Soluble Calreticulin Induces Tumor Necrosis Factor-α (TNF-α) and Interleukin (IL)-6 Production by Macrophages through Mitogen-Activated Protein Kinase (MAPK) and NFκB Signaling Pathways
Abstract
:1. Introduction
2. Results and Discussion
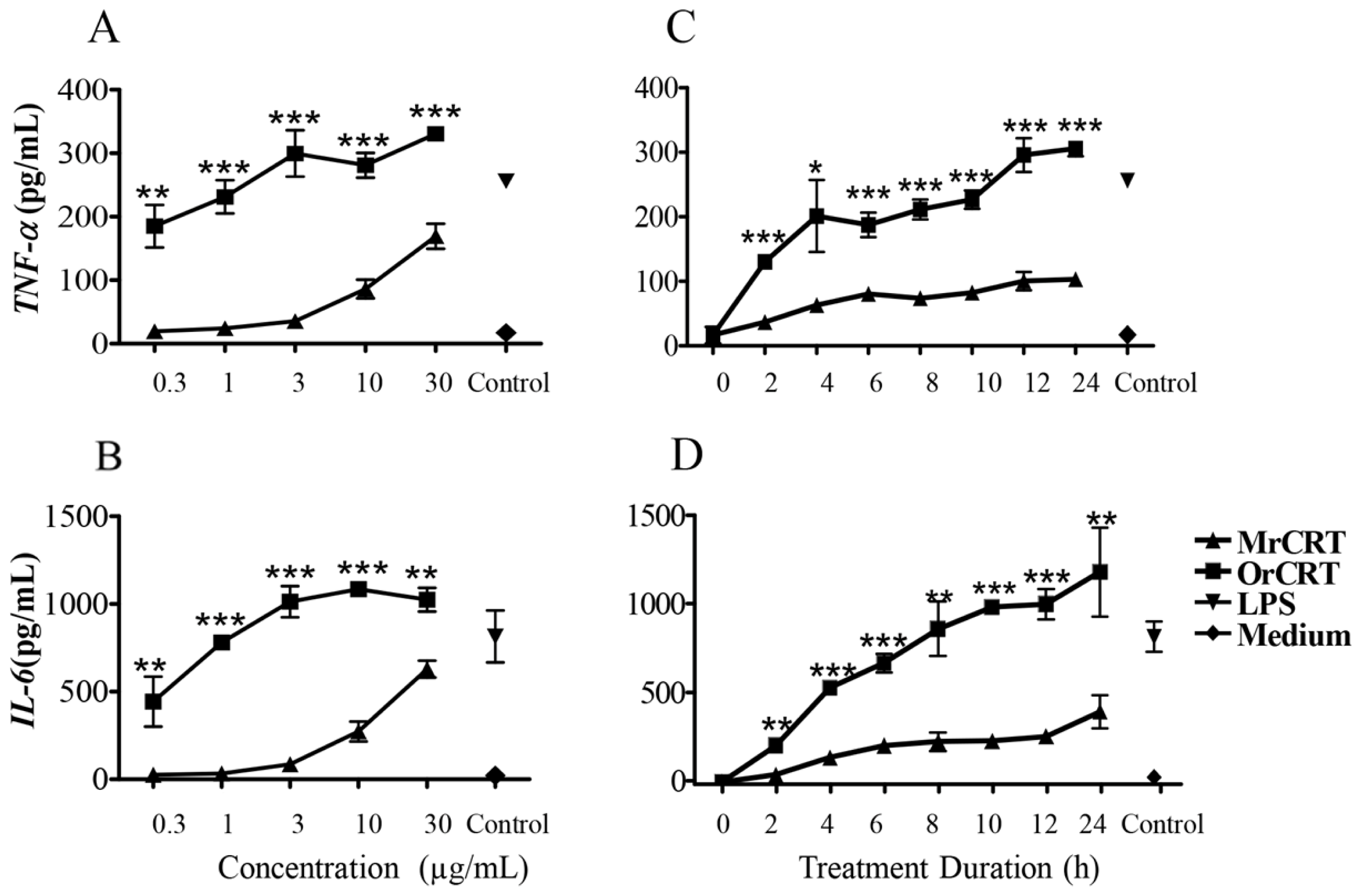
2.1. TNF-α and IL-6 Production by Murine Macrophages in Response to rCRT Stimulation
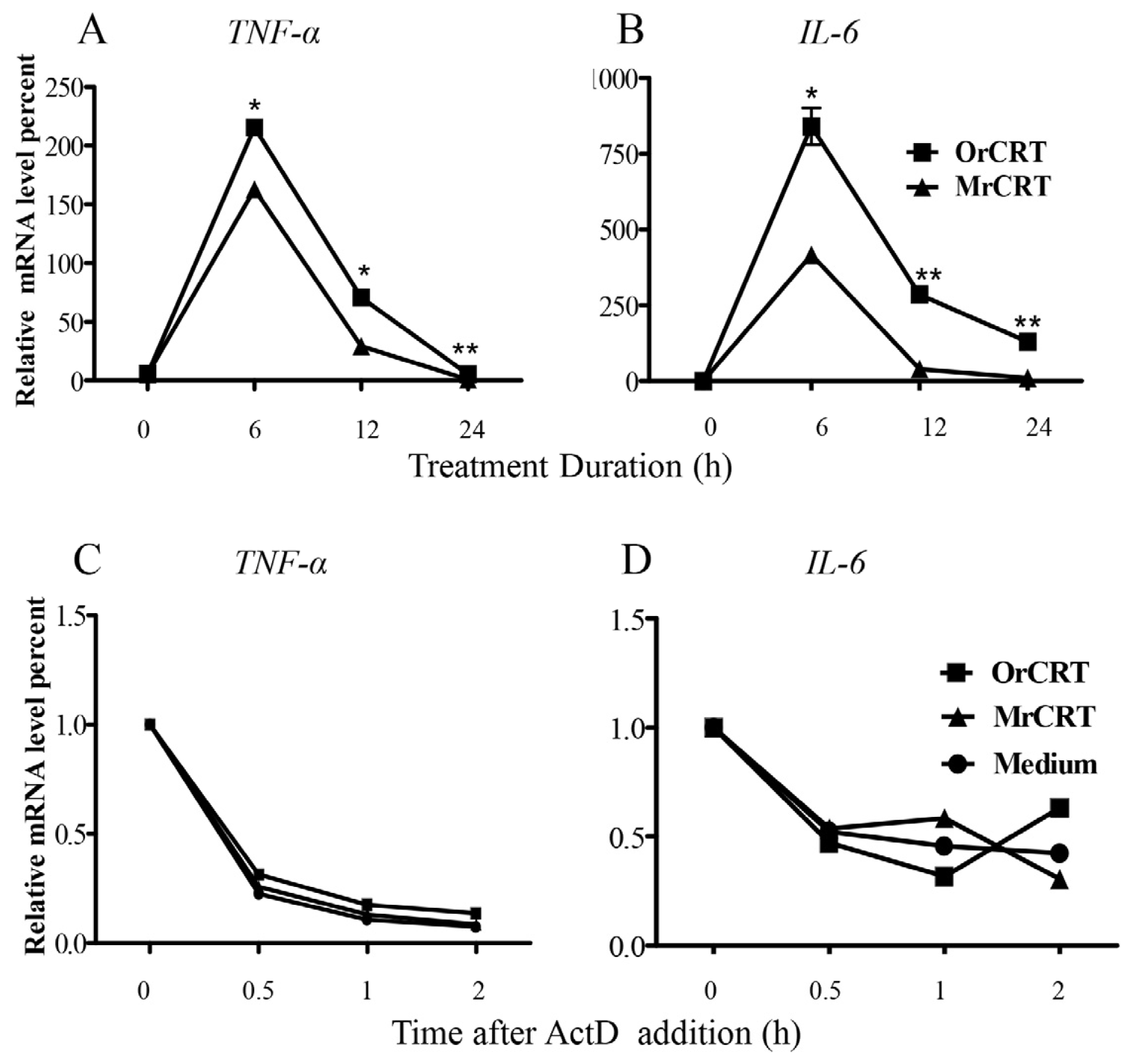
2.2. TNF-α and IL-6 mRNA Expression and Stability in Macrophages under rCRT Stimulation
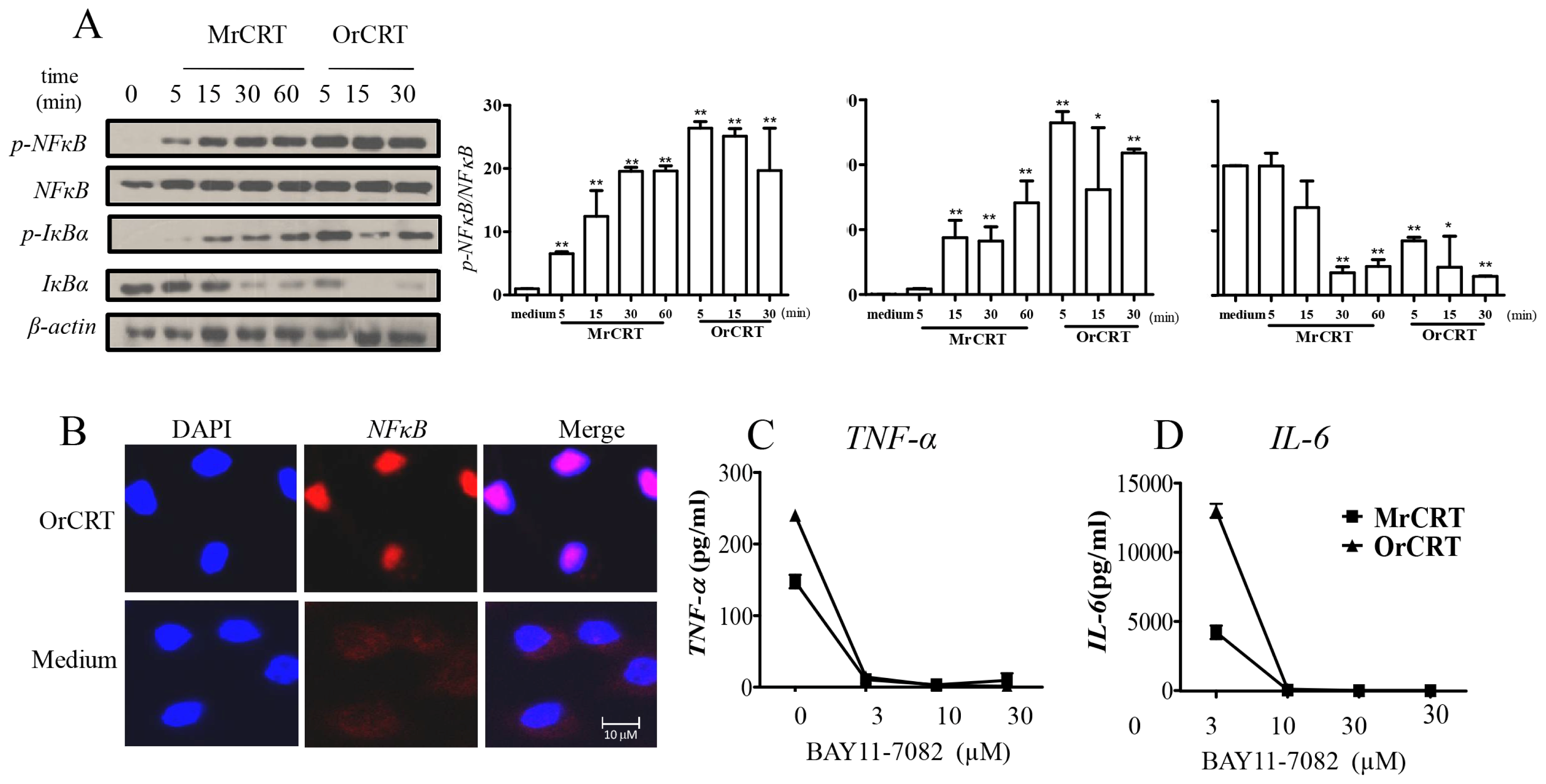
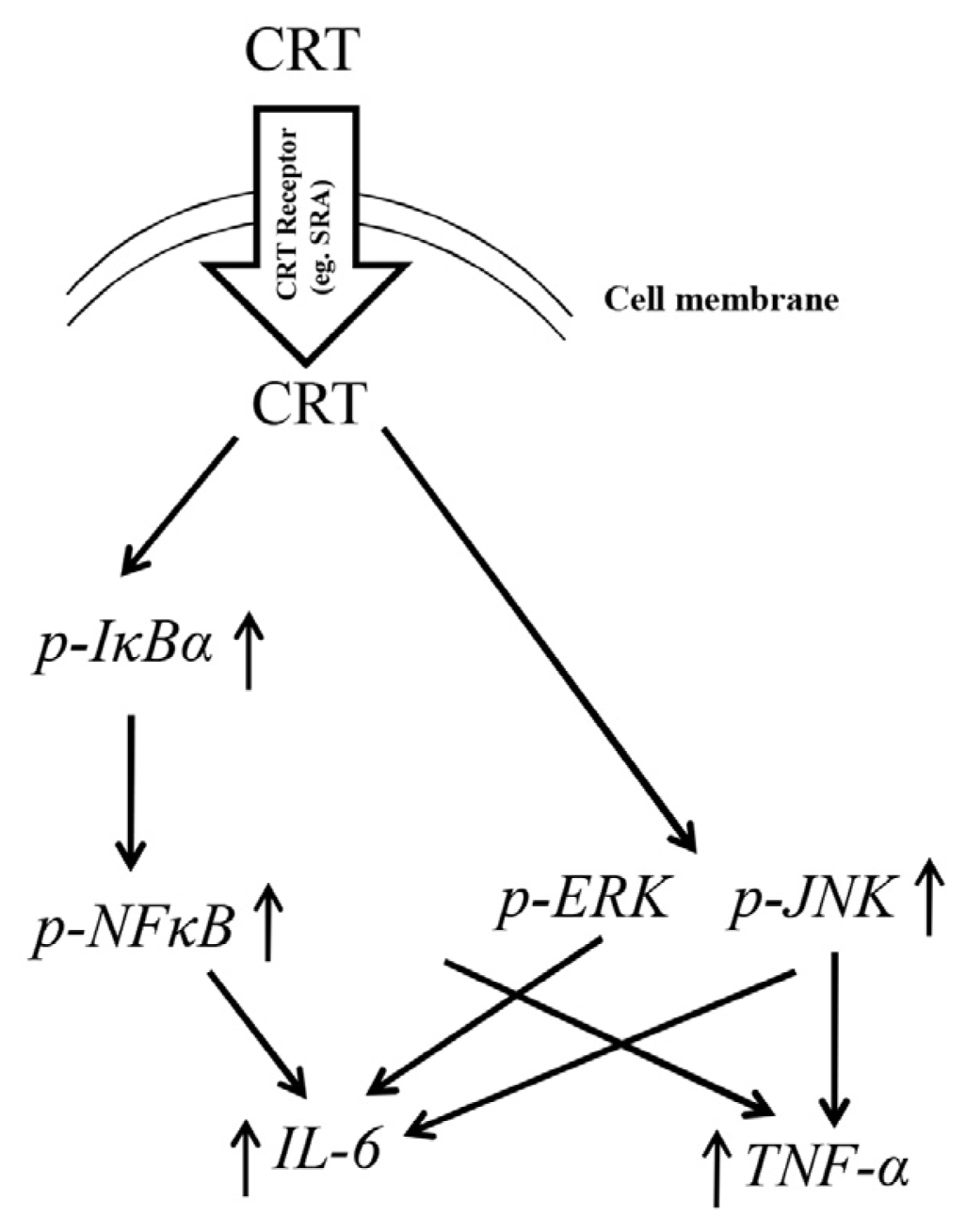
2.3. NFκB Activation during rCRT-Induced TNF-α and IL-6 Response
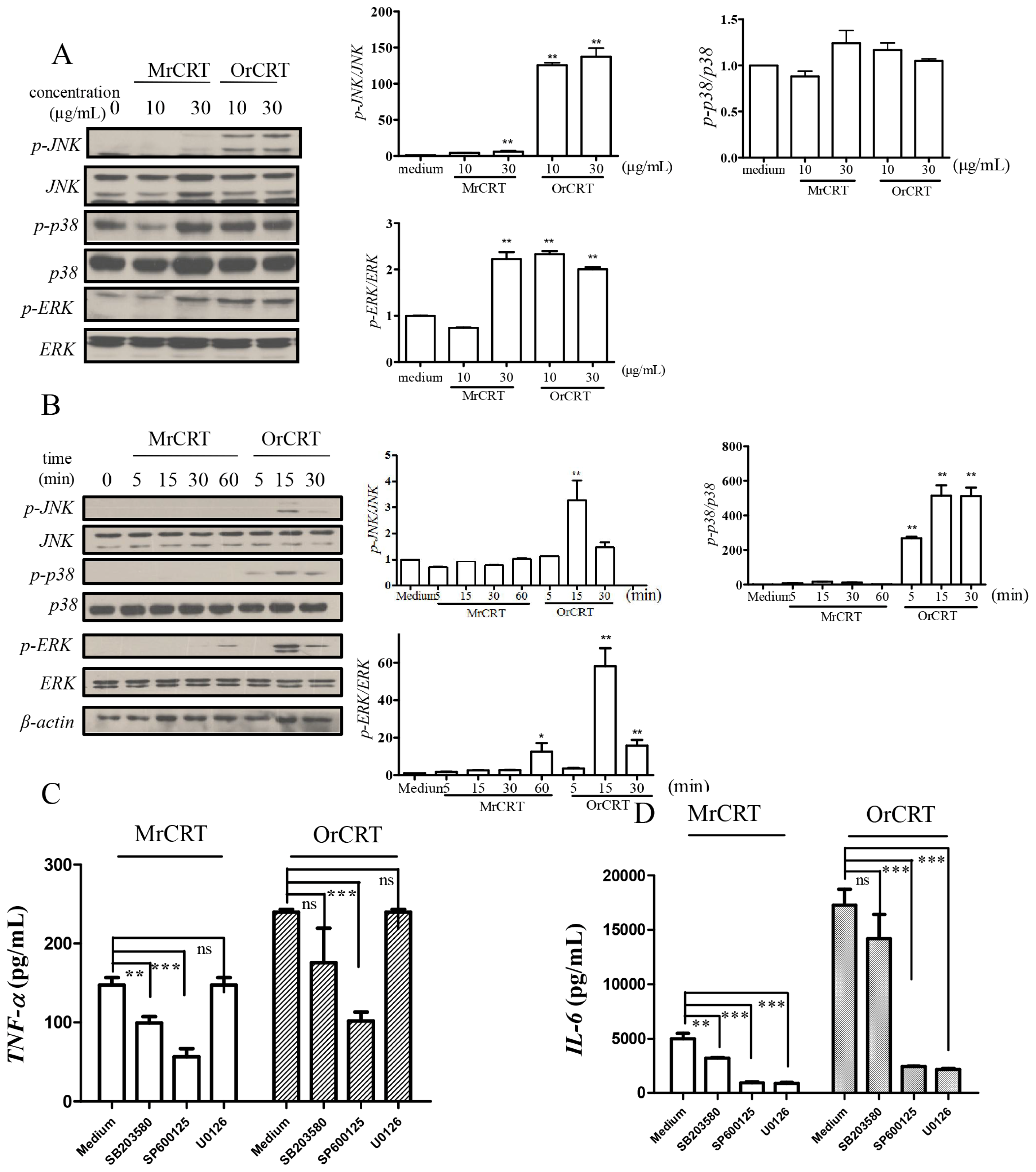
2.4. MAPK Activation during rCRT-Induced TNF-α and IL-6 Response
2.5. Inhibition of OrCRT Binding/Internalization by Fucoidan
2.6. Discussion
3. Experimental Section
3.1. Materials
3.2. Protein Purification and Separation
3.3. Isolation of Mouse Peritoneal Macrophages
3.4. Enzyme-Linked Immunosorbent Assay (ELISA) of TNF-α and IL-6
3.5. RNA Isolation and Real-Time Quantitative RT-PCR
3.6. Western Blot Assay
3.7. Assessment of TNF-α and IL-6 mRNA Stability
3.8. Flow Cytometric Analysis
3.9. Immunofluorescence Staining
