Purification and Characterization of Aporphine Alkaloids from Leaves of Nelumbo nucifera Gaertn and Their Effects on Glucose Consumption in 3T3-L1 Adipocytes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Selection of Suitable Two-Phase Solvent System for HSCCC
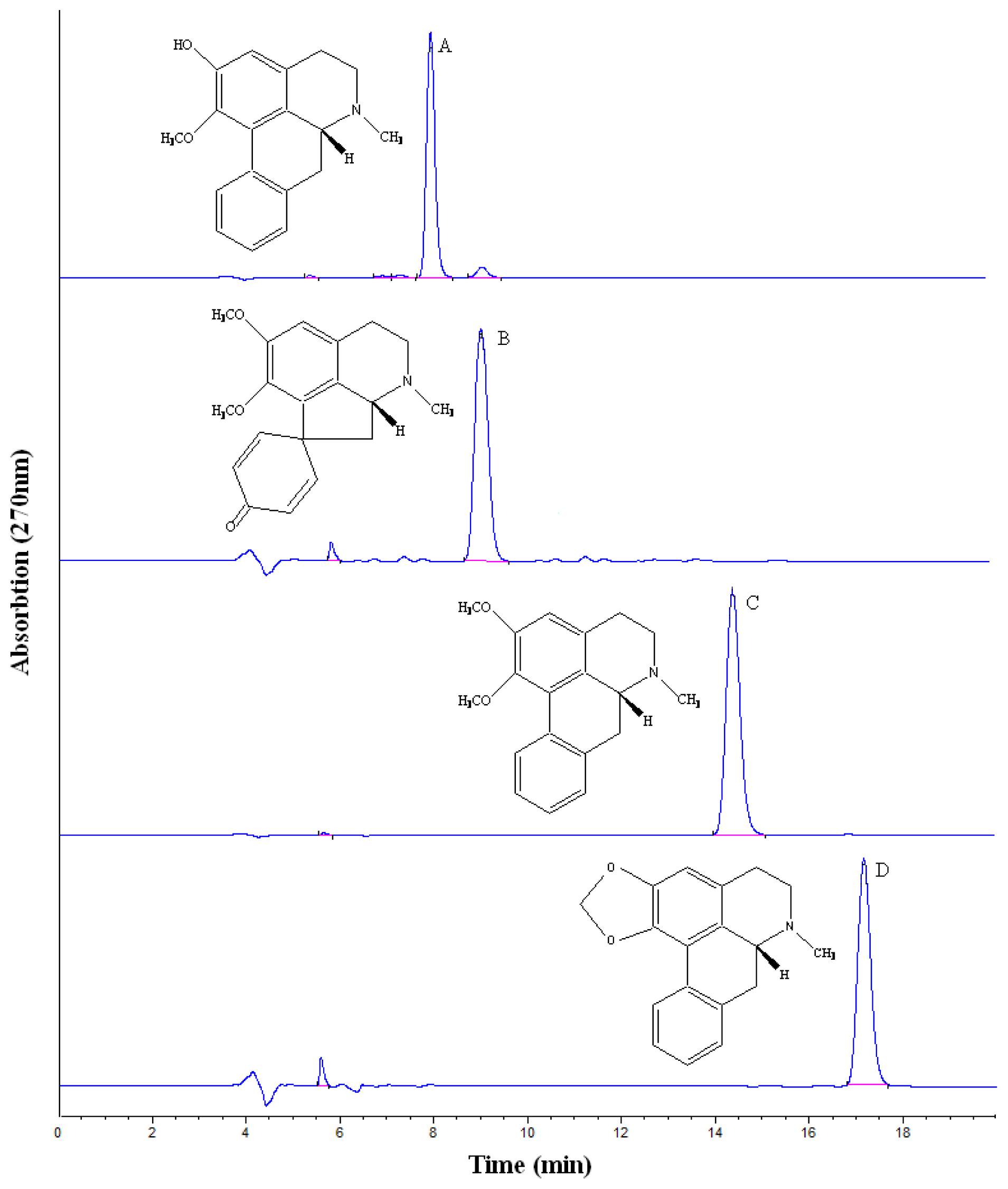
2.2. HSCCC Purification and HPLC Identification
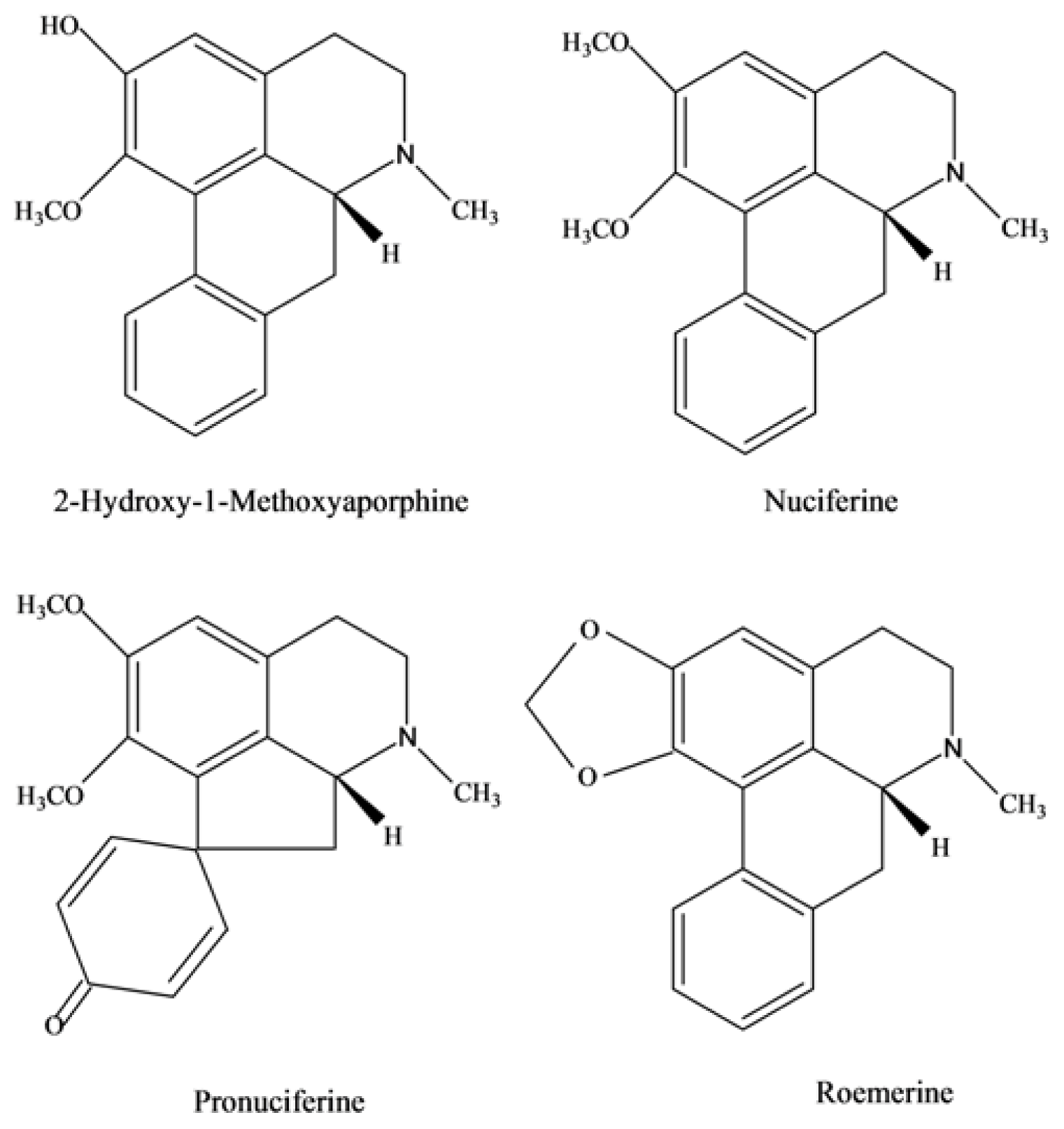
