation for Calcite from a Solid-Liquid-Gas System with an Imidazolium-Based Ionic Liquid. International Journal of Molecular Sciences. 2014; 15(7):11350-11363. https://doi.org/10.3390/ijms150711350
Chicago/Turabian StyleIbrahim, Abdul-Rauf, Jean Bosco Vuningoma, Yan Huang, Hongtao Wang, and Jun Li. 2014. "Rapid Carbonation for Calcite from a Solid-Liquid-Gas System with an Imidazolium-Based Ionic Liquid" International Journal of Molecular Sciences 15, no. 7: 11350-11363. https://doi.org/10.3390/ijms150711350






