Radioprotective and Antioxidant Effect of Resveratrol in Hippocampus by Activating Sirt1
Abstract
:1. Introduction
2. Results and Discussion
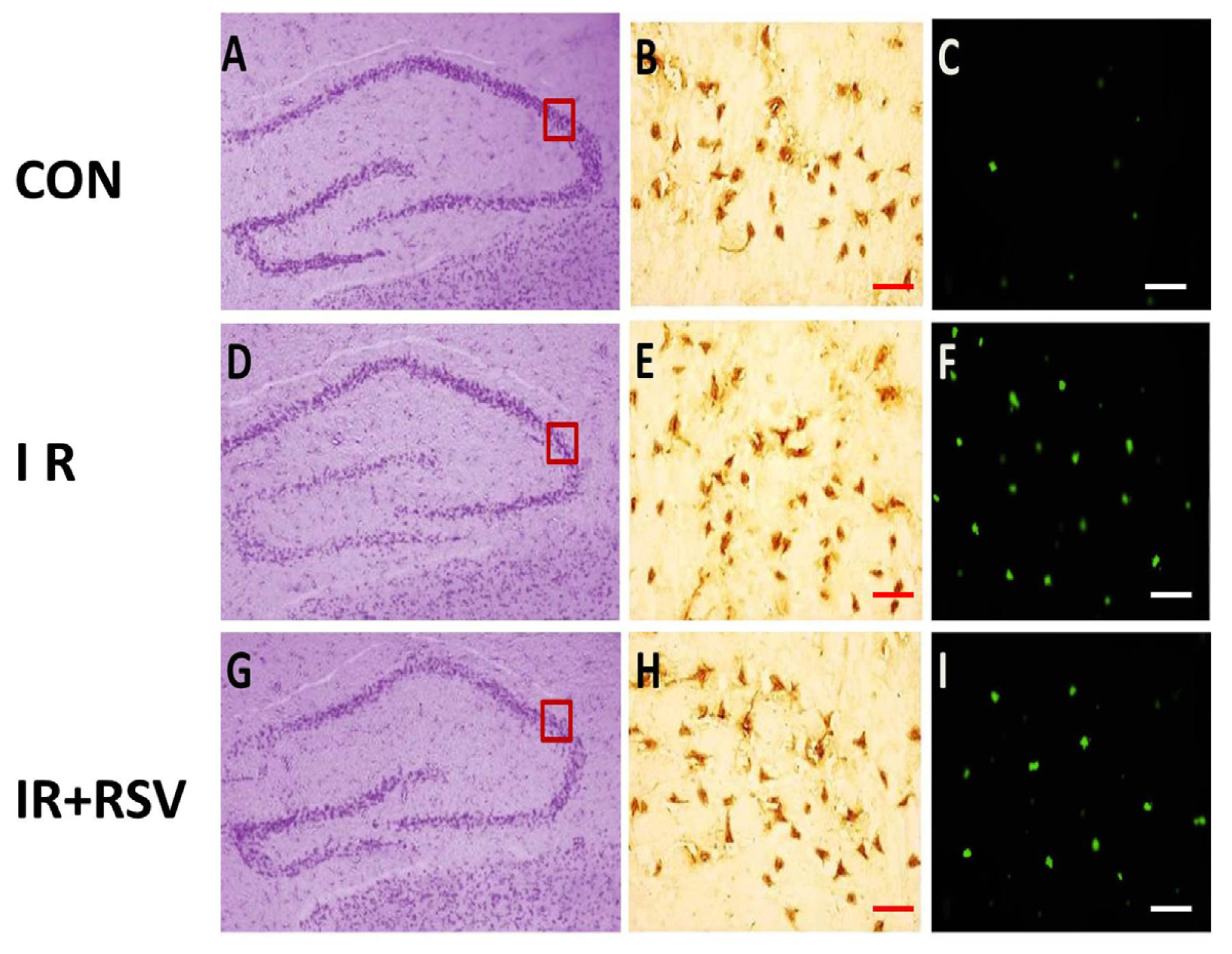
2.1. Immunohistology and TUNEL-Positive Cells within the Hippocampi
2.2. Neuroprotective Effects of Resveratrol
2.3. Sirt1 Expression by Western Blot and qRTPCR
2.4. Mean ROS Accumulation
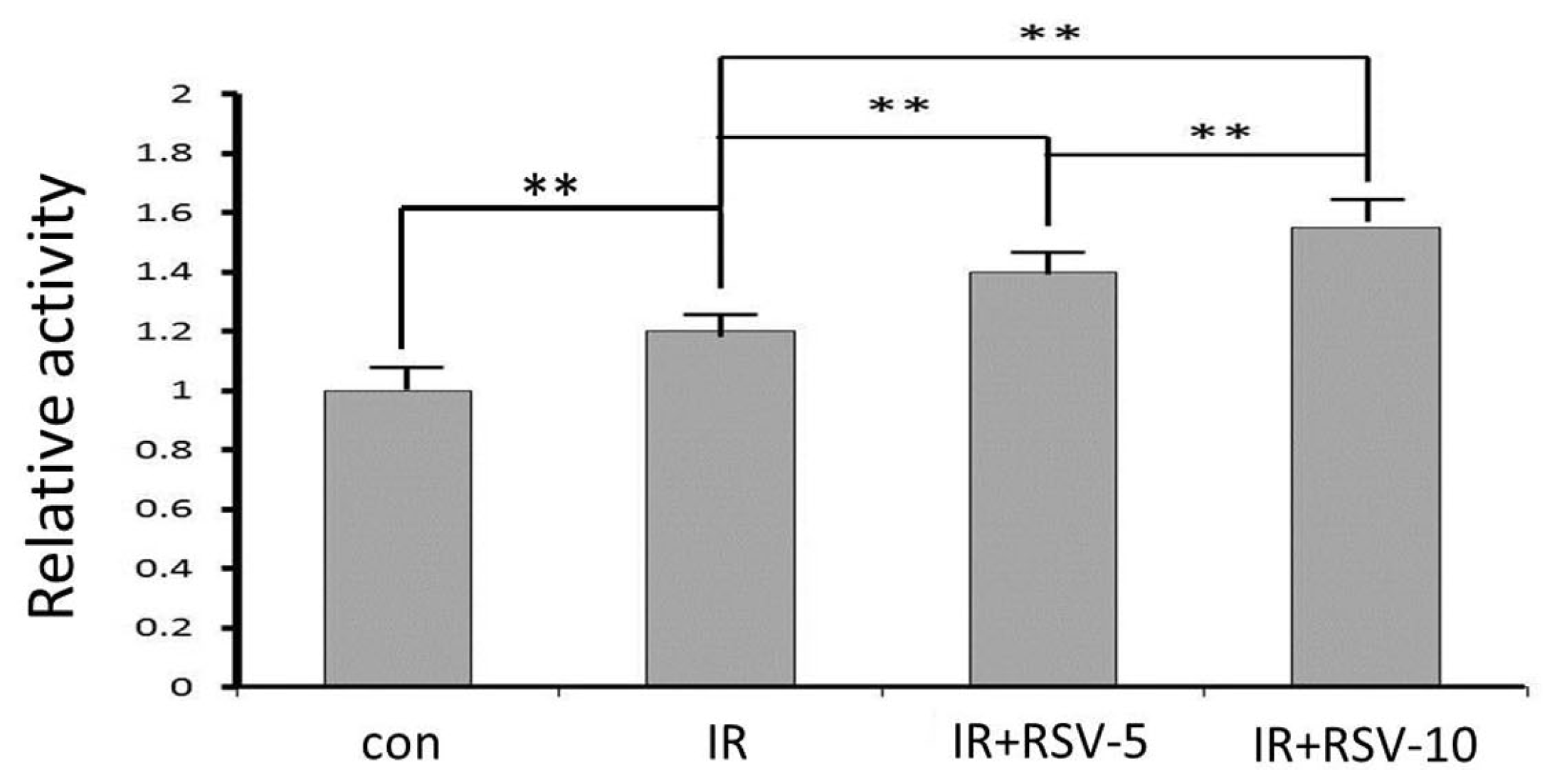
2.5. Sirt1 Activity after IR and Resveratrol Treatment
3. Experimental Section
3.1. Chemicals and Animals
3.2. Drug Treatments
3.3. Radiation Model
3.4. Terminal Deoxynucleotidyl Transferase dUTP Nick End-Labeling (TUNEL) Staining, Immunohistology and Cresyl Violet (CV) Staining
3.5. Western Blot Analysis
3.6. RNA Extraction, cDNA Synthesis, and Quantitative Real-Time PCR (qRT-PCR)
3.7. Analysis of Reactive Oxygen Species
3.8. Sirt1 Activity Assay
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Arora, R.; Gupta, D.; Chawla, R.; Sagar, R.; Sharma, A.; Kumar, R.; Prasad, J.; Singh, S.; Samanta, N.; Sharma, R.K. Radioprotection by plant products: Present status and future prospects. Phytother. Res 2005, 19, 1–22. [Google Scholar]
- Jagetia, G.C. Radioprotective potential of plants and herbs against the effects of ionizing radiation. J. Clin. Biochem. Nutr 2007, 40, 74–81. [Google Scholar]
- Maisin, J.R. Bacq and Alexander award lecture chemical radioprotection: Past, present and future prospects. Int. J. Radiat. Biol 1998, 73, 443–450. [Google Scholar]
- Nair, C.K.K.; Parida, D.K.; Nomura, T. Radioprotectorsin radiotherapy. J. Radiat. Res 2001, 42, 21–37. [Google Scholar]
- Zuo, L.; Motherwell, M.S. The impact of reactive oxygen species and genetic mitochondrial mutations in Parkinson’s disease. Gene 2013, 532, 18–23. [Google Scholar]
- Sochocka, M.; Koutsouraki, E.S.; Gasiorowski, K.; Leszek, J. Vascular oxidative stress and mitochondrial failure in the pathobiology of Alzheimer’s disease: A new approach to therapy. CNS Neurol. Disord. Drug Targets 2013, 12, 870–881. [Google Scholar]
- Hou, J.; Han, Z.P.; Jing, Y.Y.; Yang, X.; Zhang, S.S.; Sun, K.; Hao, C.; Meng, Y.; Yu, F.H.; Liu, X.Q.; et al. Autophagy prevents irradiation injury and maintains stemness through decreasing ROS generation in mesenchymal stem cells. Cell Death Dis 2013, 4, e844. [Google Scholar]