2.3. Chemical Structure Identification
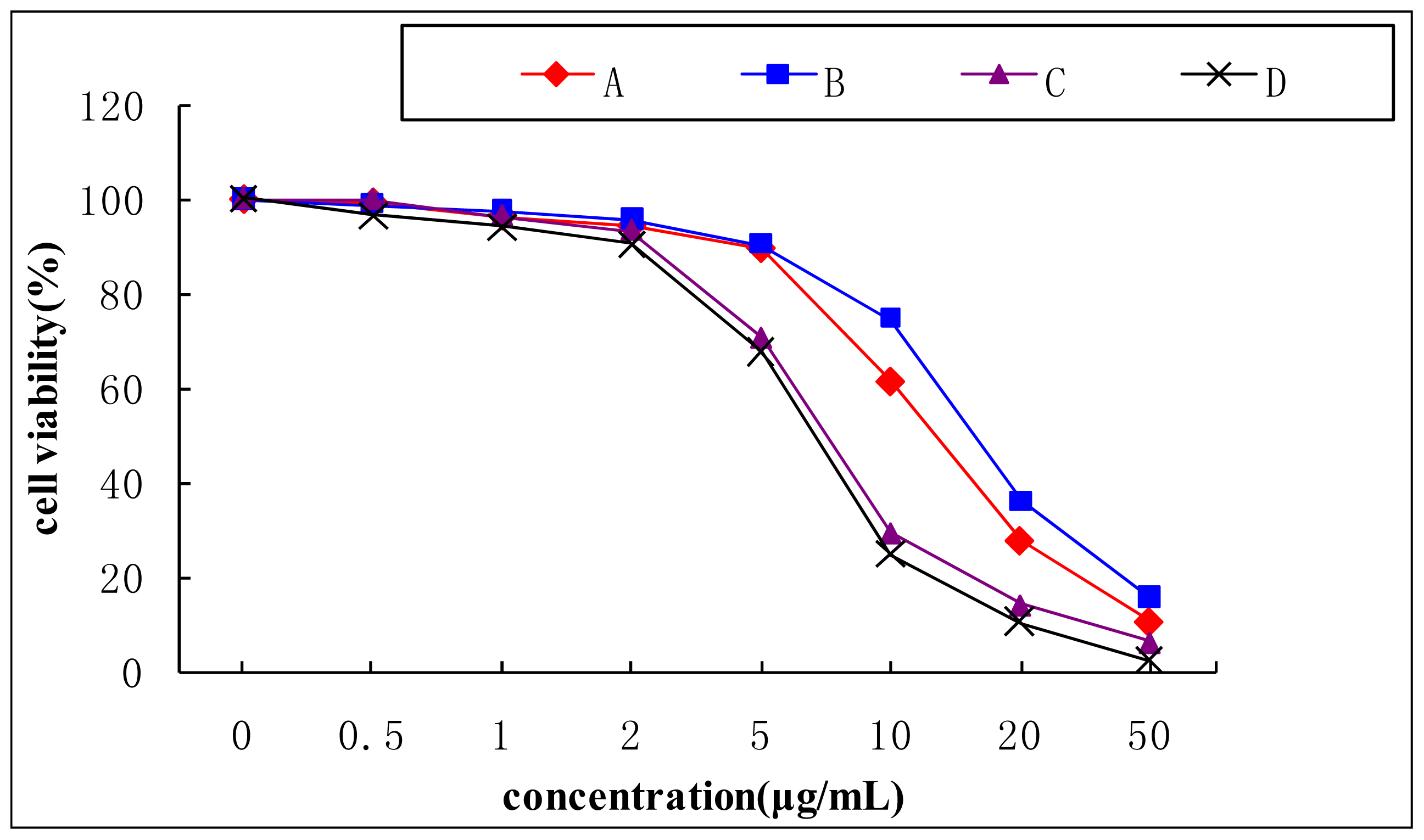
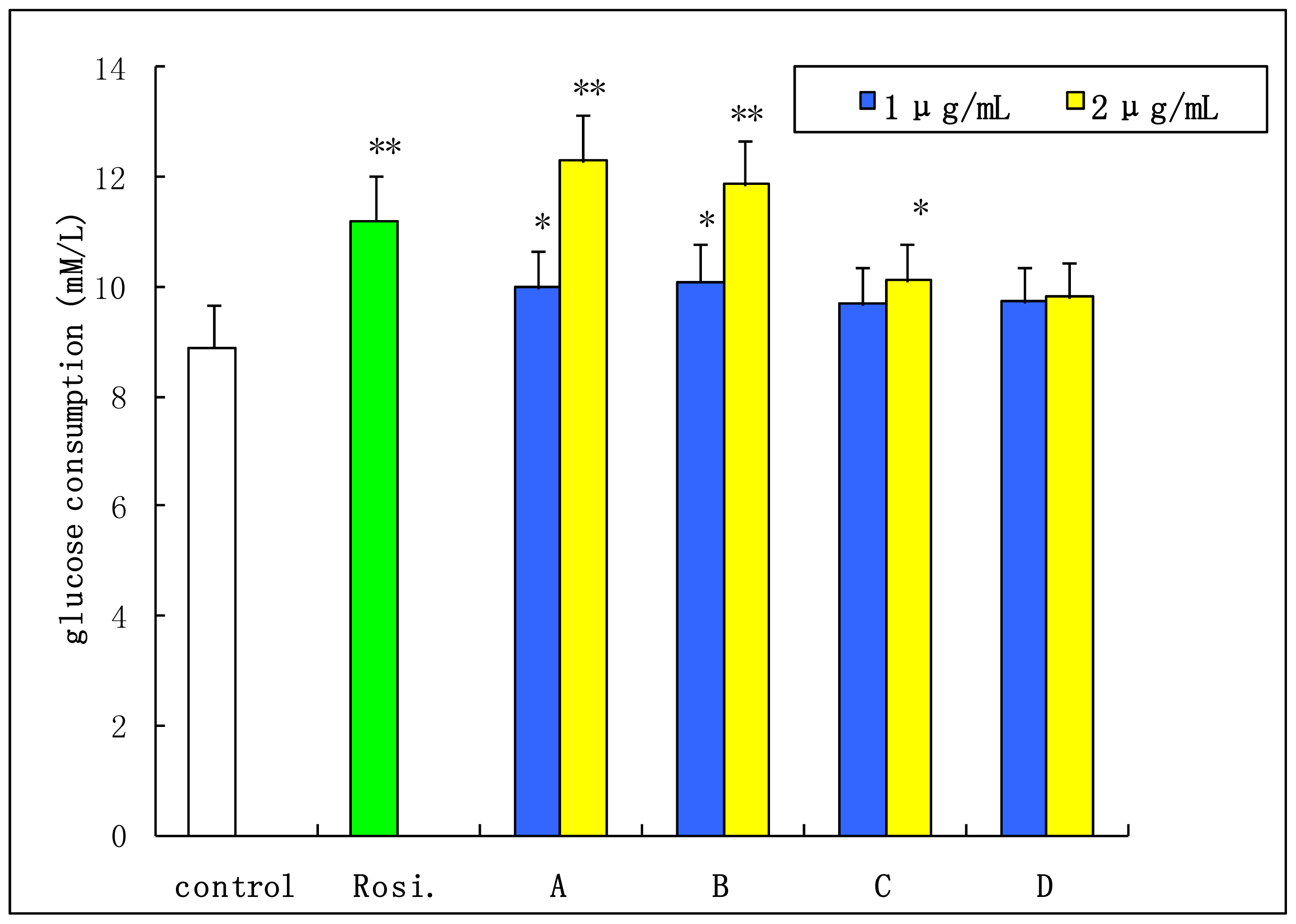
2.4. Insulin-Stimulated Glucose Consumption of 3T3-L1 Cell
3. Experimental Section
3.1. Chemical and Reagents
3.2. Apparatus
3.3. Preparation of Crude Extract from the Leaves of N. nucifera
3.4. Selection of Two-Phase Solvent Systems and Sample Solution for HSCCC
3.5. HSCCC Separation Procedure
3.6. HPLC Analysis and Identification of HSCCC Fractions
3.7. 3T3-L1 Cell Culture
3.8. Cytotoxicity Study of Aporphine Alkaloids
3.9. Insulin-Stimulated Glucose Consumption Study
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Wu, S.H.; Sun, C.R.; Cao, X.J.; Zhou, H.; Zhang, H.; Pan, Y.J. Preparative counter-current chromatography isolation of liensinine and its analogues from embryo of the seed of Nelumbo nucifera Gaertn using upright coil planet centrifuge with four multilayer coils connected in series. J. Chromatogr. A 2004, 1041, 153–162. [Google Scholar]
- China Pharmacopoeia Committee , Pharmacopoeia of the Peoples’ Republic of China; China Chemical Industry Press: Beijing, China, 2010.
