Multilineage Potential Research of Bovine Amniotic Fluid Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Results and Discussion
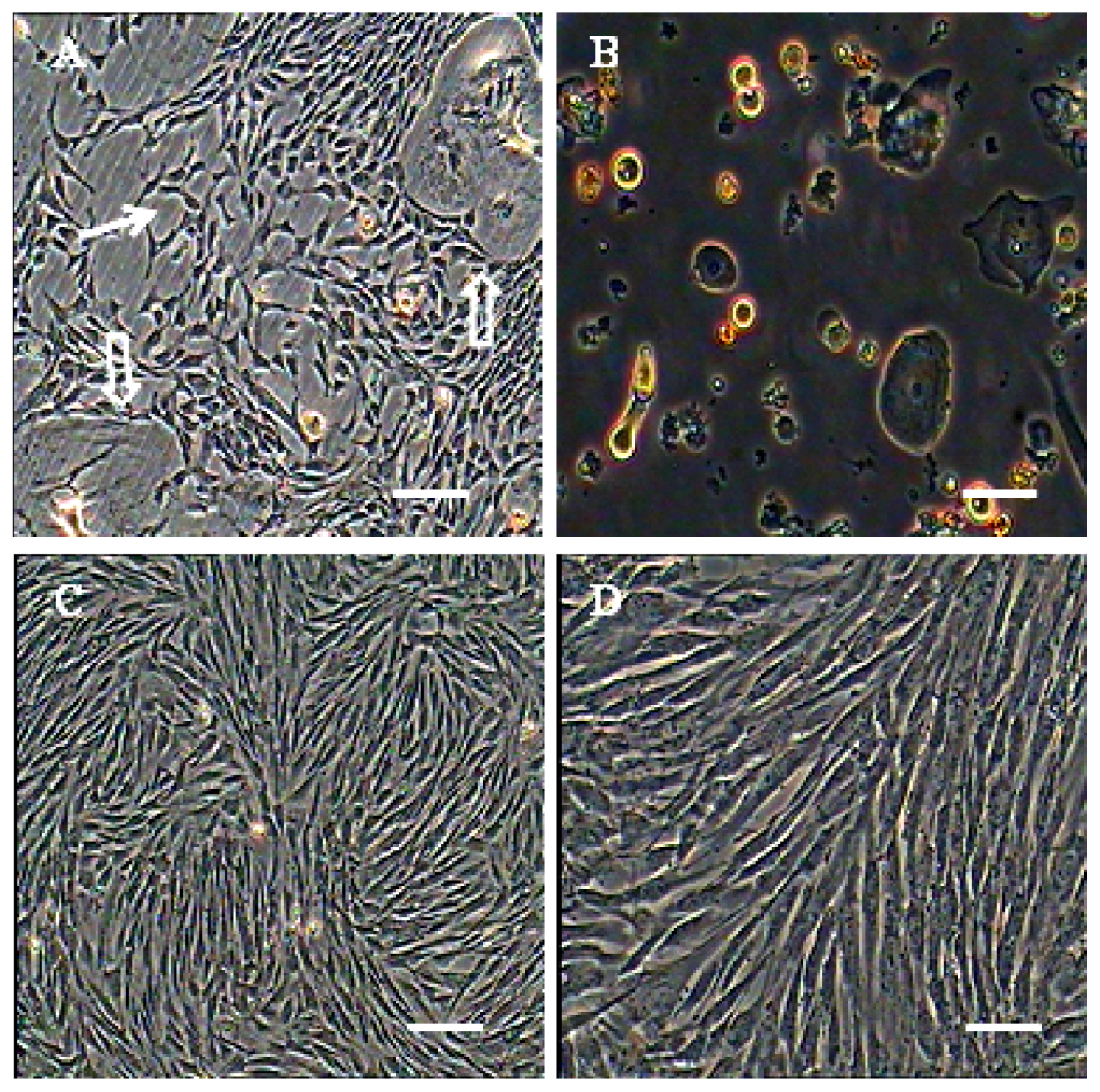
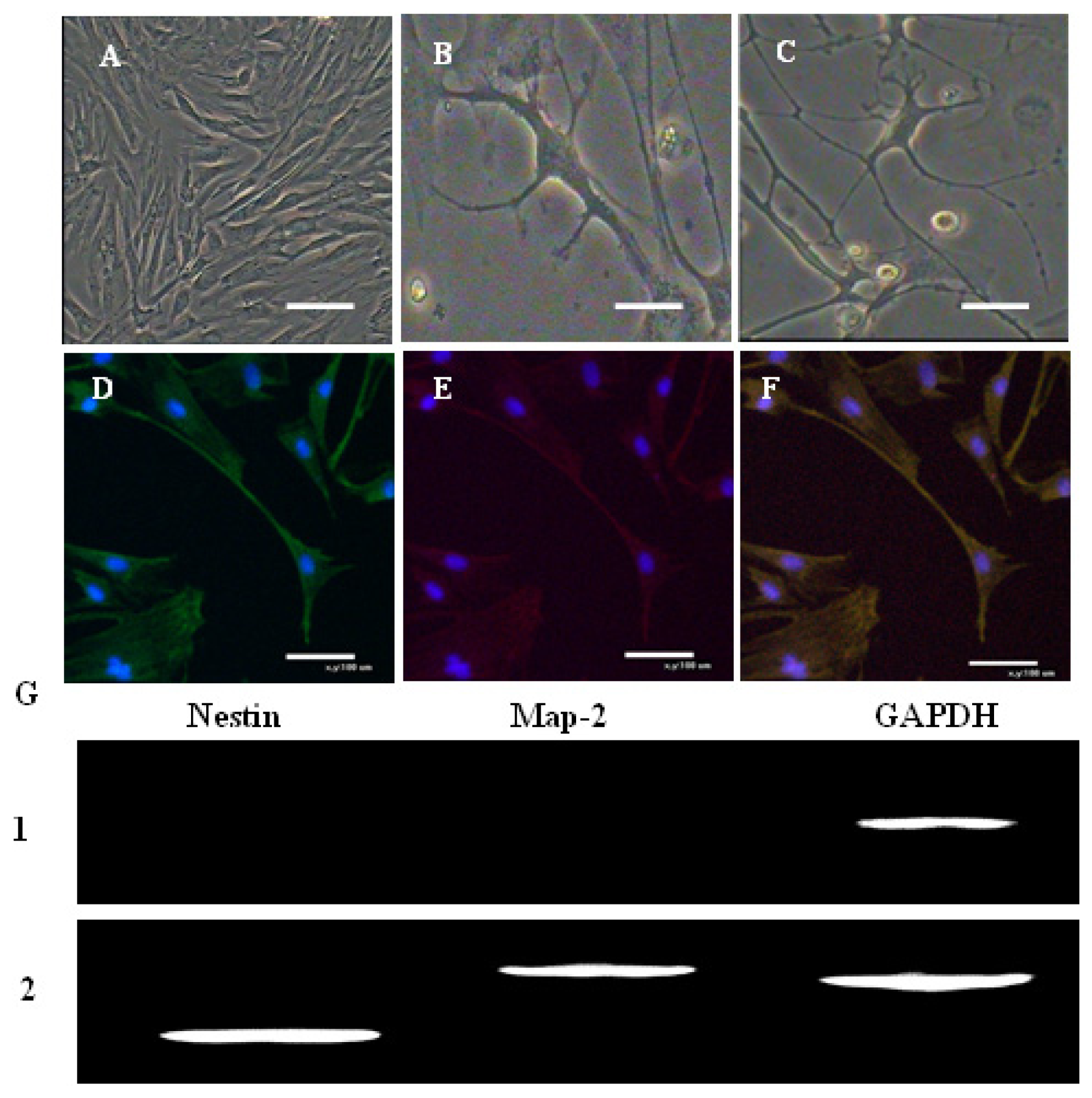
2.1. Morphological Observation of AFMSCs
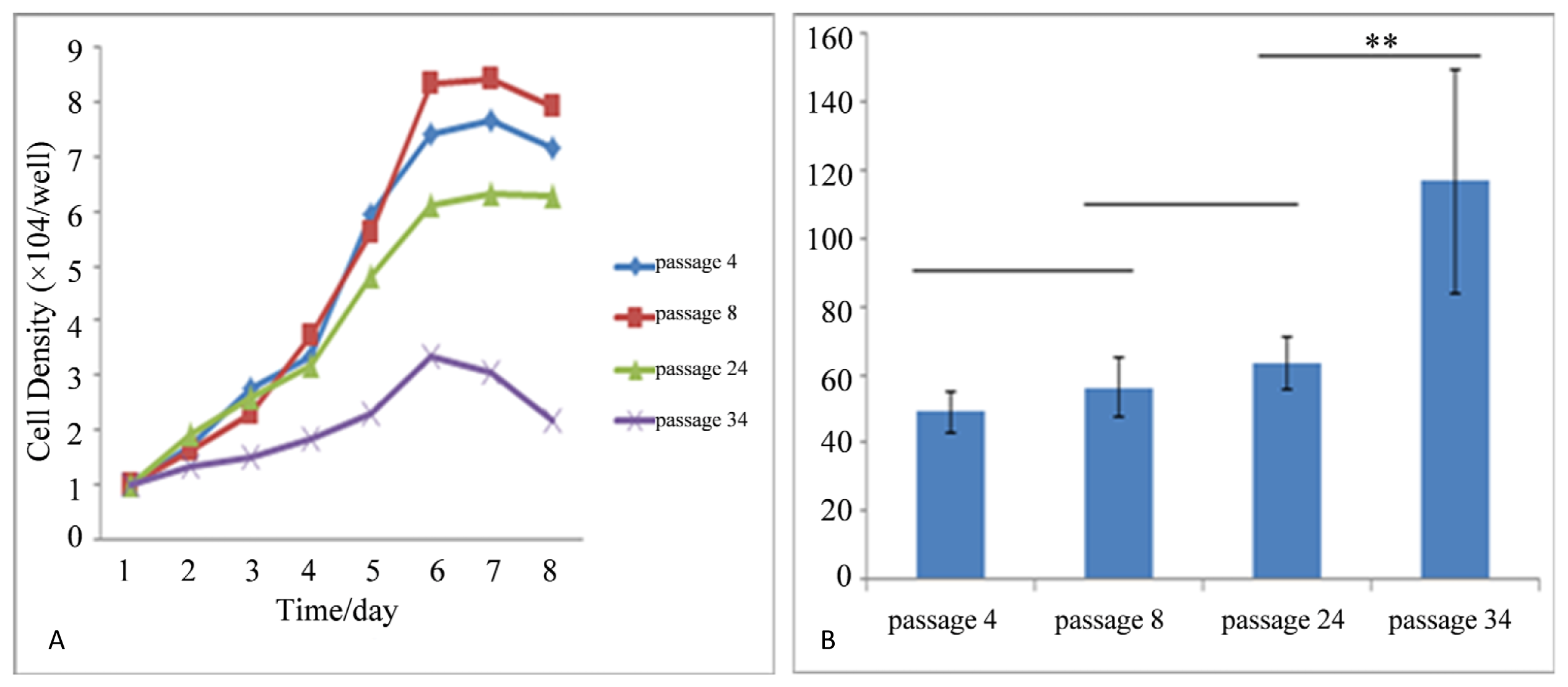
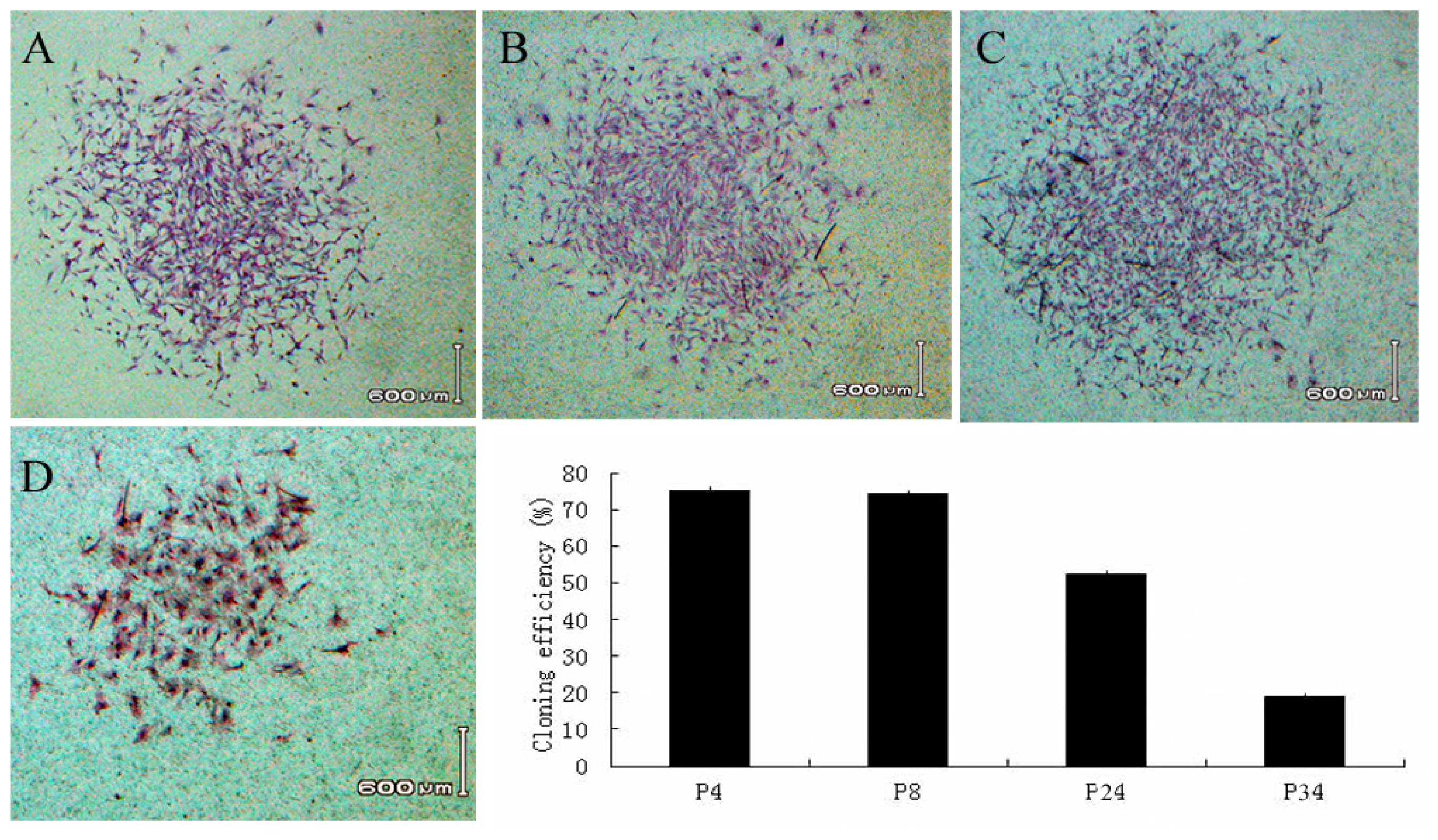
2.2. Self Renewal and Proliferation Assays
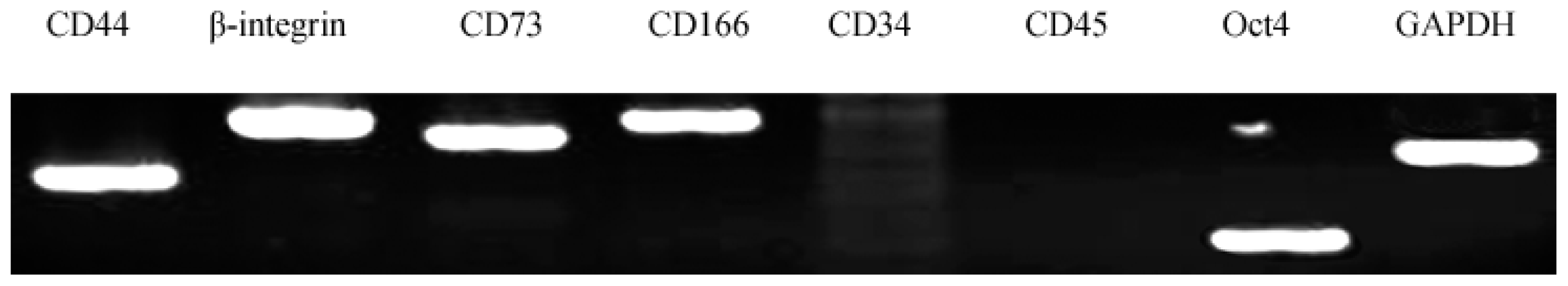
2.3. Characterization of AFMSCs
2.4. In Vitro Differentiation of AFMSCs
2.5. Discussion
3. Experimental Section
3.1. Ethics Statement
3.2. Materials
3.3. Isolation and Culture of the MSCs from Amniotic Fluid
3.4. Self-Renewal and Proliferation Assays
3.5. Characterization of AFMSCs
3.5.1. Immunofluorescence
3.5.2. RT-PCR Assays
3.6. Multiple Differentiations Potential
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kaviani, A.; Perry, T.E.; Dzakovic, A.; Jennings, R.W.; Ziegler, M.M.; Fauza, D.O. The amniotic fluid as a source of cells for fetal tissue engineering. J. Pediatr. Surg. 2001, 36, 1662–1665. [Google Scholar]