- Halliwell, B. Reactive oxygen species and the central nervous system. J. Neurochem 1992, 59, 1609–1623. [Google Scholar]
- Wong, C.S.; van der Kogel, A.J. Mechanisms of radiation injury to the central nervous system: Implications for neuroprotection. Mol. Interv 2004, 4, 273–284. [Google Scholar]
- Siegal, T.; Pfeffer, M.R.; Meltzer, A.; Shezen, E.L.; Nimrod, A.; Ezov, N.; Ovadia, H. Cellular and secretory mechanisms related to delayed radiation-induced microvessel dysfunction in the spinal cord of rats. Int. J. Radiat. Oncol. Biol. Phys 1996, 36, 649–659. [Google Scholar]
- Chong, Z.Z.; Shang, Y.C.; Wang, S.; Maiese, K. SIRT1: New avenues of discovery for disorders of oxidative stress. Expert Opin. Ther. Targets 2012, 16, 167–178. [Google Scholar]
- Nakata, R.; Takahashi, S.; Inoue, H. Recent advances in the study on resveratrol. Biol. Pharm. Bull 2012, 35, 273–279. [Google Scholar]
- Gruber, J.; Tang, S.Y.; Halliwell, B. Evidence for a trade-off between survival and fitness caused by resveratrol treatment of caenorhabditis elegans. Ann. N. Y. Acad. Sci 2007, 1100, 530–542. [Google Scholar]
- Pasinetti, G.M.; Wang, J.; Marambaud, P.; Ferruzzi, M.; Gregor, P.; Knable, L.A.; Ho, L. Neuroprotective and metabolic effects of resveratrol: Therapeutic implications for Huntington’s disease and other neurodegenerative disorders. Exp. Neurol 2011, 232, 1–6. [Google Scholar]
- Brasnyó, P.L.; Molnár, G.A.; Mohás, M.; Markó, L.; Laczy, B.; Cseh, J.; Mikolás, E.; Szijártó, I.A.; Mérei, A.; Halmai, R.; et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr 2011, 106, 383–389. [Google Scholar]
- Bradamante, S.; Barenghi, L.; Villa, A. Cardiovascular protective effects of resveratrol. Cardiovasc. Drug Rev 2004, 22, 169–188. [Google Scholar]
- Zhang, H.; Zhai, Z.; Wang, Y.; Zhang, J.; Wu, H.; Wang, Y.; Li, C.; Li, D.; Lu, L.; Wang, X.; et al. Resveratrol ameliorates ionizing irradiation-induced long-term hematopoietic stem cell injury in mice. Free Radic. Biol. Med 2013, 54, 40–50. [Google Scholar]