- Nakamura, S.; Nakashima, S.; Tanabe, G.; Oda, Y.; Yokota, N.; Fujimoto, K.; Matsumoto, T.; Sakuma, R.; Ohta, T.; Ogawa, K.; et al. Alkaloid constituents from flower buds and leaves of sacred lotus (Nelumbo nucifera Nymphaeaceae) with melanogenesis inhibitory activity in B16 melanoma cells. Bioorg. Med. Chem 2013, 21, 779–789. [Google Scholar]
- Xiao, G.Q.; Lu, X.Y.; Tian, Y.; Yi, K.; Zhou, X.M. The research advance of alkaloids from lotus leaf. Chem. Bioen 2006, 23, 1–2. [Google Scholar]
- Luo, X.; Chen, B.; Liu, J.; Yao, S. Simultaneous analysis of N-nornuciferine O-nornuciferine nuciferine and roemerine in leaves of Nelumbo nucifera Gaertn by high-performance liquid chromatography-photodiode array detection-electrospray mass spectrometry. Anal. Chim. Acta 2005, 538, 129–133. [Google Scholar]
- Wang, L.L.; Liu, B.; Shi, R.B. Study on chemical constituents of folium nelumbinis. Nat. Prod. Res. Dev 2009, 21, 416–419. [Google Scholar]
- Yang, Z.D.; Zhang, X.; Du, J.; Ma, Z.J.; Guo, F.; Li, S.; Yao, X.J. An aporphine alkaloid from Nelumbo nucifera as an acetylcholinesterase inhibitor and the primary investigation for structure-activity correlations. Nat. Prod. Res 2012, 26, 387–392. [Google Scholar]
- Kashiwada, Y.; Aoshima, A.; Ikeshiro, Y.; Chen, Y.P.; Furukawa, H.; Itoigawa, M.; Fujioka, T.; Mihashi, K.; Cosentino, L.M.; Morris-Natschke, S.L.; et al. Anti-HIV benzylisoquinoline alkaloids and flavonoids from the leaves of Nelumbo nucifera and structure-activity correlations with related alkaloids. Bioorg. Med. Chem 2005, 13, 443–448. [Google Scholar]
- Lacour, B.; Molgaard, P.; Yi, Z. Traditional Chinese medicine in treatment of heperlipidemia. J. Ethnopharmacol 1995, 46, 125–129. [Google Scholar]
- Chen, K.S.; Ko, F.N.; Teng, C.M.; Wu, Y.C. Antiplatelet and vasorelaxing actions of some benzylisoquinoline and phenanthrene alkaloids. J. Nat. Prod 1996, 59, 531–534. [Google Scholar]
- Morales, M.A.; Bustamante, S.E.; Brito, G.; Pazd, D.; Cassels, B.K. Cardiovascular effect of plant secondary metabolites norarmepavine coclaurine and norcoclaurine. Phytother. Res 1998, 12, 103–109. [Google Scholar]
- Cho, E.J.; Yokozawa, T.; Rhyu, D.Y.; Kim, S.C.; Shibahara, N.; Park, J.C. Study on the inhibitory effects of Korean medicinal plant and their main compounds on the 11-diphenyl-2-picrylhydrazy radical. Phytomedicine 2003, 10, 544–551. [Google Scholar]
- Agnihotri, V.K.; Elsohly, H.N.; Khan, S.I.; Jacob, M.R.; Joshi, V.C.; Smillie, T.; Khan, I.A.; Walker, L.A. Constituents of Nelumbo nucifera leaves and their antimalarial and antifungal activity. Phytochem. Lett 2008, 1, 89–93. [Google Scholar]
- Ohkoshi, E.; Miyazaki, H.; Shindo, K.; Watanabe, H.; Yoshida, A.; Yajima, H. Constituents from the leaves of Nelumbo nucifera stimulate lipolysis in the white adipose tissue of mice. Planta Med 2007, 73, 1255–1259. [Google Scholar]
- Ono, Y.; Hattori, E.; Fukaya, Y.; Imai, S.; Ohizumi, Y. Anti-obesity effect of Nelumbo nucifera leaves extract in mice and rats. J. Ethnopharmacol 2006, 106, 238–244. [Google Scholar]
- Nguyen, K.H.; Ta, T.N.; Pham, T.H.; Nguyen, Q.T.; Pham, H.D.; Mishra, S.; Nyomba, B.L. Nuciferine stimulates insulin secretion from beta cells-an in vitro comparison with glibenclamide. J. Ethnopharmacol 2012, 142, 488–495. [Google Scholar]
