HuR and TIA1/TIAL1 Are Involved in Regulation of Alternative Splicing of SIRT1 Pre-mRNA
Abstract
:1. Introduction
2. Results and Discussion
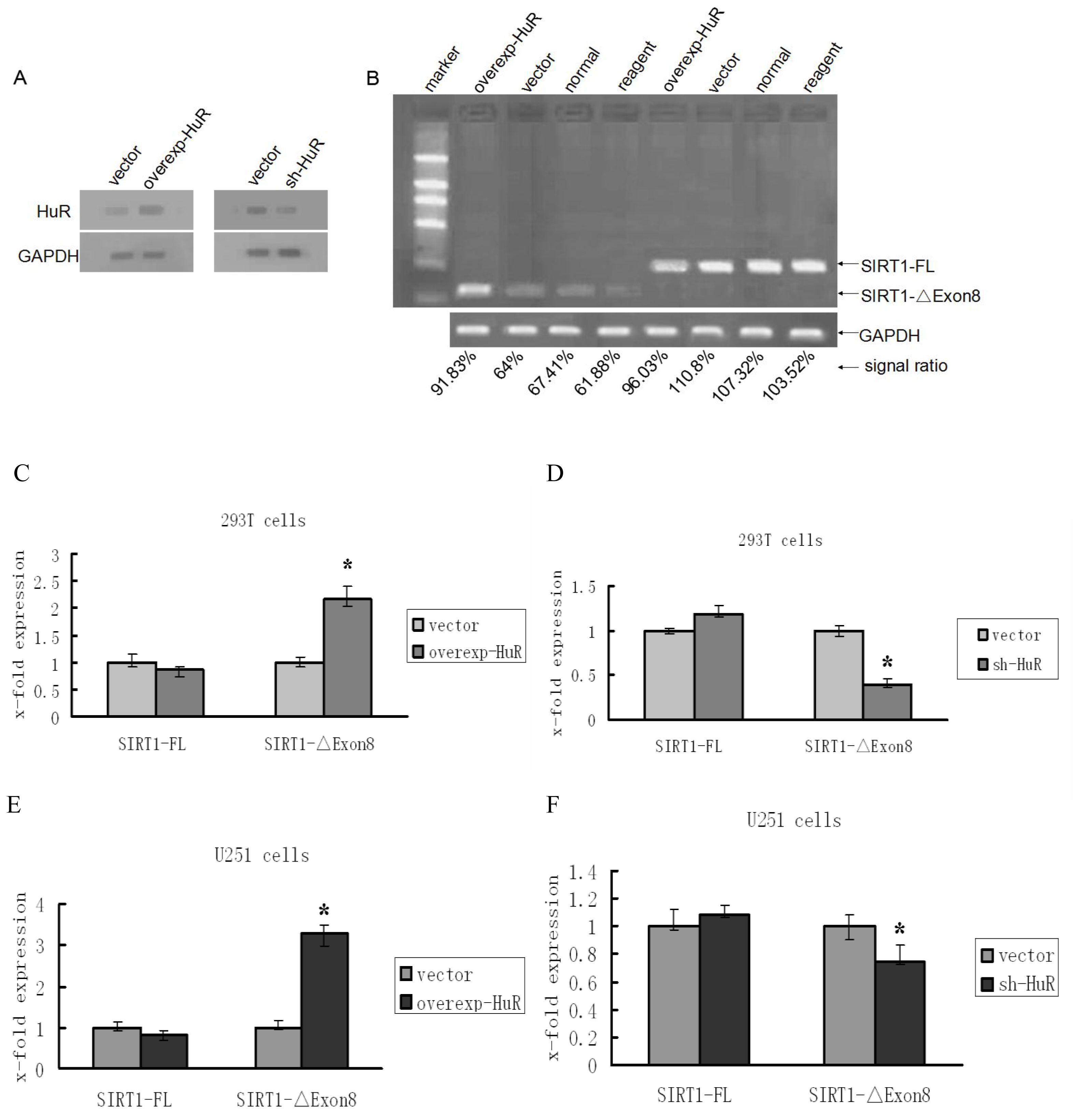
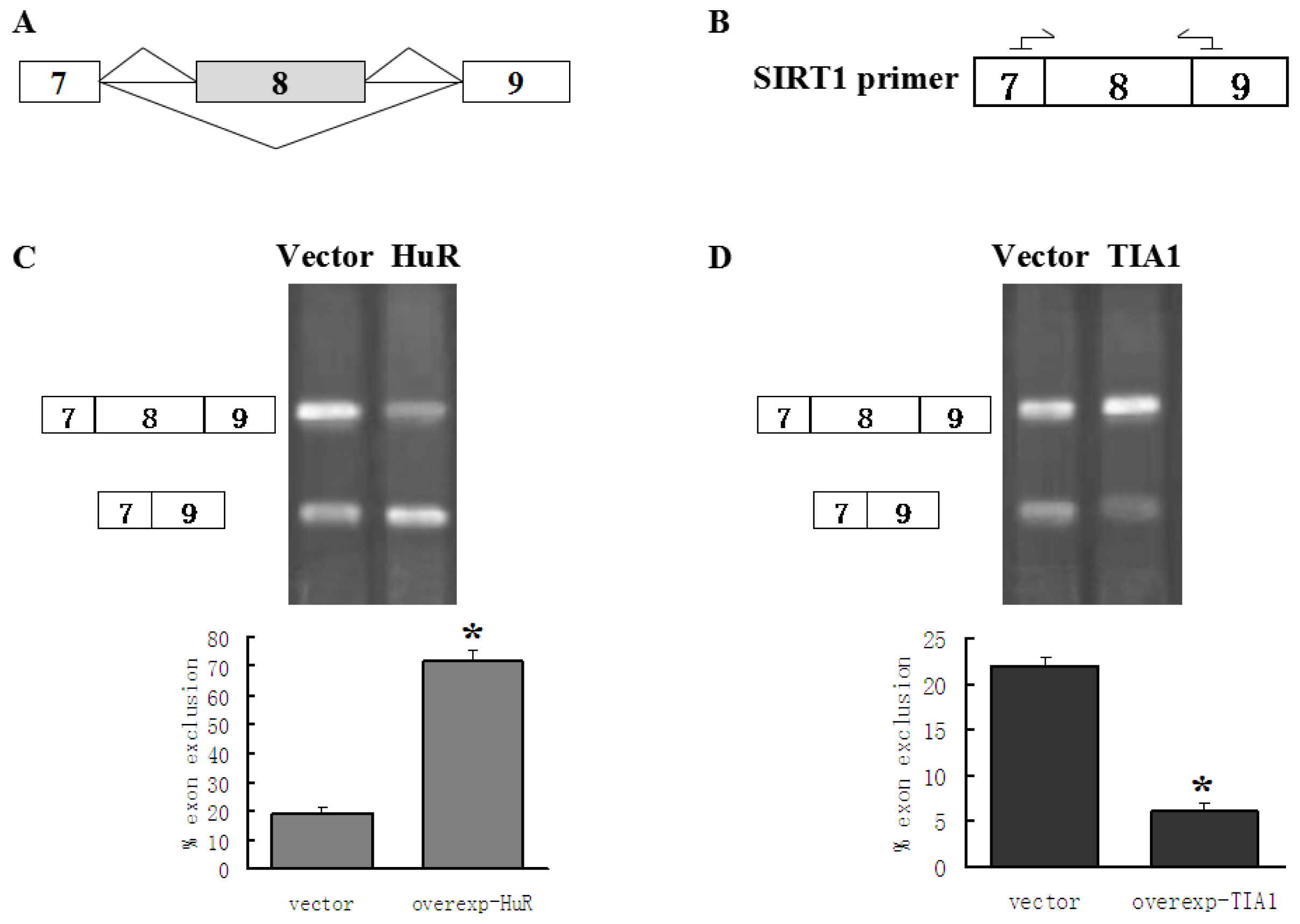
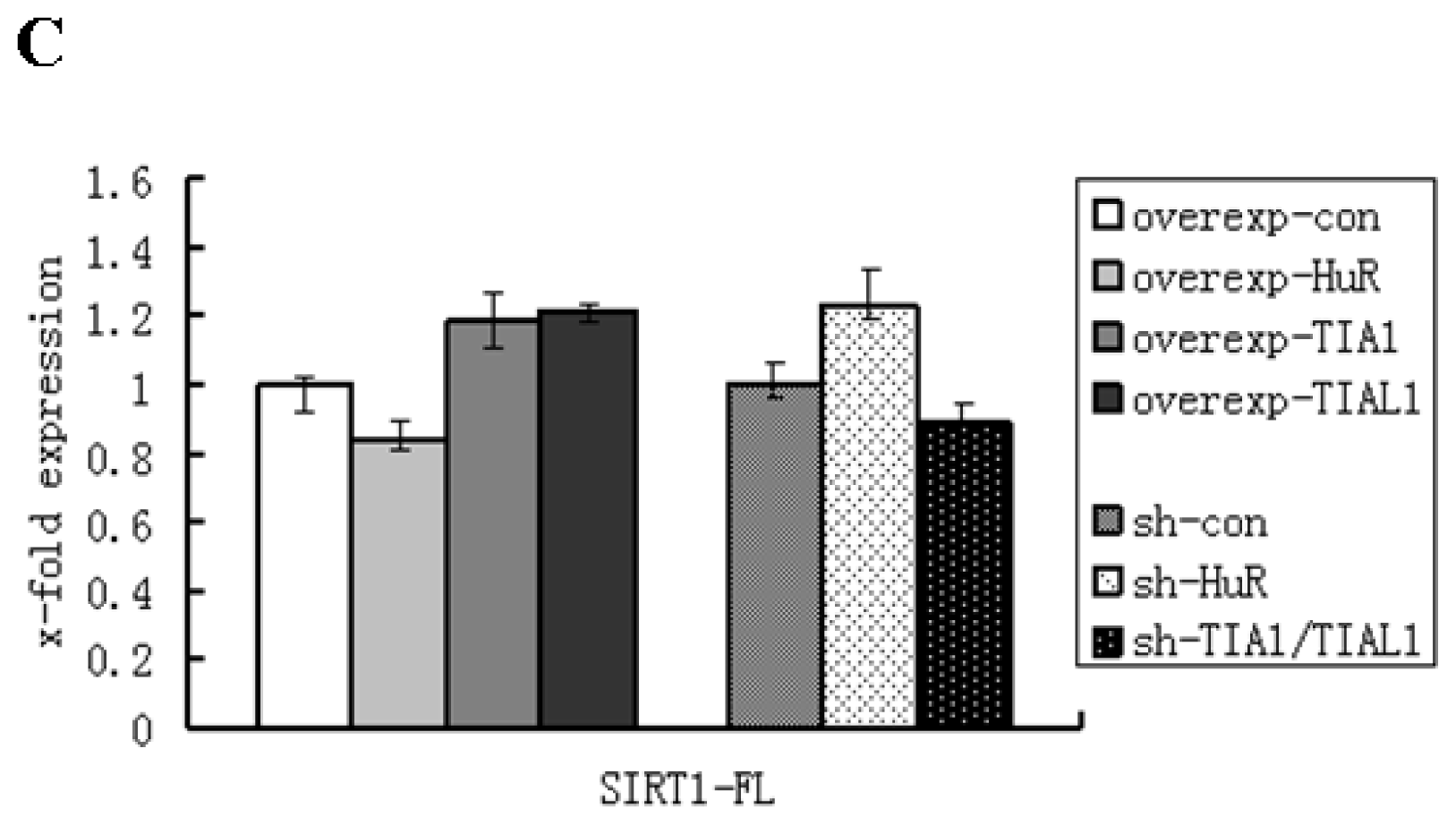
2.1. HuR Promotes Exclusion of SIRT1 Exon 8
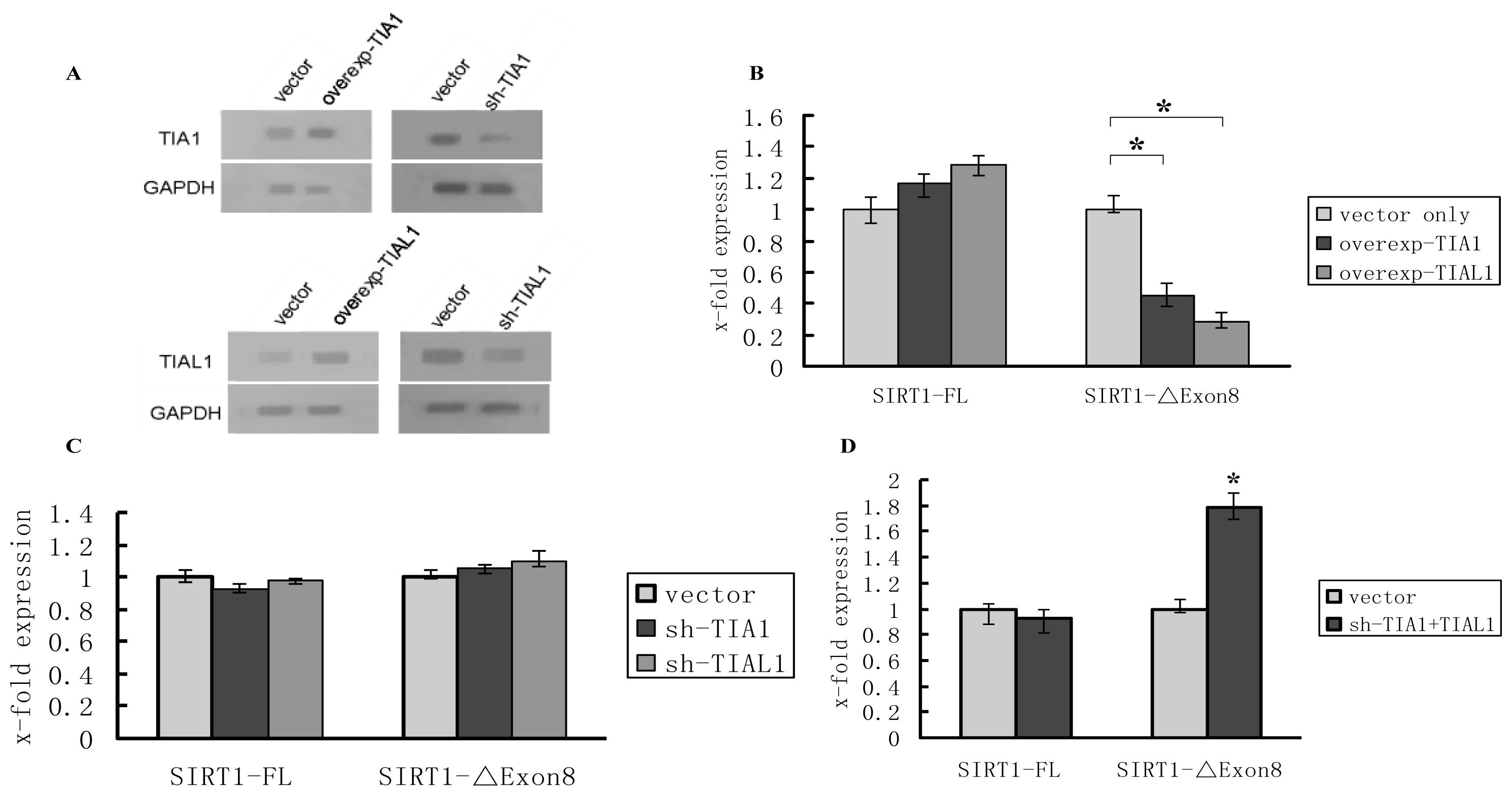
2.2. TIA1/TIAL1 Inhibits Exclusion of SIRT1 Exon 8
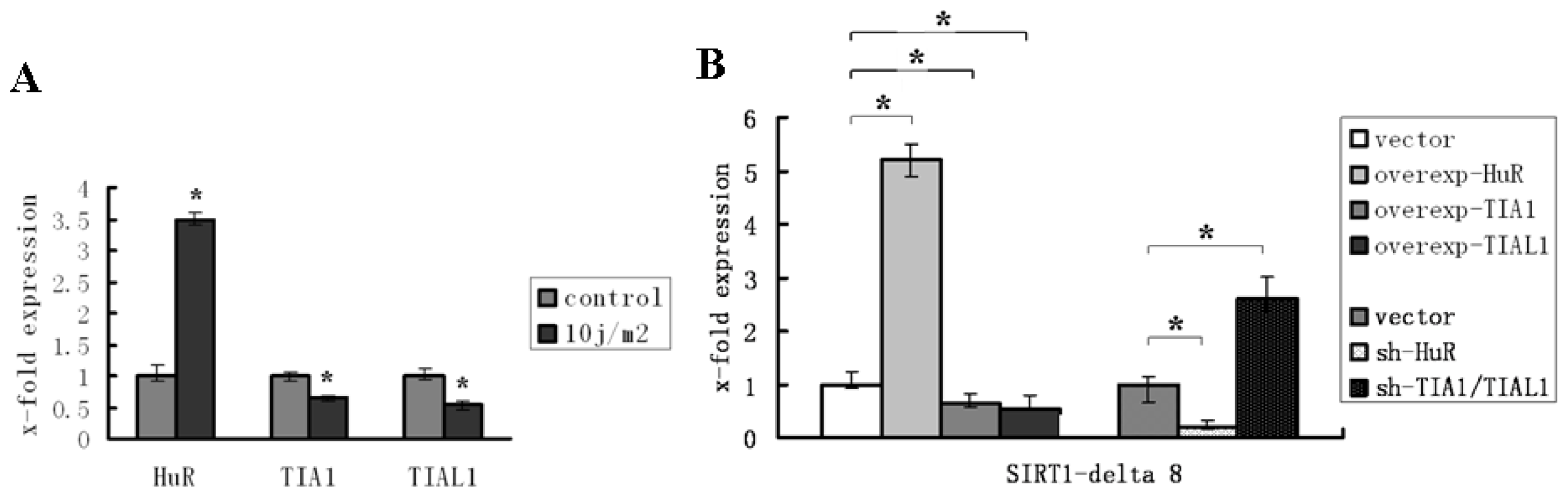
2.3. HuR and TIA1/TIAL1 Regulate the Alternative Splicing of SIRT1 Exon 8 under Injury Circumstance
3. Experimental Section
3.1. Cell Culture and Generation of Cell Lines
3.2. shRNA and Plasmids
3.3. Transfection
3.4. Western Blot Analysis
3.5. RNA Analysis
3.6. Ultraviolet-Induced Stress
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- McBurney, M.W.; Yang, X.; Jardine, K.; Hixon, M.; Boekelheide, K.; Webb, J.R.; Lansdorp, P.M.; Lemieux, M. The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis. Mol. Cell. Biol 2003, 23, 38–54. [Google Scholar]
- Asher, G.; Gatfield, D.; Stratmann, M.; Reinke, H.; Dibner, C.; Kreppel, F.; Mostoslavsky, R.; Alt, F.W.; Schibler, U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 2008, 134, 317–328. [Google Scholar]
- Feige, J.N.; Auwerx, J. Transcriptional targets of sirtuins in the coordination of mammalian physiology. Curr. Opin. Cell Biol 2008, 20, 303–309. [Google Scholar]