- Brace, R.A.; Wolf, E.J. Normal amniotic fluid volume changes throughout pregnancy. Am. J. Obstet. Gynecol. 1989, 161, 382–388. [Google Scholar]
- Reinisch, A.; Bartmann, C.; Rohde, E.; Schallmoser, K.; Bjelic-Radisic, V.; Lanzer, G.; Linkesch, W.; Strunk, D. Humanized system to propagate cord blood-derived multipotent mesenchymal stromal cells for clinical application. Regen. Med. 2007, 2, 371–382. [Google Scholar]
- Bosnakovski, D.; Mizuno, M.; Kim, G.; Ishiguro, T.; Okumura, M.; Iwanaga, T.; Kadosawa, T.; Fujinaga, T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells in pellet cultural system. Exp. Hematol. 2004, 32, 502–509. [Google Scholar]
- Lu, T.; Huang, Y.; Wang, H.; Ma, Y.; Guan, W. Multi-lineage potential research of Bone Marrow-Derived Stromal Cells (BMSCs) from cattle. Appl. Biochem. Biotechnol. 2013. [Google Scholar] [CrossRef]
- Phermthai, T.; Odglun, Y.; Julavijitphong, S.; Titapant, V.; Chuenwattana, P.; Vantanasiri, C.; Pattanapanyasat, K. A novel method to derive amniotic fluid stem cells for therapeutic purposes. BMC Cell Biol. 2010. [Google Scholar] [CrossRef]