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Michalak, M.; Corbett, E.F.; Mesaeli, N.; Nakamura, K.; Opas, M. Calreticulin: One protein, one gene, many functions. Biochem. J 1999, 344, 281–292. [Google Scholar]
- Arosa, F.A.; de Jesus, O.; Porto, G.; Carmo, A.M.; de Sousa, M. Calreticulin is expressed on the cell surface of activated human peripheral blood T lymphocytes in association with major histocompatibility complex class I molecules. J. Biol. Chem 1999, 274, 16917–16922. [Google Scholar]
- Hong, C.; Qiu, X.; Li, Y.; Huang, Q.; Zhong, Z.; Zhang, Y.; Liu, X.; Sun, L.; Lv, P.; Gao, X.M. Functional analysis of recombinant calreticulin fragment 39–272: Implications for immunobiological activities of calreticulin in health and disease. J. Immunol 2010, 185, 4561–4569. [Google Scholar]
- Liu, R.; Gong, J.; Chen, J.; Li, Q.; Song, C.; Zhang, J.; Li, Y.; Liu, Z.; Dong, Y.; Chen, L.; et al. Calreticulin as a potential diagnostic biomarker for lung cancer. Cancer Immunol. Immunother 2012, 61, 855–864. [Google Scholar]
- Huang, S.H.; Zhao, L.X.; Hong, C.; Duo, C.C.; Guo, B.N.; Zhang, L.J.; Gong, Z.; Xiong, S.D.; Gong, F.Y.; Gao, X.M. Self-oligomerization is essential for enhanced immunological activities of soluble recombinant calreticulin. PLoS One 2013, 8, e64951. [Google Scholar]