- Ghosh, H.S. The anti-aging, metabolism potential of SIRT1. Curr. Opin. Investig. Drugs 2008, 9, 1095–1102. [Google Scholar]
- Lavu, S.; Boss, O.; Elliott, P.J.; Lambert, P.D. Sirtuins: Novel therapeutic targets to treat age-associated diseases. Nat. Rev. Drug Discov 2008, 7, 841–853. [Google Scholar]
- Lynch, C.J.; Shah, Z.H.; Allison, S.J.; Ahmed, S.U.; Ford, J.; Warnock, L.J.; Li, H.; Serrano, M.; Milner, J. SIRT1 undergoes alternative splicing in a novel auto-regulatory loop with p53. PLoS One 2010, 5, e13502. [Google Scholar]
- Shah, Z.H.; Ahmed, S.U.; Ford, J.R.; Allison, S.J.; Knight, J.R.; Milner, J. A deacetylase-deficient SIRT1 variant opposes full-length SIRT1 in regulating tumor suppressor p53 and governs expression of cancer-related genes. Mol. Cell. Biol 2012, 32, 704–716. [Google Scholar]
- Lukong, K.E.; Chang, K.W.; Khandjian, E.W.; Richard, S. RNA-binding proteins in human genetic disease. Trends Genet 2008, 24, 416–425. [Google Scholar]
- Moore, M.J. From birth to death: the complex lives of eukaryotic mRNAs. Science 2005, 309, 1514–1518. [Google Scholar]
- Wilkie, G.S.; Dickson, K.S.; Gray, N.K. Regulation of mRNA translation by 5′- and 3′-UTR-binding factors. Trends Biochem. Sci 2003, 28, 182–188. [Google Scholar]
