Synthesis of Cellulose-2,3-bis(3,5-dimethylphenylcarbamate) in an Ionic Liquid and Its Chiral Separation Efficiency as Stationary Phase
Abstract
:1. Introduction
2. Results and Discussion
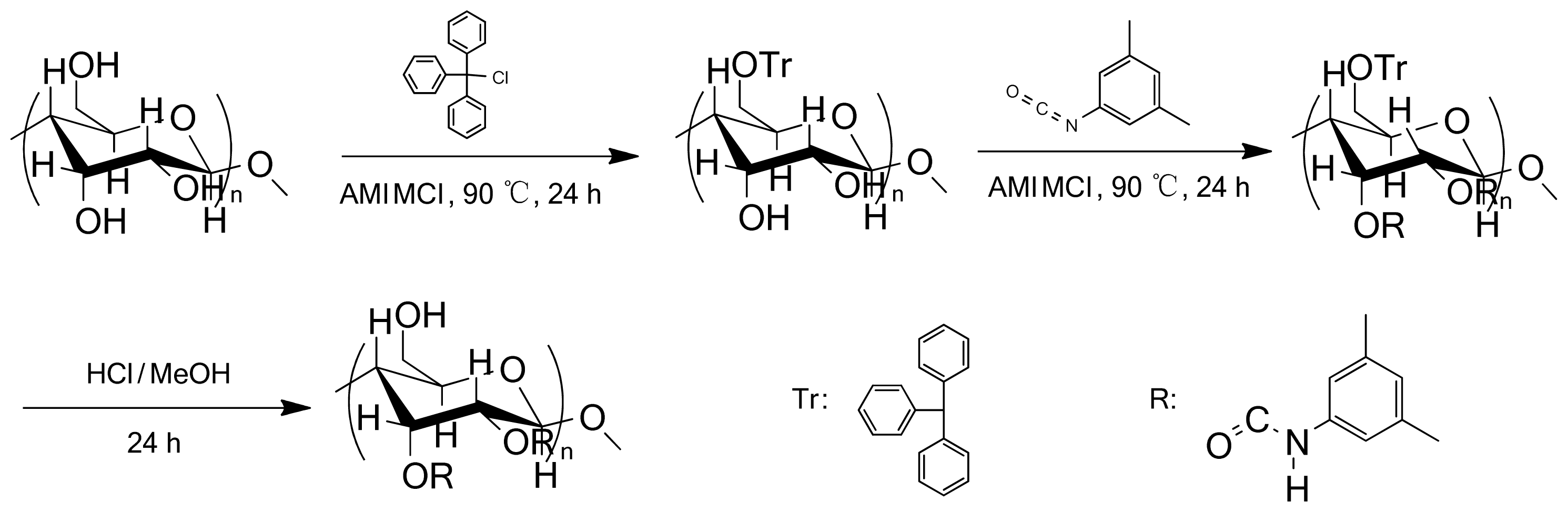
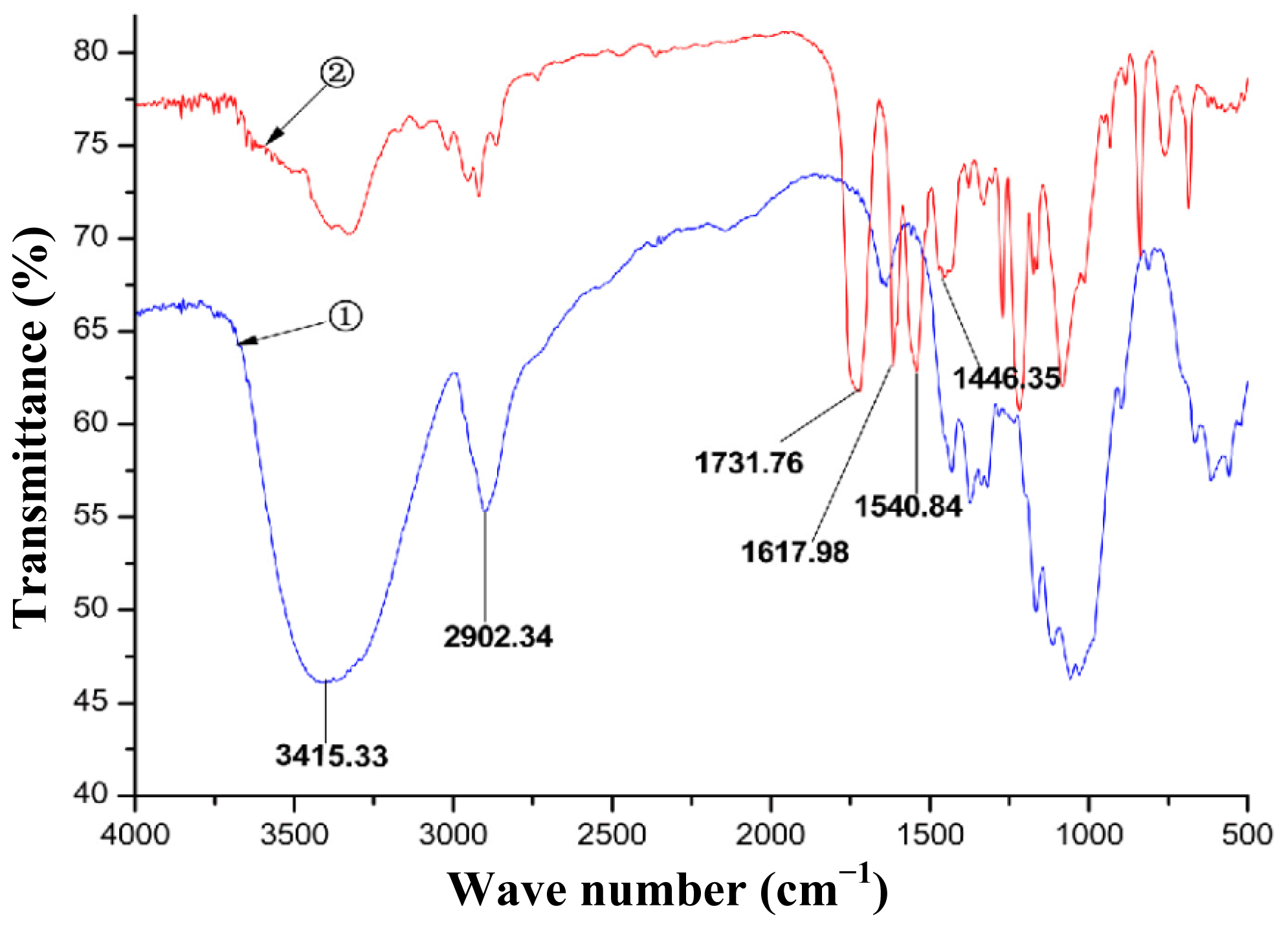
2.1. Synthesis and Characterization of CBDMPC
2.2. Enantioseparation of Three Fungicides Using the Prepared Chiral Column
2.3. Recycling and Characterization of the AMIMCl
3. Experimental Section
3.1. Chemicals and Equipments
3.2. Synthesis of Cellulose-2,3-bis(3,5-dimethylphenylcarbamate)
3.3. Preparation of Bonded-Type Chiral Stationary Phase
3.4. Packing
4. Conclusions
Supplementary Information
ijms-15-06161-s001.pdfAcknowledgments
Conflicts of Interest
- Author ContributionsR.L. and Y.Z. designed the experiment under the supervision of L.B.; R.L., Y.Z. and Y.Z. performed the experiments and analyzed the data with the advice of M.H. and Y.Z.; R.L. and Y.Z. discussed the results; L.B., Y.Z., J.C. and M.H. gave conceptual advice; R.L. wrote the manuscript; all authors commented on the manuscript at all stages.