- Do, T.C.; Nguyen, T.D.; Tran, H.; Stuppner, H.; Ganzera, M. Analysis of alkaloids in Lotus (Nelumbo nucifera Gaertn) leaves by non-aqueous capillary electrophoresis using ultraviolet and mass spectrometric detection. J. Chromatogr. A 2013, 1302, 174–180. [Google Scholar]
- Ahn, J.H.; Kim, E.S.; Lee, Ch.; Kim, S.; Cho, S.H.; Hwang, B.Y.; Lee, M.K. Chemical constituents from Nelumbo nucifera leaves and their anti-obesity effects. Bioorg. Med. Chem. Lett 2013, 23, 3604–3608. [Google Scholar]
- Chen, J.; Ma, X.; Gao, K.; Wang, Y.; Zhao, H.; Wu, H.; Wang, J.; Xie, H.; OuYang, Y.; Luo, L.; et al. The active ingredients of Jiang-Zhi-Ning: Study of the Nelumbo nucifera alkaloids and their main bioactive metabolites. Molecules 2012, 17, 9855–9867. [Google Scholar]
- Ito, Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J. Chromatogr. A 2005, 1065, 145–168. [Google Scholar]
- Fang, L.; Liu, Y.; Yang, B.; Wang, X.; Huang, L. Separation of alkaloids from herbs using high-speed counter-current chromatography. J. Sep. Sci 2011, 34, 2545–2558. [Google Scholar]
- Zheng, Z.J.; Wang, M.L.; Wang, D.J.; Duan, W.J.; Wang, X.; Zheng, C.C. Preparative separation of alkaloids from Nelumbo nucifera leaves by conventional and Ph-zone-refining counter-current chromatography. J. Chromatogr. B 2010, 878, 1647–1651. [Google Scholar]
- Inoue, K.; Nomura, C.; Ito, S.; Nagatsu, A.; Hino, T.; Oka, H. Purification of curcumin demethoxycurcumin and bisdemethoxycurcumin by high-speed countercurrent chromatography. J. Agric. Food Chem 2008, 56, 9328–9336. [Google Scholar]
- Li, Z.C.; Zuo, C.X.; Yang, S.J.; Zhong, Y.; Ding, X.B. Study on chemical constituents of folium nelumbinis. Chin. Tradit. Herb. Drugs 1996, 27, 50–52. [Google Scholar]
- Wu, H.; Liu, B.; Wang, W.; Shi, R.B. Studies on the chemical reference substance 2-hydroxy-1-methoxyaporphine of folium nelumbinis. Chin. J. Pharm. Anal 2010, 30, 1650–1653. [Google Scholar]
- Xu, M.E.; Xiao, S.Z.; Sun, Y.H.; Ou-yang, Y.; Guan, C.; Zheng, X.X. A preadipocyte differentiation assay as a method for screening potential anti-type II diabetes drugs from herbal extracts. Planta Med 2006, 72, 14–19. [Google Scholar]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar]
- Jang, Y.Y.; Song, J.H.; Shin, Y.K.; Han, E.S.; Lee, C.S. Protective effect of boldine on oxidative mitochondrial damage in streptozotocin-induced diabetic rats. Pharmacol. Res 2000, 42, 361–371. [Google Scholar]
- Yu, B.; Cook, C.; Santanam, N. The aporphine alkaloid boldine induces adiponectin expression and regulation in 3T3-L1 cells. J. Med. Food 2009, 12, 1074–1083. [Google Scholar]
- Lau, Y.S.; Tian, X.Y.; Mustafa, M.R.; Murugan, D.; Liu, J.; Zhang, Y.; Lau, C.W.; Huang, Y. Boldine improves endothelial function in diabetic db/db mice through inhibition of angiotensin II-mediated BMP4-oxidative stress cascade. Br. J. Pharmacol 2013. [Google Scholar] [CrossRef]
- Wu, X.; Motoshima, H.; Mahadev, K.; Stalker, T.J.; Scalia, R.; Goldstein, B.J. Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes 2003, 52, 1355–1363. [Google Scholar]