- Hasegawa, K.; Wakino, S.; Yoshioka, K.; Tatematsu, S.; Hara, Y.; Minakuchi, H.; Washida, N.; Tokuyama, H.; Hayashi, K.; Itoh, H. Sirt1 protects against oxidative stress-induced renal tubular cell apoptosis by the bidirectional regulation of catalase expression. Biochem. Biophys. Res. Commun 2008, 372, 51–56. [Google Scholar]
- Chong, Z.Z.; Lin, S.H.; Li, F.; Maiese, K. The Sirtuin inhibitor nicotinamide enhances neuronal cell survival during acute anoxic injury through Akt, Bad, PARP, and mitochondrial associated “antiapoptotic” pathways. Curr. Neurovasc. Res 2005, 2, 271–285. [Google Scholar]
- Chong, Z.Z.; Maiese, K. Enhanced tolerance against early and late apoptotic oxidative stress in mammalian neurons through nicotinamidase and Sirtuin mediated pathways. Curr. Neurovasc. Res 2008, 5, 159–170. [Google Scholar]
- Tanno, M.; Kuno, A.; Yano, T.; Miura, T.; Hisahara, S.; Ishikawa, S.; Shimamoto, K.; Horio, Y. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J. Biol. Chem 2010, 285, 8375–8382. [Google Scholar]
- Kume, S.; Haneda, M.; Kanasaki, K.; Sugimoto, T.; Araki, S.; Isono, M.; Isshiki, K.; Uzu, T.; Kashiwagi, A.; Koya, D. Silent information regulator 2 (SIRT1) attenuates oxidative stress-induced mesangial cell apoptosis via p53 deacetylation. Free Radic. Biol. Med 2006, 40, 2175–2182. [Google Scholar]
- Hou, J.; Wang, S.; Shang, Y.C.; Chong, Z.Z.; Maiese, K. Erythropoietin employs cell longevity pathways of SIRT1 to foster endothelial vascular integrity during oxidant stress. Curr. Neurovasc. Res 2011, 8, 220–235. [Google Scholar]
- Orallo, F. Trans-resveratrol: A magical elixir of eternal youth? Curr. Med. Chem 2008, 15, 1887–1898. [Google Scholar]
- Balestrazzi, A.; Bonadei, M.; Calvio, C.; Mattivi, F.; Carbonera, D. Leaf-associated bacteria from transgenic white poplar producing resveratrol-like compounds: Isolation, molecular characterization, and evaluation of oxidative stress tolerance. Can. J. Microbiol 2009, 55, 829–840. [Google Scholar]
- Vidavalur, R.; Otani, H.; Singal, P.K.; Maulik, N. Significance of wine and resveratrol in cardiovascular disease: French paradox revisited. Exp. Clin. Cardiol 2006, 11, 217–225. [Google Scholar]