- Dayer, J.M.; Choy, E. Therapeutic targets in rheumatoid arthritis: The interleukin-6 receptor. Rheumatology 2010, 49, 15–24. [Google Scholar]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med 2011, 365, 2205–2219. [Google Scholar]
- Jorgensen, C.S.; Ryder, L.R.; Steino, A.; Hojrup, P.; Hansen, J.; Beyer, N.H.; Heegaard, N.H.; Houen, G. Dimerization and oligomerization of the chaperone calreticulin. Eur. J. Biochem 2003, 270, 4140–4148. [Google Scholar]
- Kracht, M.; Saklatvala, J. Transcriptional and post-transcriptional control of gene expression in inflammation. Cytokine 2002, 20, 91–106. [Google Scholar]
- Wang, S.L.; Li, Y.; Wen, Y.; Chen, Y.F.; Na, L.X.; Li, S.T.; Sun, C.H. Curcumin, a potential inhibitor of up-regulation of TNF-α and IL-6 induced by palmitate in 3T3-L1 adipocytes through NF-κB and JNK pathway. Biomed. Environ. Sci 2009, 22, 32–39. [Google Scholar]
- Krishnan, N.; Bencze, G.; Cohen, P.; Tonks, N.K. The anti-inflammatory compound bay-11-7082 is a potent inhibitor of protein tyrosine phosphatases. FEBS J 2013, 280, 2830–2841. [Google Scholar]
