Mechanisms Underlying Apoptosis-Inducing Effects of Kaempferol in HT-29 Human Colon Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
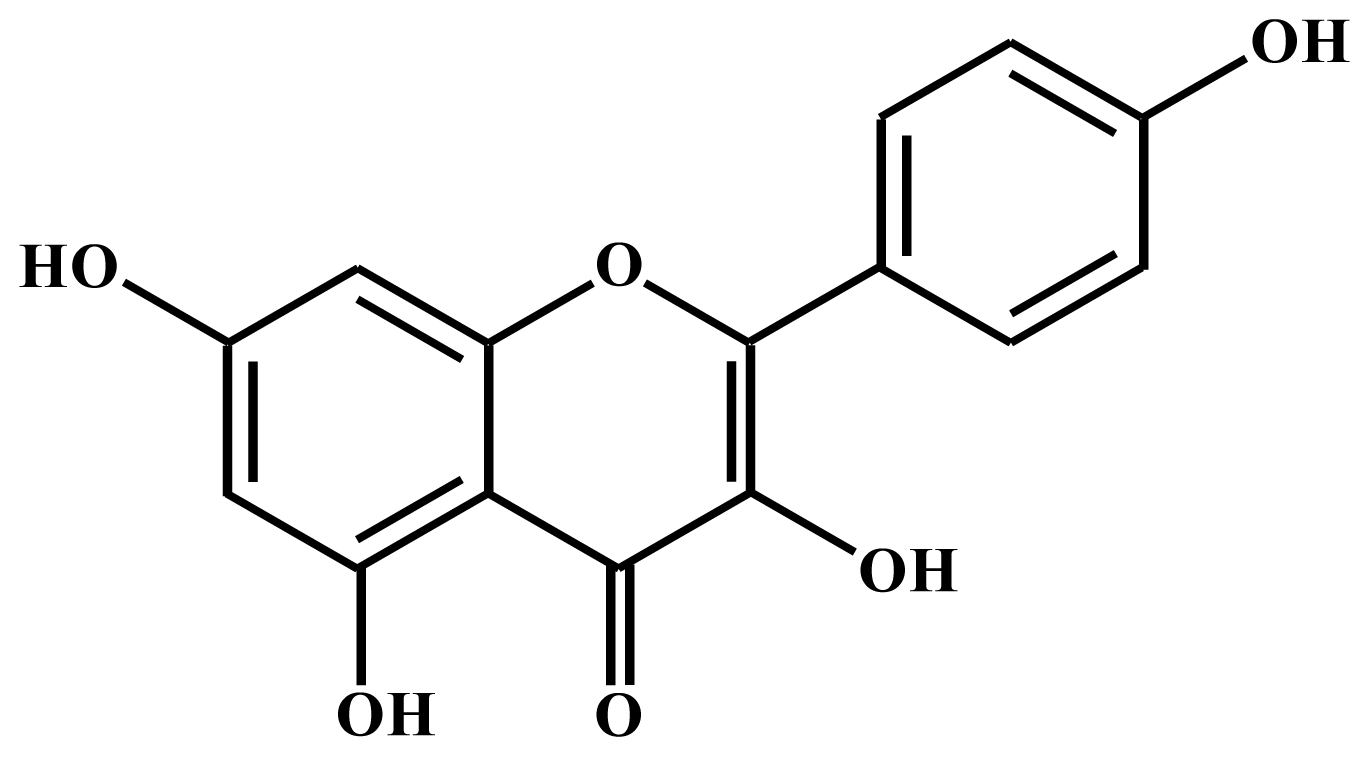
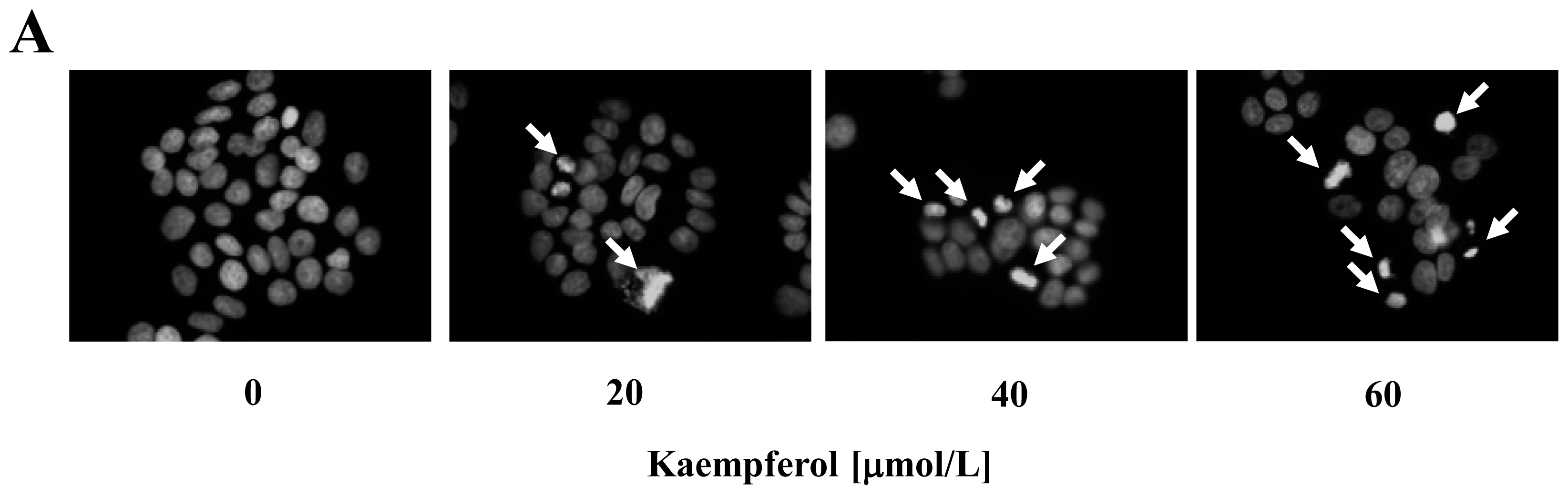
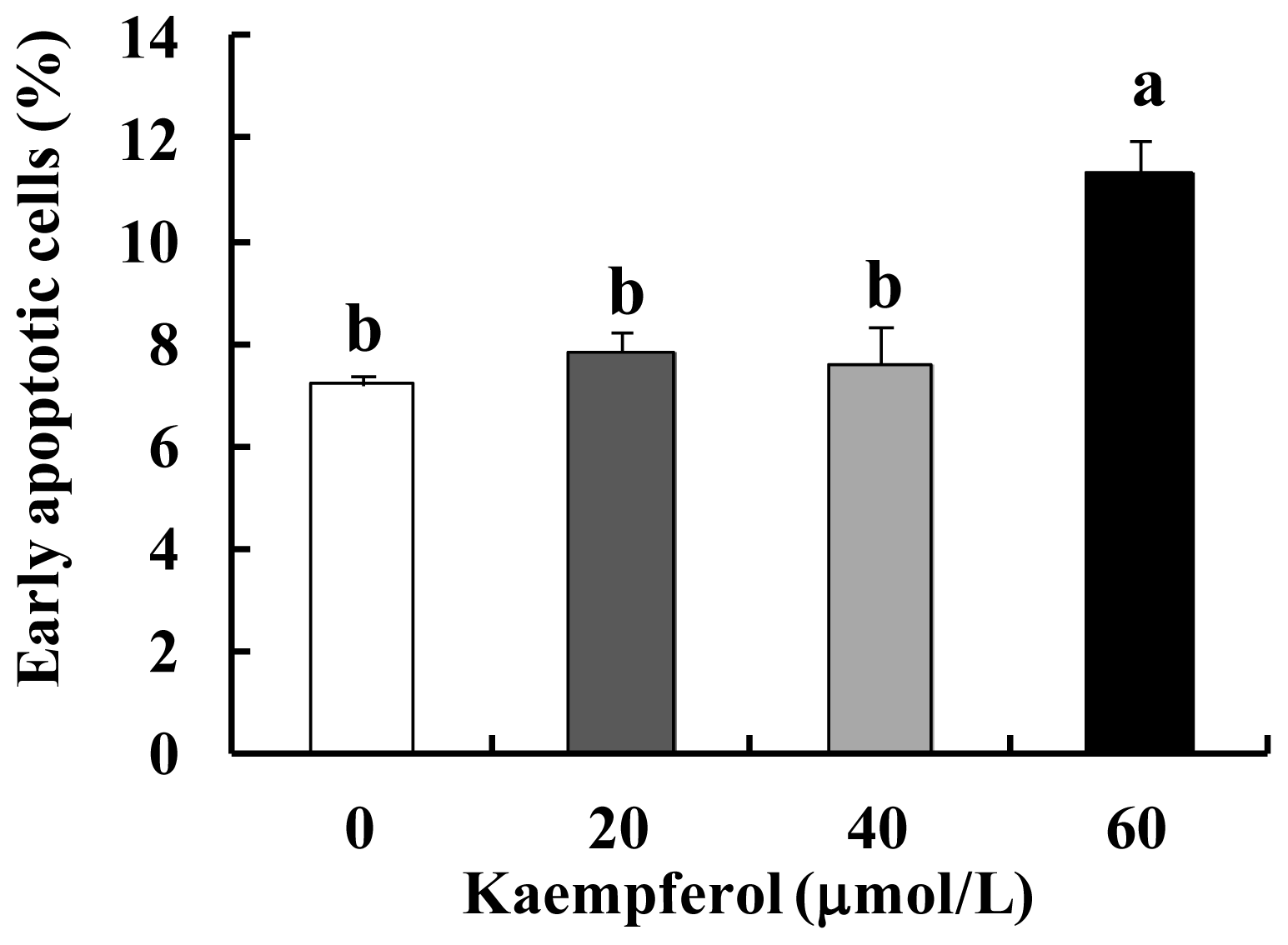
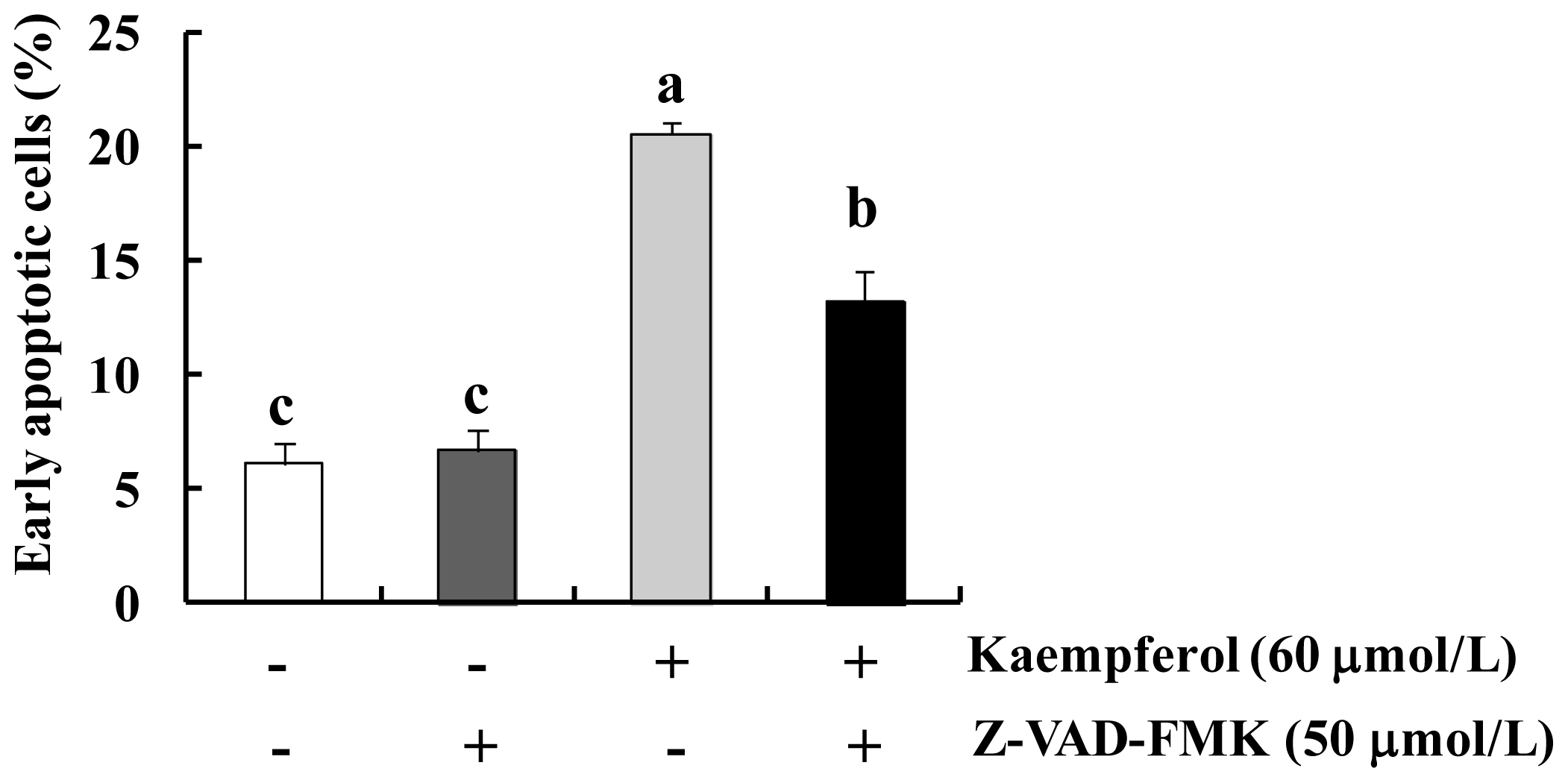
2.1. Kaempferol Induces Apoptosis in HT-29 Cells
2.2. Kaempferol Activates the Caspase Cascade in HT-29 Cells
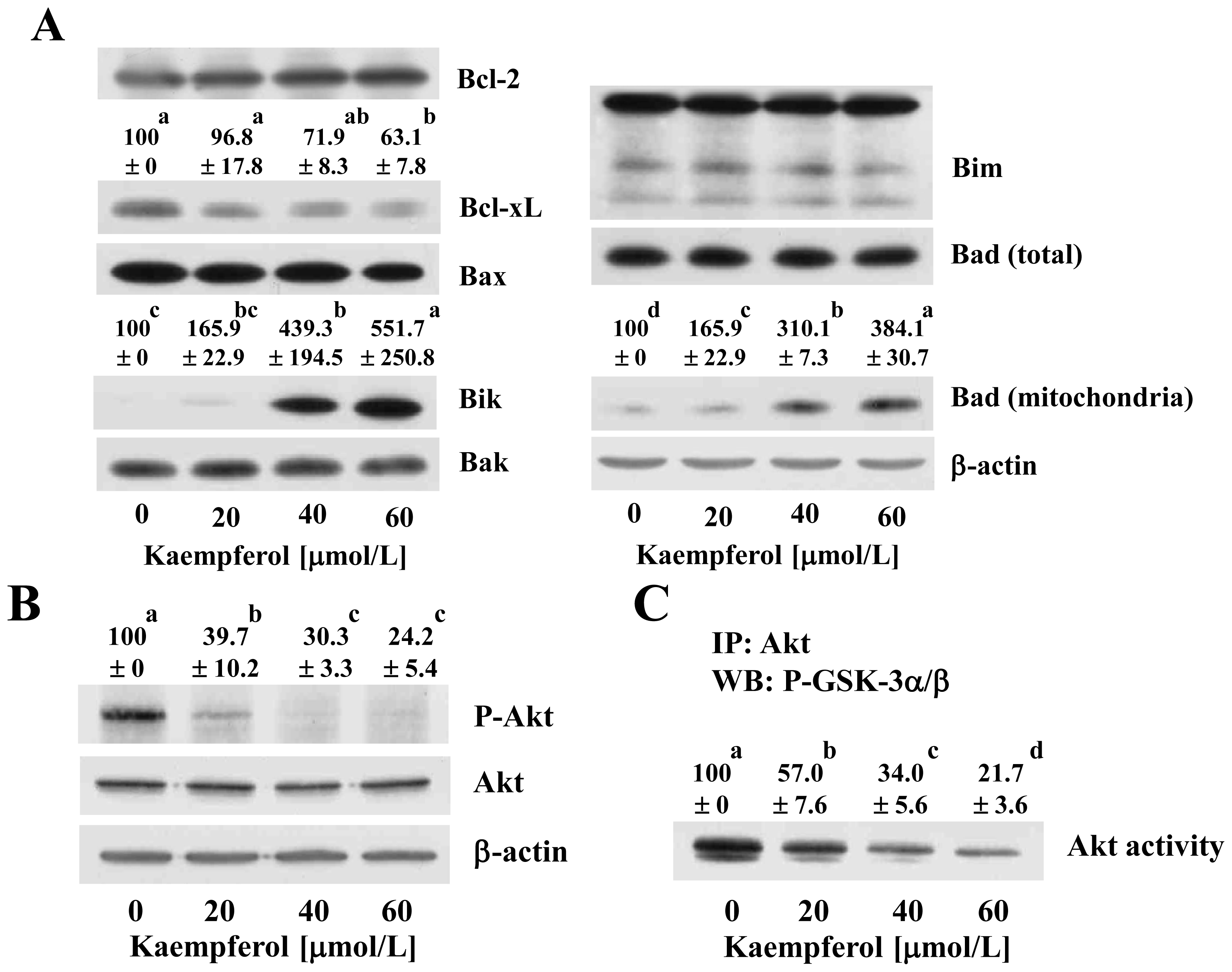
2.3. Kaempferol Modulates the Levels and Localization of Bcl-2 Family Proteins in HT-29 Cells
2.4. Kaempferol Induces Depolarization of the Mitochondria and the Release of Cytochrome C from the Mitochondria in HT-29 Cells
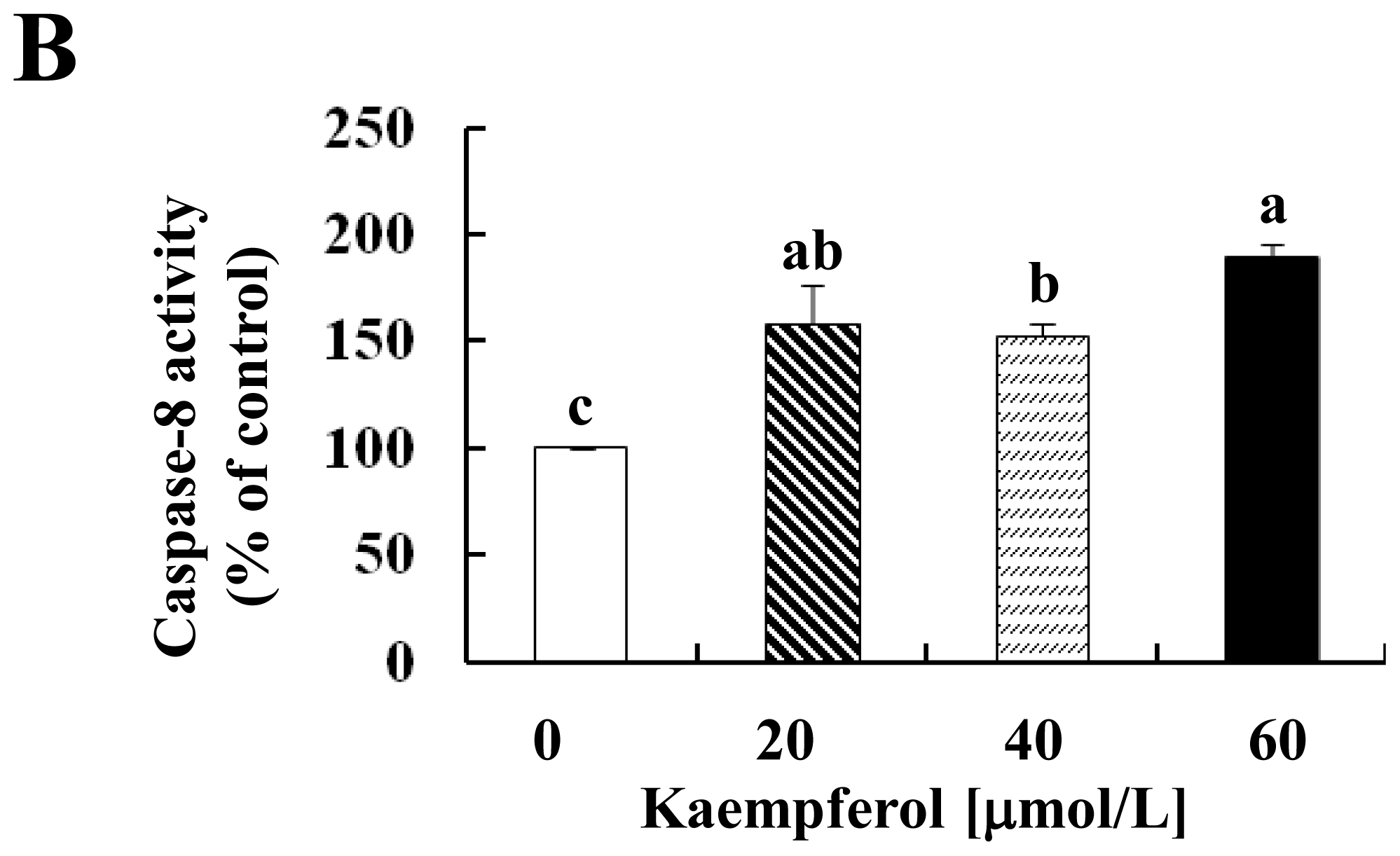
2.5. Kaempferol Induces Changes in the Levels of Proteins Involved in the Regulation of the Extrinsic Apoptotic Pathway in HT-29 Cells
3. Experimental Section
3.1. Materials
3.2. Cell Culture