- Fremont, L. Biological effects of resveratrol. Life Sci 2000, 66, 663–673. [Google Scholar]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov 2006, 5, 493–506. [Google Scholar]
- Robich, M.P.; Osipov, R.M.; Nezafat, R.; Feng, J.; Clements, R.T.; Bianchi, C.; Boodhwani, M.; Coady, M.A.; Laham, R.J.; Sellke, F.W. Resveratrol improves myocardial perfusion in a swine model of hypercholesterolemia and chronic myocardial ischemia. Circulation 2010, 122, 142–149. [Google Scholar]
- Sun, A.Y.; Wang, Q.; Simonyi, A.; Sun, G.Y. Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol. Neurobiol 2010, 41, 375–383. [Google Scholar]
- Chung, J.H.; Manganiello, V.; Dyck, J.R.B. Resveratrol as a calorie restriction mimetic: Therapeutic implications. Trends Cell Biol 2012, 22, 546–554. [Google Scholar]
- Ogawa, K.; Tsuruma, K.; Tanaka, J.; Kakino, M.; Kobayashi, S.; Shimazawa, M.; Hara, H. The protective effects of bilberry and lingonberry extracts against UV light-induced retinal photoreceptor cell damage in vitro. J. Agric. Food Chem. 2013, 61, 10345–10353. [Google Scholar]
- Simsek, Y.; Gurocak, S.; Turkoz, Y.; Akpolat, N.; Celik, O.; Ozer, A.; Yılmaz, E.; Turhan, U.; Ozyalin, F. Ameliorative effects of resveratrol on acute ovarian toxicityinduced by total body irradiation in young adult rats. J. Pediatr. Adolesc. Gynecol 2012, 25, 262–266. [Google Scholar]
- Mudò, G.; Mäkelä, J.; di Liberto, V.; Tselykh, T.V.; Olivieri, M.; Piepponen, P.; Eriksson, O.; Mälkiä, A.; Bonomo, A.; Kairisalo, M.; et al. Transgenic expression and activation of PGC-1α protect dopaminergic neurons in the MPTP mouse model of Parkinson’s disease. Cell. Mol. Life Sci 2012, 69, 1153–1165. [Google Scholar]
- Chung, S.; Yao, H.; Caito, S.; Hwang, J.W.; Arunachalam, G.; Rahman, I. Regulation of SIRT1 in cellular functions: Role of polyphenols. Arch. Biochem. Biophys 2010, 501, 79–90. [Google Scholar]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar]
- Kang, Y.; Jung, W.Y.; Lee, H.; Lee, E.; Kim, A.; Kim, B.H. Expression of SIRT1 and DBC1 in Gastric Adenocarcinoma. Korean J. Pathol 2012, 46, 523–531. [Google Scholar]