- Fauza, D. Amniotic fluid and placental stem cells. Best Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 877–891. [Google Scholar]
- Steigman, S.A.; Fauza, D.O. Isolation of mesenchymal stem cells from amniotic fluid and placenta. Curr. Protoc. Stem Cell Biol. 2007. [Google Scholar] [CrossRef]
- Lin, A.W.; Barradas, M.; Stone, J.C.; van Aelst, L.; Serrano, M.; Lowe, S.W. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 1998, 12, 3008–3019. [Google Scholar]
- Roubelakis, M.G.; Trohatou, O.; Anagnou, N.P. Amniotic fluid and amniotic membrane stem cells: Marker discovery. Stem Cells Int. 2012. [Google Scholar] [CrossRef]
- Prusa, A.R.; Hengstschlager, M. Amniotic fluid cells and human stem cell research: A new connection. Med. Sci. Monit. 2002, 8, RA253–RA257. [Google Scholar]
- Bernhardt, A.; Lode, A.; Peters, F.; Gelinsky, M. Optimization of culture conditions for osteogenically-induced mesenchymal stem cells in beta-tricalcium phosphate ceramics with large interconnected channels. J. Tissue Eng. Regen. Med. 2011, 5, 444–453. [Google Scholar]
- Tropel, P.; Platet, N.; Platel, J.C.; Noel, D.; Albrieux, M.; Benabid, A.L.; Berger, F. Functional neuronal differentiation of bone marrow-derived mesenchymal stem cells. Stem Cells 2006, 24, 2868–2876. [Google Scholar]
- McBurney, M.W.; Reuhl, K.R.; Ally, A.I.; Nasipuri, S.; Bell, J.C.; Craig, J. Differentiation and maturation of embryonal carcinoma-derived neurons in cell culture. J. Neurosci. 1988, 8, 1063–1073. [Google Scholar]
- Liu, Y.; Liu, L.; Ma, X.; Yin, Y.; Tang, B.; Li, Z. Characteristics and neural-like differentiation of mesenchymal stem cells derived from foetal porcine bone marrow. Biosc. Rep. 2013. [Google Scholar] [CrossRef]
- Gao, Y.; Pu, Y.; Wang, D.; Hou, L.; Guan, W.; Ma, Y. Isolation and biological characterization of chicken amnion epithelial cells. Eur. J. Histochem. 2012. [Google Scholar] [CrossRef]
- Owens, M.A.; Horten, B.C.; Da Silva, M.M. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin. Breast Cancer 2004, 5, 63–69. [Google Scholar]
- Tsai, M.S.; Lee, J.L.; Chang, Y.J.; Hwang, S.M. Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum. Reprod. 2004, 19, 1450–1456. [Google Scholar]