References
- Armstrong, D.W. The evolution of chiral stationary phases for liquid chromatography. J. Chin. Chem. Soc 1998, 45, 581–590. [Google Scholar]
- Lämmerhofer, M.; Svec, F.; Fréchet, J.M. Separation of enantiomers by capillary electrochromatography. J. Trends Anal. Chem 2000, 19, 676–698. [Google Scholar]
- Chankvetadze, B.; Yamamoto, C.; Okamoto, Y. Enantioseparation of selected chiral sulfoxides using polysaccharide-type chiral stationary phases and polar organic, polar aqueous–organic and normal-phase eluents. J. Chromatogr. A 2001, 922, 127–137. [Google Scholar]
- Li, W.H.; Liu, C.H.; Tan, G.G.; Zhang, X.R.; Zhu, Z.Y.; Chai, Y.F. Molecular modeling study of chiral separation and recognition mechanism of β-adrenergic antagonists by capillary electrophoresis. Int. J. Mol. Sci 2012, 13, 710–725. [Google Scholar]
- Okamoto, Y.; Kaida, Y. Resolution by high-performance liquid chromatography using polysaccharide carbamates and benzoates as chiral stationary phases. J. Chromatogr. A 1994, 666, 403–419. [Google Scholar]
- Franco, P.; Minguillón, C.; Oliveros, L. Solvent versatility of bonded cellulose-derived chiral stationary phases for high-performance liquid chromatography and its consequences in column loadability. J. Chromatogr. A 1998, 793, 239–247. [Google Scholar]
- Oliveros, L.; Senso, A.; Minguillon, C. Benzoates of cellulose bonded on silica gel: Chiral discrimination ability as high-performance liquid chromatographic chiral stationary phases. Chirality 1997, 9, 145–149. [Google Scholar]
- Bai, L.Y.; Zhang, Y.P.; Deng, P.H.; Zhang, Y.J.; Chen, J. Enantioseparation of typical pesticides using cellulose carbamate stationary phases by capillary liquid chromatography. Asian J. Chem 2012, 24, 4917–4922. [Google Scholar]
- Zhang, J.M.; Wu, J.; Cao, Y.; Sang, S.M.; Zhang, J.; He, J.S. Synthesis of cellulose benzoates under homogeneous conditions in an ionic liquid. Cellulose 2009, 16, 299–308. [Google Scholar]
- Granström, M.; Olszewska, A.; Makela, V.; Heikkinen, S.; Kilpelainen, I. A new protection group strategy for cellulose in an ionic liquid: Simultaneous protection of two sites to yield 2,6-di-O-substituted mono-p-methoxytrityl cellulose. Tetrahedron Lett 2009, 50, 1744–1747. [Google Scholar]
- Xu, X.T.; Duan, W.G.; Huang, M.; Li, G.H. Synthesis of cellulose dehydroabietate in ionic liquid [bmim] Br. Carbohydr. Res 2011, 346, 2024–2027. [Google Scholar]