- Bao, S.; Li, Y.; Lei, X.; Wohltmann, M.; Jin, W.; Bohrer, A.; Semenkovich, C.F.; Ramanadham, S.; Tabas, I.; Turk, J. Attenuated free cholesterol loading-induced apoptosis but preserved phospholipid composition of peritoneal macrophages from mice that do not express group via phospholipase A2. J. Biol. Chem 2007, 282, 27100–27114. [Google Scholar]
- Zhang, T.T.; Xia, Y.; Zhang, L.J.; Bao, W.R.; Hong, C.; Gao, X.M. Cd1dhicd5+ B cells differentiate into antibody-secreting cells under the stimulation with calreticulin fragment. Protein Cell 2013, 4, 872–881. [Google Scholar]
- Cho, J.H.; Homma, K.J.; Kanegasaki, S.; Natori, S. Activation of human monocyte cell line U937 via cell surface calreticulin. Cell Stress Chaperones 2001, 6, 148–152. [Google Scholar]
- Pawaria, S.; Binder, R.J. Cd91-dependent programming of T-helper cell responses following heat shock protein immunization. Nat. Commun 2011, 2, 521. [Google Scholar]
- Gardai, S.J.; McPhillips, K.A.; Frasch, S.C.; Janssen, W.J.; Starefeldt, A.; Murphy-Ullrich, J.E.; Bratton, D.L.; Oldenborg, P.A.; Michalak, M.; Henson, P.M. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 2005, 123, 321–334. [Google Scholar]