- Scintu, F.; Reali, C.; Pillai, R.; Badiali, M.; Sanna, M.A.; Argiolu, F.; Ristaldi, M.S.; Sogos, V. Differentiation of human bone marrow stem cells into cells with a neural phenotype: Diverse effects of two specific treatments. BMC Neurosci. 2006. [Google Scholar] [CrossRef]



| Gene | Primers | Products (bp) |
|---|---|---|
| CD44 | F: 5′-CGGAACATAGGGTTTGAGA-3′ R: 5′-GGTTGATGTCTTCTGGGTTA-3′ | 301 |
| β-integrin | F: 5′-GAAACTTGGTGGCATCGT-3′ R: 5′-CTCAGTGAAGCCCAGAGG-3′ | 493 |
| CD73 | F: 5′-CAATGGCACGATTACCTG-3′ R: 5′-GACCTTCAACTGCTGGATA-3′ | 428 |
| CD34 | F: 5′-CCTCATCAGCTTTGCGACTT-3′ R: 5′-CCAGGAGCAAGGAGCACA-3′ | 314 |
| CD45 | F: 5′-CTACCCAACCTTCTACTCAA-3′ R: 5′-TTCACATCCAGGAGGTTC-3′ | 221 |
| CD166 | F: 5′-TATCAGGATGCTGGAAAC-3′ R: 5′-TAGCCAATAGACGACACC-3′ | 498 |
| OCT4 | F: 5′-CTCTTTGGAAAGGTGTTCAG-3′ R: 5′-GTCTCTGCCTTGCATATCTC-3′ | 155 |
| Coll I | F: 5′-AGAAGCATGTCTGGGTAGGAG-3′ R: 5′-AGGATAGGCAGGCGAGATR-3′ | 358 |
| OPN | F: 5′-CCAATGAAAGCCCTGAG-3′ R: 5′-TCCTCCTCTGTGGCATC-3′ | 310 |
| PPAR-γ | F: 5′-ATCCCTGTTCCGTGCTG-3′ R: 5′-GGGATACAGGCTCCACTT-3′ | 356 |