3.3. Hoechst 33258 Staining
3.4. Fluorescence-Activated Cell Sorting (FACS) Analysis
3.5. Mitochondrial Membrane Potential Indicator Loading Procedure
3.6. Western Blot Analysis and in Vitro Kinase Assay
3.7. Caspase-8 Activity
3.8. Statistical Analysis
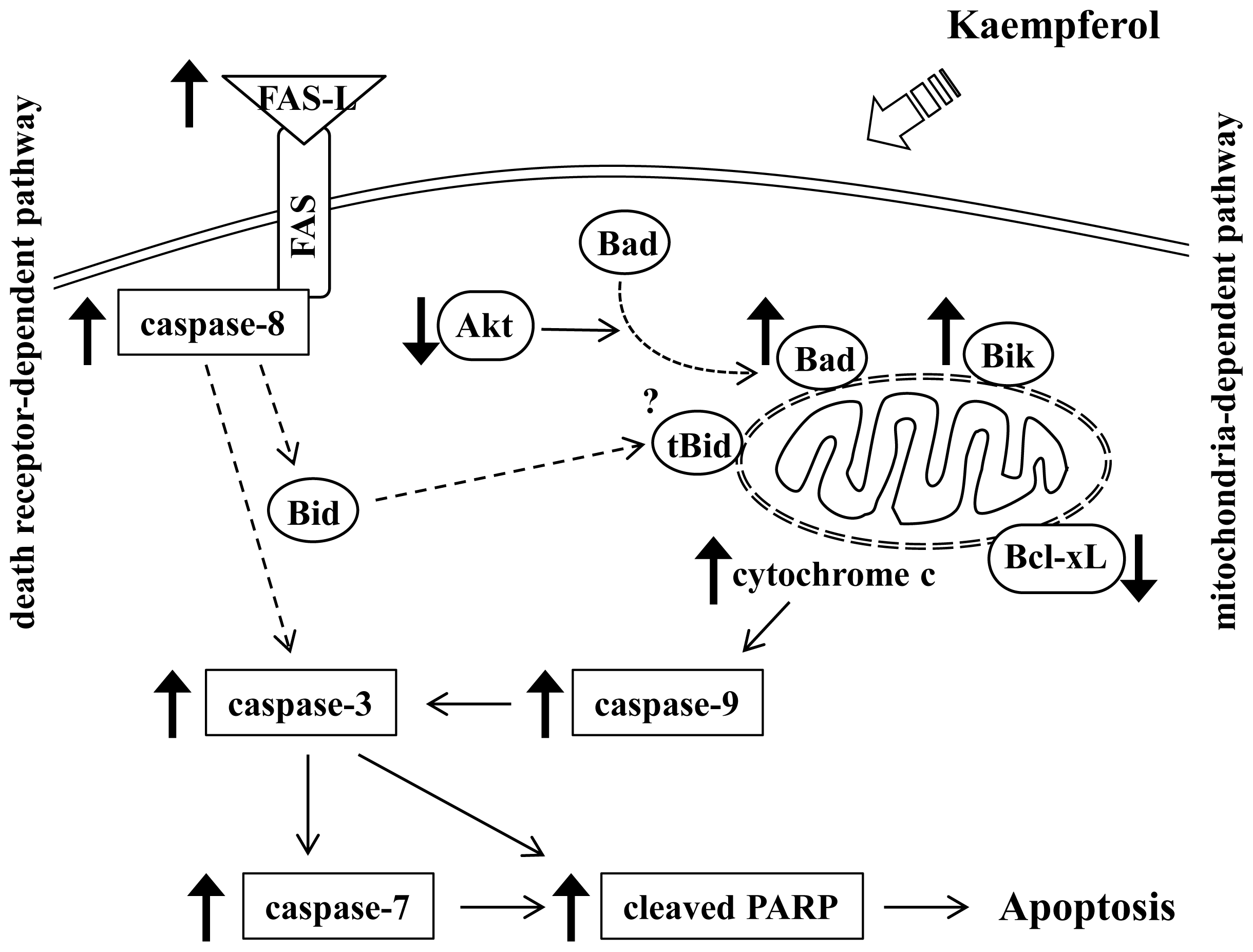
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kamangar, F.; Dores, G.M.; Anderson, W.F. Patterns of cancer incidence, mortality, and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol 2006, 24, 2137–2150. [Google Scholar]
- Sung, J.J.; Lau, J.Y.; Goh, K.L.; Leung, W.K. Increasing incidence of colorectal cancer in Asia: Implications for screening. Lancet Oncol 2005, 6, 871–876. [Google Scholar]
- Surh, Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar]
- Hollman, P.C.; Katan, M.B. Dietary flavonoids: Intake, health effects and bioavailability. Food Chem. Toxicol 1999, 37, 937–942. [Google Scholar]