- Basu, S.; Binder, R.J.; Suto, R.; Anderson, K.M.; Srivastava, P.K. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-κB pathway. Int. Immunol 2000, 12, 1539–1546. [Google Scholar]
- Ohashi, K.; Burkart, V.; Flohe, S.; Kolb, H. Cutting edge: Heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J. Immunol 2000, 164, 558–561. [Google Scholar]
- Binder, R.J.; Han, D.K.; Srivastava, P.K. Cd91: A receptor for heat shock protein GP96. Nat. Immunol 2000, 1, 151–155. [Google Scholar]
- Giraldo, E.; Martin-Cordero, L.; Garcia, J.J.; Gehrmann, M.; Multhoff, G.; Ortega, E. Exercise-induced extracellular 72 KDa heat shock protein (HSP72) stimulates neutrophil phagocytic and fungicidal capacities via TLR-2. Eur. J. Appl. Physiol 2010, 108, 217–225. [Google Scholar]
- Delneste, Y.; Magistrelli, G.; Gauchat, J.; Haeuw, J.; Aubry, J.; Nakamura, K.; Kawakami-Honda, N.; Goetsch, L.; Sawamura, T.; Bonnefoy, J.; et al. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity 2002, 17, 353–362. [Google Scholar]
- Panjwani, N.N.; Popova, L.; Srivastava, P.K. Heat shock proteins GP96 and HSP70 activate the release of nitric oxide by APCs. J. Immunol 2002, 168, 2997–3003. [Google Scholar]