- Ma, C.J.; Li, G.; Zhang, J.; Zheng, Q.S.; Fan, X.; Wang, Z.H. An efficient combination of supercritical fluid extraction and high-speed counter-current chromatography to extract and purify homoisoflavonoids from Ophiopogon japonicas (Thunb) Ker-Gawler. J. Sep. Sci 2009, 32, 1949–1956. [Google Scholar]


| Category | HSCCC | Traditional Methods |
|---|---|---|
| Partition method | liquid-liquid partition | solid-liquid partition |
| Separation time | time-saving | tedious and time-consuming |
| Separation process | one step | multiple steps |
| Dosage of organic solvent | smaller volume | larger volume |
| Sample adsorbed loss | smaller | larger |
| Selection range of solvent systems | wider | limited |
| Sample condition | crude sample | multiple steps pre-treatment sample |
| Two-Phase Solvents | Partition Coefficient (K) | ||||
|---|---|---|---|---|---|
| Ratio (v/v) | A | B | C | D | |
| chloroform/methanol/water | 10/0/10 | 0 | 0 | 0 | 0.01 |
| 10/1/9 | 0 | 0 | 0.01 | 0.09 | |
| 10/3/7 | 0.02 | 0.05 | 0.10 | 0.19 | |
| 10/5/5 | 0.07 | 0.14 | 0.25 | 0.38 | |
| 10/6/4 | 0.04 | 0.17 | 0.21 | 0.29 | |
| 10/7/3 | 0.02 | 0.15 | 0.18 | 0.20 | |
| ethyl acetate/methanol/water | 10/1/9 | 10.46 | 9.53 | 16.93 | 22.50 |
| 10/4/6 | 3.38 | 4.08 | 7.08 | 15.67 | |
| 10/6/4 | 2.05 | 3.91 | 4.74 | 8.21 | |
| 10/8/3 | 1.71 | 3.01 | 3.89 | 7.08 | |
| n-hexane/ethyl acetate/methanol/water | 5/5/5/5 | 1.01 | 1.19 | 1.60 | 2.85 |
| 5/3/3/5 | 0.94 | 1.41 | 2.02 | 3.90 | |
| 5/2/2/5 | 0.80 | 1.88 | 2.91 | 4.32 | |
| 5/1/1/5 | 1.28 | 2.69 | 3.76 | 6.54 | |
| n-hexane/ethyl acetate/methanol/acetonitrile/water | 5/3/3/0.5/5 | 0.89 | 1.22 | 1.74 | 3.16 |
| 5/3/3/1.0/5 | 0.77 | 1.03 | 1.59 | 2.44 | |
| 5/3/3/1.5/5 | 0.69 | 0.89 | 1.42 | 2.17 | |
| 5/3/3/2.0/5 | 0.56 | 0.77 | 1.25 | 1.94 | |
| 5/3/3/2.5/5 | 0.51 | 0.76 | 1.20 | 1.87 | |
| 5/3/3/3.0/5 | 0.48 | 0.66 | 1.22 | 1.79 | |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ma, C.; Wang, J.; Chu, H.; Zhang, X.; Wang, Z.; Wang, H.; Li, G. Purification and Characterization of Aporphine Alkaloids from Leaves of Nelumbo nucifera Gaertn and Their Effects on Glucose Consumption in 3T3-L1 Adipocytes. Int. J. Mol. Sci. 2014, 15, 3481-3494. https://doi.org/10.3390/ijms15033481
Ma C, Wang J, Chu H, Zhang X, Wang Z, Wang H, Li G. Purification and Characterization of Aporphine Alkaloids from Leaves of Nelumbo nucifera Gaertn and Their Effects on Glucose Consumption in 3T3-L1 Adipocytes. International Journal of Molecular Sciences. 2014; 15(3):3481-3494. https://doi.org/10.3390/ijms15033481
Chicago/Turabian StyleMa, Chengjun, Jinjun Wang, Hongmei Chu, Xiaoxiao Zhang, Zhenhua Wang, Honglun Wang, and Gang Li. 2014. "Purification and Characterization of Aporphine Alkaloids from Leaves of Nelumbo nucifera Gaertn and Their Effects on Glucose Consumption in 3T3-L1 Adipocytes" International Journal of Molecular Sciences 15, no. 3: 3481-3494. https://doi.org/10.3390/ijms15033481
APA StyleMa, C., Wang, J., Chu, H., Zhang, X., Wang, Z., Wang, H., & Li, G. (2014). Purification and Characterization of Aporphine Alkaloids from Leaves of Nelumbo nucifera Gaertn and Their Effects on Glucose Consumption in 3T3-L1 Adipocytes. International Journal of Molecular Sciences, 15(3), 3481-3494. https://doi.org/10.3390/ijms15033481