- Matsushita, T.; Sasaki, H.; Takayama, K.; Ishida, K.; Matsumoto, T.; Kubo, S.; Matsuzaki, T.; Nishida, K.; Kurosaka, M.; Kuroda, R. The overexpression of SIRT1 inhibited osteoarthritic gene expression changes induced by interleukin-1β in human chondrocytes. J. Orthop. Res 2013, 31, 531–537. [Google Scholar]
- Chen, F.; Xu, C.; Du, L.; Wang, Y.; Cao, J.; Fu, Y.; Guo, Y.; Liu, Q.; Fan, F. Tat-SmacN7 induces radiosensitization in cancer cells through the activation of caspases and induction of apoptosis. Int. J. Oncol 2013, 42, 985–992. [Google Scholar]
- Fu, Y.; Wang, Y.; Du, L.; Xu, C.; Cao, J.; Fan, T.; Liu, J.; Su, X.; Fan, S.; Liu, Q.; et al. Resveratrol inhibits ionising irradiation-induced inflammation in MSCs by activating SIRT1 and limiting NLRP-3 inflammasome activation. Int. J. Mol. Sci 2013, 14, 14105–14118. [Google Scholar]
- LeBel, C.P.; Ali, S.F.; McKee, M.; Bondy, S.C. Organometal-induced increases in oxygen reactive species: The potential of 2′,7′-dichlorofluorescin diacetate as an index of neurotoxic damage. Toxicol. Appl. Pharmacol 1990, 104, 17–24. [Google Scholar]
- McGahon, B.M.; Murray, C.A.; Horrobin, D.F.; Lynch, M.A. Age-related changes in oxidative mechanisms and LTP are reversed by dietary manipulation. Neurobiol. Aging 1999, 20, 643–653. [Google Scholar]


© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, J.; Feng, L.; Xing, Y.; Wang, Y.; Du, L.; Xu, C.; Cao, J.; Wang, Q.; Fan, S.; Liu, Q.; et al. Radioprotective and Antioxidant Effect of Resveratrol in Hippocampus by Activating Sirt1. Int. J. Mol. Sci. 2014, 15, 5928-5939. https://doi.org/10.3390/ijms15045928
Li J, Feng L, Xing Y, Wang Y, Du L, Xu C, Cao J, Wang Q, Fan S, Liu Q, et al. Radioprotective and Antioxidant Effect of Resveratrol in Hippocampus by Activating Sirt1. International Journal of Molecular Sciences. 2014; 15(4):5928-5939. https://doi.org/10.3390/ijms15045928
Chicago/Turabian StyleLi, Jianguo, Li Feng, Yonghua Xing, Yan Wang, Liqing Du, Chang Xu, Jia Cao, Qin Wang, Saijun Fan, Qiang Liu, and et al. 2014. "Radioprotective and Antioxidant Effect of Resveratrol in Hippocampus by Activating Sirt1" International Journal of Molecular Sciences 15, no. 4: 5928-5939. https://doi.org/10.3390/ijms15045928