- Bobe, G.; Sansbury, L.B.; Albert, P.S.; Cross, A.J.; Kahle, L.; Ashby, J.; Slattery, M.L.; Caan, B.; Paskett, E.; Iber, F.; et al. Dietary flavonoids and colorectal adenoma recurrence in the Polyp Prevention Trial. Cancer Epidemiol. Biomark. Prev 2008, 17, 1344–1353. [Google Scholar]
- Gates, M.A.; Tworoger, S.S.; Hecht, J.L.; De Vivo, I.; Rosner, B.; Hankinson, S.E. A prospective study of dietary flavonoid intake and incidence of epithelial ovarian cancer. Int. J. Cancer 2007, 121, 2225–2232. [Google Scholar]
- Nothlings, U.; Murphy, S.P.; Wilkens, L.R.; Henderson, B.E.; Kolonel, L.N. Flavonols and pancreatic cancer risk: The multiethnic cohort study. Am. J. Epidemiol 2007, 166, 924–931. [Google Scholar]
- Garcia-Closas, R.; Gonzalez, C.A.; Agudo, A.; Riboli, E. Intake of specific carotenoids and flavonoids and the risk of gastric cancer in Spain. Cancer Causes Control 1999, 10, 71–75. [Google Scholar]
- Leung, H.W.; Lin, C.J.; Hour, M.J.; Yang, W.H.; Wang, M.Y.; Lee, H.Z. Kaempferol induces apoptosis in human lung non-small carcinoma cells accompanied by an induction of antioxidant enzymes. Food Chem. Toxicol 2007, 45, 2005–2013. [Google Scholar]
- Nguyen, T.T.; Tran, E.; Ong, C.K.; Lee, S.K.; Do, P.T.; Huynh, T.T.; Nguyen, T.H.; Lee, J.J.; Tan, Y.; Ong, C.S.; et al. Kaempferol-induced growth inhibition and apoptosis in A549 lung cancer cells is mediated by activation of MEK-MAPK. J. Cell. Physiol 2003, 197, 110–121. [Google Scholar]
- Zhang, Q.; Zhao, X.H.; Wang, Z.J. Flavones and flavonols exert cytotoxic effects on a human oesophageal adenocarcinoma cell line (OE33) by causing G2/M arrest and inducing apoptosis. Food Chem. Toxicol 2008, 46, 2042–2053. [Google Scholar]
- De Leo, M.; Braca, A.; Sanogo, R.; Cardile, V.; DeTommasi, N.; Russo, A. Antiproliferative activity of Pteleopsis suberosa leaf extract and its flavonoid components in human prostate carcinoma cells. Planta Med 2006, 72, 604–610. [Google Scholar]