- Keene, J.D.; Lager, P.J. Post-transcriptional operons and regulons co-ordinating gene expression. Chromosome Res 2005, 13, 327–337. [Google Scholar]
- Abdelmohsen, K.; Kuwano, Y.; Kim, H.H.; Gorospe, M. Posttranscriptional gene regulation by RNA-binding proteins during oxidative stress: Implications for cellular senescence. Biol. Chem 2008, 389, 243–255. [Google Scholar]
- Masuda, K.; Abdelmohsen, K.; Gorospe, M. RNA-binding proteins implicated in the hypoxic response. J. Cell. Mol. Med 2009, 13, 2759–2769. [Google Scholar]
- Apponi, L.H.; Corbett, A.H.; Pavlath, G.K. RNA-binding proteins and gene regulation in myogenesis. Trends Pharmacol. Sci 2011, 32, 652–658. [Google Scholar]
- Ma, W.J.; Cheng, S.; Campbell, C.; Wright, A.; Furneaux, H. Cloning and characterization of HuR, a ubiquitously expressed Elav-like protein. J. Biol. Chem 1996, 271, 8144–8151. [Google Scholar]
- King, P.H.; Levine, T.D.; Fremeau, R.T., Jr.; Keene, J.D. Mammalian homologs of Drosophila ELAV localized to a neuronal subset can bind in vitro to the 3′ UTR of mRNA encoding the Id transcriptional repressor. J. Neurosci 1994, 14, 1943–1952. [Google Scholar]
- Good, P.J. A conserved family of elav-like genes in vertebrates. Proc. Natl. Acad. Sci. USA 1995, 92, 4557–4561. [Google Scholar]
- Antic, D.; Keene, J.D. Embryonic lethal abnormal visual RNA-binding proteins involved in growth, differentiation, and posttranscriptional gene expression. Am. J. Hum. Genet 1997, 61, 273–278. [Google Scholar]