| LPL | F: 5′-GAACTGGATGGCGGATG-3′ R: 5′-CTGGATTCCGATACTTCGACCT-3′ | 256 |
| Nestin | F: 5′-TGAAACACCTGTGCCAACCT-3′ R: 5′-GCTTCAGCCCACATGACTTC-3′ | 204 |
| MAP2 | F: 5′-GAGAACGGAATCAACGGAGAAC-3′ R: 5′-CCAAACAGAGTGGGAGGTGC-3′ | 467 |
| GAPDH | F: 5′-GGCAAGTTCAACGGCACAGTCA-3′ R: 5′-TAAGTCCCTCCACGATGCCAAAG-3′ | 364 |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gao, Y.; Zhu, Z.; Zhao, Y.; Hua, J.; Ma, Y.; Guan, W. Multilineage Potential Research of Bovine Amniotic Fluid Mesenchymal Stem Cells. Int. J. Mol. Sci. 2014, 15, 3698-3710. https://doi.org/10.3390/ijms15033698
Gao Y, Zhu Z, Zhao Y, Hua J, Ma Y, Guan W. Multilineage Potential Research of Bovine Amniotic Fluid Mesenchymal Stem Cells. International Journal of Molecular Sciences. 2014; 15(3):3698-3710. https://doi.org/10.3390/ijms15033698
Chicago/Turabian StyleGao, Yuhua, Zhiqiang Zhu, Yuhua Zhao, Jinlian Hua, Yuehui Ma, and Weijun Guan. 2014. "Multilineage Potential Research of Bovine Amniotic Fluid Mesenchymal Stem Cells" International Journal of Molecular Sciences 15, no. 3: 3698-3710. https://doi.org/10.3390/ijms15033698