- Yoshida, T.; Konishi, M.; Horinaka, M.; Yasuda, T.; Goda, A.E.; Taniguchi, H.; Yano, K.; Wakada, M.; Sakai, T. Kaempferol sensitizes colon cancer cells to TRAIL-induced apoptosis. Biochem. Biophys. Res. Commun 2008, 375, 129–133. [Google Scholar]
- Mutoh, M.; Takahashi, M.; Fukuda, K.; Matsushima-Hibiya, Y.; Mutoh, H.; Sugimura, T.; Wakabayashi, K. Suppression of cyclooxygenase-2 promoter-dependent transcriptional activity in colon cancer cells by chemopreventive agents with a resorcin-type structure. Carcinogenesis 2000, 21, 959–963. [Google Scholar]
- Kang, J.W.; Kim, J.H.; Song, K.; Kim, S.H.; Yoon, J.H.; Kim, K.S. Kaempferol and quercetin, components of Ginkgo biloba extract (EGb 761), induce caspase-3-dependent apoptosis in oral cavity cancer cells. Phytother. Res 2010, 24, S77–S82. [Google Scholar]
- Choi, E.J.; Ahn, W.S. Kaempferol induced the apoptosis via cell cycle arrest in human breast cancer MDA-MB-453 cells. Nutr. Res. Pract 2008, 2, 322–325. [Google Scholar]
- Violette, S.; Poulain, L.; Dussaulx, E.; Pepin, D.; Faussat, A.M.; Chambaz, J.; Lacorte, J.M.; Staedel, C.; Lesuffleur, T. Resistance of colon cancer cells to long-term 5-fluorouracil exposure is correlated to the relative level of Bcl-2 and Bcl-X(L) in addition to Bax and p53 status. Int. J. Cancer 2002, 98, 498–504. [Google Scholar]
- Li, W.; Du, B.; Wang, T.; Wang, S.; Zhang, J. Kaempferol induces apoptosis in human HCT116 colon cancer cells via the Ataxia-Telangiectasia Mutated-p53 pathway with the involvement of p53 Upregulated Modulator of Apoptosis. Chem. Biol. Interact 2009, 177, 121–127. [Google Scholar]
- Cho, H.J.; Park, J.H.Y. Kaempferol Induces cell cycle arrest in HT-29 human colon cancer cells. J. Cancer Prev 2013, 18, 257–263. [Google Scholar]