- Fan, X.C.; Steitz, J.A. Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J 1998, 17, 3448–3460. [Google Scholar]
- Lopez de Silanes, I.; Lal, A.; Gorospe, M. HuR: Post-transcriptional paths to malignancy. RNA Biol 2005, 2, 11–13. [Google Scholar]
- Cherry, J.; Karschner, V.; Jones, H.; Pekala, P.H. HuR, an RNA-binding protein, involved in the control of cellular differentiation. In Vivo 2006, 20, 17–23. [Google Scholar]
- Vázquez-Chantada, M.; Fernández-Ramos, D.; Embade, N.; Martinez-Lopez, N.; Varela-Rey, M.; Woodhoo, A.; Luka, Z.; Wagner, C.; Anglim, P.P.; Finnell, R.H.; et al. HuR/methyl-HuR and AUF1 regulate the MAT expressed during liver proliferation, differentiation, and carcinogenesis. Gastroenterology 2010, 138, 1943–1953. [Google Scholar]
- Srikantan, S.; Gorospe, M. HuR function in disease. Front. Biosci 2012, 17, 189–205. [Google Scholar]
- Zhu, H.; Hasman, R.A.; Barron, V.A.; Luo, G.; Lou, H. A nuclear function of Hu proteins as neuron-specific alternative RNA processing regulators. Mol. Biol. Cell 2006, 17, 5105–5114. [Google Scholar]
- Zhu, H.; Zhou, H.L.; Hasman, R.A.; Lou, H. Hu proteins regulate polyadenylation by blocking sites containing U-rich sequences. J. Biol. Chem 2007, 282, 2203–2210. [Google Scholar]