- Chankvetadze, B.; Ikai, T.; Yamamoto, C.; Okamoto, Y. High-performance liquid chromatographic enantioseparations on monolithic silica columns containing a covalently attached 3,5-dimethylphenylcarbamate derivative of cellulose. J. Chromatogr. A 2004, 1042, 55–60. [Google Scholar]
- Yashima, E.; Fukaya, H.; Okamoto, Y. 3,5-Dimethylphenylcarbamates of cellulose and amylase regioselectively bonded to silica gel as chiral stationary phases for high-performance liquid chromatography. J. Chromatogr. A 1994, 677, 11–19. [Google Scholar]
- Ahmed, A.Y.; Mangelings, D.; Heyden, Y.V. Chiral separations in normal-phase liquid chromatography: Enantioselectivity of recently commercialized polysaccharide-based selectors. Part II. Optimization of enantioselectivity. J. Pharm. Biomed. Anal 2011, 56, 521–537. [Google Scholar]
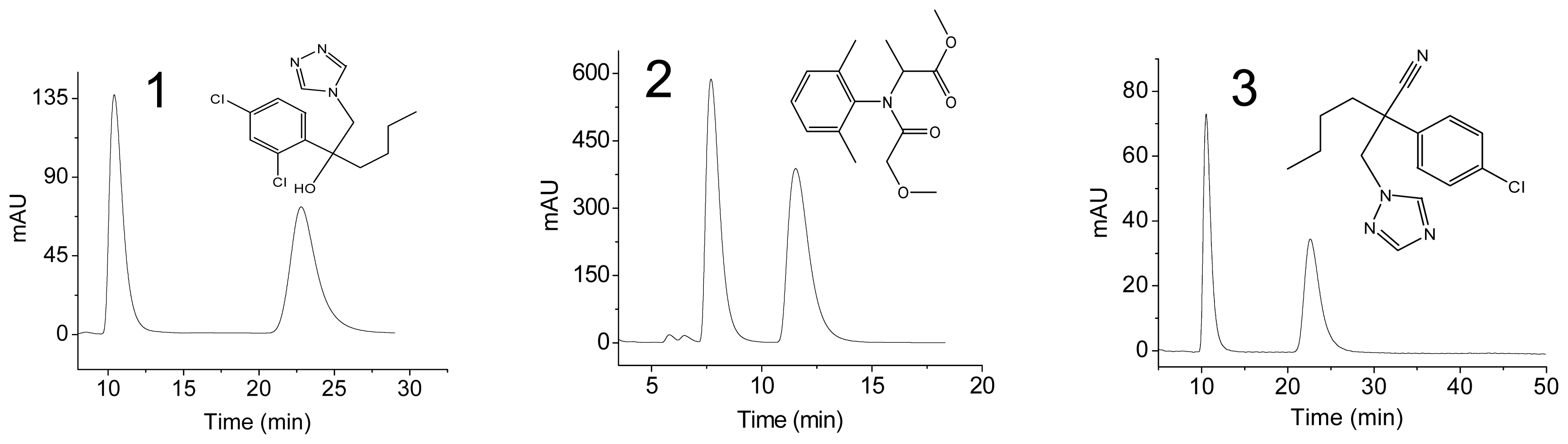
| Compound | n-Hexane/Isopropanol (v/v) | k′1 | k′2 | α | Rs |
|---|---|---|---|---|---|
| Hexaconazole | 70/30 | 2.81 | 7.35 | 2.62 | 5.23 |
| 75/25 | 3.68 | 9.70 | 2.64 | 5.50 | |
| 80/20 | 4.84 | 13.18 | 2.73 | 6.05 | |
| 85/15 | 7.52 | 21.14 | 2.81 | 9.30 | |
| 90/10 | 12.10 | 35.51 | 2.94 | 11.23 | |
| Metalaxyl | 75/25 | 1.92 | 3.37 | 1.76 | 2.64 |
| 80/20 | 2.58 | 4.73 | 1.83 | 3.62 | |
| 85/15 | 3.27 | 6.09 | 1.86 | 3.74 | |
| 90/10 | 4.84 | 9.45 | 1.95 | 3.89 | |
| 95/5 | 9.59 | 20.25 | 2.11 | 4.37 | |
| 98/2 | 22.02 | 49.12 | 2.23 | 11.74 | |
| Myclobutanil | 70/30 | 2.85 | 7.28 | 2.55 | 5.01 |
| 75/25 | 3.69 | 9.50 | 2.57 | 5.29 | |
| 80/20 | 5.29 | 13.97 | 2.64 | 6.08 | |
| 85/15 | 6.86 | 19.84 | 2.89 | 6.39 | |
| 90/10 | 12.01 | 34.89 | 2.91 | 7.09 | |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, R.; Zhang, Y.; Bai, L.; Huang, M.; Chen, J.; Zhang, Y. Synthesis of Cellulose-2,3-bis(3,5-dimethylphenylcarbamate) in an Ionic Liquid and Its Chiral Separation Efficiency as Stationary Phase. Int. J. Mol. Sci. 2014, 15, 6161-6168. https://doi.org/10.3390/ijms15046161
Liu R, Zhang Y, Bai L, Huang M, Chen J, Zhang Y. Synthesis of Cellulose-2,3-bis(3,5-dimethylphenylcarbamate) in an Ionic Liquid and Its Chiral Separation Efficiency as Stationary Phase. International Journal of Molecular Sciences. 2014; 15(4):6161-6168. https://doi.org/10.3390/ijms15046161
Chicago/Turabian StyleLiu, Runqiang, Yijun Zhang, Lianyang Bai, Mingxian Huang, Jun Chen, and Yuping Zhang. 2014. "Synthesis of Cellulose-2,3-bis(3,5-dimethylphenylcarbamate) in an Ionic Liquid and Its Chiral Separation Efficiency as Stationary Phase" International Journal of Molecular Sciences 15, no. 4: 6161-6168. https://doi.org/10.3390/ijms15046161