- Binder, R.J.; Zhou, Y.J.; Messmer, M.N.; Pawaria, S. Cd91-dependent modulation of immune responses by heat shock proteins: A role in autoimmunity. Autoimmune Dis 2012, 2012, 863041. [Google Scholar]
- Berwin, B.; Hart, J.P.; Rice, S.; Gass, C.; Pizzo, S.V.; Post, S.R.; Nicchitta, C.V. Scavenger receptor-a mediates GP96/GRP94 and calreticulin internalization by antigen-presenting cells. EMBO J 2003, 22, 6127–6136. [Google Scholar]
- Duus, K.; Hansen, E.W.; Tacnet, P.; Frachet, P.; Arlaud, G.J.; Thielens, N.M.; Houen, G. Direct interaction between CD91 and C1Q. FEBS J 2010, 277, 3526–3537. [Google Scholar]
- Peiser, L.; Gordon, S. The function of scavenger receptors expressed by macrophages and their role in the regulation of inflammation. Microbes Infect 2001, 3, 149–159. [Google Scholar]

© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Duo, C.-C.; Gong, F.-Y.; He, X.-Y.; Li, Y.-M.; Wang, J.; Zhang, J.-P.; Gao, X.-M. Soluble Calreticulin Induces Tumor Necrosis Factor-α (TNF-α) and Interleukin (IL)-6 Production by Macrophages through Mitogen-Activated Protein Kinase (MAPK) and NFκB Signaling Pathways. Int. J. Mol. Sci. 2014, 15, 2916-2928. https://doi.org/10.3390/ijms15022916