- Izquierdo, J.M. Hu Antigen R (HuR) functions as an alternative pre-mRNA splicing regulator of fas apoptosis-promoting receptor on exon definition. J. Biol. Chem 2008, 283, 19077–19084. [Google Scholar]
- Zhu, H.; Hinman, M.N.; Hasman, R.A.; Mehta, P.; Lou, H. Regulation of neuron-specific alternative splicing of neurofibromatosis type 1 pre-mRNA. Mol. Cell. Biol 2008, 28, 1240–1251. [Google Scholar]
- Izquierdo, J.M. Heterogeneous ribonucleoprotein C displays a repressor activity mediated by T-cell intracellular antigen-1-related/like protein to modulate Fas exon 6 splicing through a mechanism involving Hu antigen R. Nucleic Acids Res 2010, 38, 8001–8014. [Google Scholar]
- Zhou, H.L.; Hinman, M.N.; Barron, V.A.; Geng, C.; Zhou, G.; Luo, G.; Siegel, R.E.; Lou, H. Hu proteins regulate alternative splicing by inducing localized histone hyperacetylation in an RNA-dependent manner. Proc. Natl. Acad. Sci. USA 2011, 108, E627–E635. [Google Scholar]
- Tian, Q.; Streuli, M.; Saito, H.; Schlossman, S.F.; Anderson, P. A polyadenylate binding protein localized to the granules of cytolytic lymphocytes induces DNA fragmentation in target cells. Cell 1991, 67, 629–639. [Google Scholar]
- Kawakami, A.; Tian, Q.; Duan, X.; Streuli, M.; Schlossman, S.F.; Anderson, P. Identification and functional characterization of a TIA-1-related nucleolysin. Proc. Natl. Acad. Sci. USA 1992, 89, 8681–8685. [Google Scholar]
- Keene, J.D. Why is Hu where? Shuttling of early-response-gene messenger RNA subsets. Proc. Natl. Acad. Sci. USA 1999, 96, 5–7. [Google Scholar]
- Brennan, C.M.; Steitz, J.A. HuR and mRNA stability. Cell. Mol. Life Sci 2001, 58, 266–277. [Google Scholar]
- Barreau, C.; Paillard, L.; Osborne, H.B. AU-rich elements and associated factors: Are there unifying principles? Nucleic Acids Res 2005, 33, 7138–7150. [Google Scholar]
- Damgaard, C.K.; Lykke-Andersen, J. Translational coregulation of 5′TOP mRNAs by TIA-1 and TIAR. Genes. Dev 2011, 25, 2057–2068. [Google Scholar]
- Izquierdo, J.M.; Majós, N.; Bonnal, S.; Martínez, C.; Castelo, R.; Guigó, R.; Bilbao, D.; Valcárcel, J. Regulation of Fas alternative splicing by antagonistic effects of TIA1 and PTB on exon definition. Mol. Cell 2005, 19, 475–484. [Google Scholar]
- Abdelmohsen, K.; Pullmann, R., Jr.; Lal, A.; Kim, H.H.; Galban, S.; Yang, X.; Blethrow, J.D.; Walker, M.; Shubert, J.; Gillespie, D.A.; et al. Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol. Cell 2007, 25, 543–557. [Google Scholar]
- Abramoff, M.D.; Magelhaes, P.J.; Ram, S.J. Image processing with image J. Biophoton Int 2004, 11, 36–42. [Google Scholar]
- Dai, W.; Zhang, G.; Makeyev, E.V. RNA-binding protein HuR autoregulates its expression by promoting alternative polyadenylation site usage. Nucleic Acids Res 2012, 40, 787–800. [Google Scholar]