Duo C-C, Gong F-Y, He X-Y, Li Y-M, Wang J, Zhang J-P, Gao X-M. Soluble Calreticulin Induces Tumor Necrosis Factor-α (TNF-α) and Interleukin (IL)-6 Production by Macrophages through Mitogen-Activated Protein Kinase (MAPK) and NFκB Signaling Pathways. International Journal of Molecular Sciences. 2014; 15(2):2916-2928. https://doi.org/10.3390/ijms15022916
Chicago/Turabian StyleDuo, Cui-Cui, Fang-Yuan Gong, Xiao-Yan He, Yan-Mei Li, Jun Wang, Jin-Ping Zhang, and Xiao-Ming Gao. 2014. "Soluble Calreticulin Induces Tumor Necrosis Factor-α (TNF-α) and Interleukin (IL)-6 Production by Macrophages through Mitogen-Activated Protein Kinase (MAPK) and NFκB Signaling Pathways" International Journal of Molecular Sciences 15, no. 2: 2916-2928. https://doi.org/10.3390/ijms15022916
APA StyleDuo, C.-C., Gong, F.-Y., He, X.-Y., Li, Y.-M., Wang, J., Zhang, J.-P., & Gao, X.-M. (2014). Soluble Calreticulin Induces Tumor Necrosis Factor-α (TNF-α) and Interleukin (IL)-6 Production by Macrophages through Mitogen-Activated Protein Kinase (MAPK) and NFκB Signaling Pathways. International Journal of Molecular Sciences, 15(2), 2916-2928. https://doi.org/10.3390/ijms15022916