- Mansfield, K.D.; Keene, J.D. Neuron-specific ELAV/Hu proteins suppress HuR mRNA during neuronal differentiation by alternative polyadenylation. Nucleic Acids Res 2012, 40, 2734–2746. [Google Scholar]
- Zhao, J.F.; Zhao, W.H.; Hou, M.M.; Wang, Y.; Zhang, Y.; Guo, D.W.; Zhang, C. Epigenetic regulation and significance of the alternative splicing of SIRT1 gene. 2014, unpublished work. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhao, W.; Zhao, J.; Hou, M.; Wang, Y.; Zhang, Y.; Zhao, X.; Zhang, C.; Guo, D. HuR and TIA1/TIAL1 Are Involved in Regulation of Alternative Splicing of SIRT1 Pre-mRNA. Int. J. Mol. Sci. 2014, 15, 2946-2958. https://doi.org/10.3390/ijms15022946
Zhao W, Zhao J, Hou M, Wang Y, Zhang Y, Zhao X, Zhang C, Guo D. HuR and TIA1/TIAL1 Are Involved in Regulation of Alternative Splicing of SIRT1 Pre-mRNA. International Journal of Molecular Sciences. 2014; 15(2):2946-2958. https://doi.org/10.3390/ijms15022946
Chicago/Turabian StyleZhao, Wenhui, Jinfeng Zhao, Miaomiao Hou, Yue Wang, Yang Zhang, Xin Zhao, Ce Zhang, and Dawei Guo. 2014. "HuR and TIA1/TIAL1 Are Involved in Regulation of Alternative Splicing of SIRT1 Pre-mRNA" International Journal of Molecular Sciences 15, no. 2: 2946-2958. https://doi.org/10.3390/ijms15022946