- Matlashewski, G.; Lamb, P.; Pim, D.; Peacock, J.; Crawford, L.; Benchimol, S. Isolation and characterization of a human p53 cDNA clone: Expression of the human p53 gene. EMBO J 1984, 3, 3257–3262. [Google Scholar]
- Naccarati, A.; Polakova, V.; Pardini, B.; Vodickova, L.; Hemminki, K.; Kumar, R.; Vodicka, P. Mutations and polymorphisms in tp53 gene—An overview on the role in colorectal cancer. Mutagenesis 2012, 27, 211–218. [Google Scholar]
- Sampson, L.; Rimm, E.; Hollman, P.C.; de Vries, J.H.; Katan, M.B. Flavonol and flavone intakes in US health professionals. J. Am. Diet. Assoc 2002, 102, 1414–1420. [Google Scholar]
- Arai, Y.; Watanabe, S.; Kimira, M.; Shimoi, K.; Mochizuki, R.; Kinae, N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J. Nutr 2000, 130, 2243–2250. [Google Scholar]
- Marfe, G.; Tafani, M.; Indelicato, M.; Sinibaldi-Salimei, P.; Reali, V.; Pucci, B.; Fini, M.; Russo, M.A. Kaempferol induces apoptosis in two different cell lines via Akt inactivation, Bax and SIRT3 activation, and mitochondrial dysfunction. J. Cell. Biochem 2009, 106, 643–650. [Google Scholar]
- Kasibhatla, S.; Gourdeau, H.; Meerovitch, K.; Drewe, J.; Reddy, S.; Qiu, L.; Zhang, H.; Bergeron, F.; Bouffard, D.; Yang, Q.; et al. Discovery and mechanism of action of a novel series of apoptosis inducers with potential vascular targeting activity. Mol. Cancer Ther 2004, 3, 1365–1374. [Google Scholar]
- Nicholson, D.W. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell. Death Differ 1999, 6, 1028–1042. [Google Scholar]
- Burkle, A.; Brabeck, C.; Diefenbach, J.; Beneke, S. The emerging role of poly(ADP-ribose) polymerase-1 in longevity. Int. J. Biochem. Cell Biol 2005, 37, 1043–1053. [Google Scholar]
- Andrabi, S.A.; Kim, N.S.; Yu, S.W.; Wang, H.; Koh, D.W.; Sasaki, M.; Klaus, J.A.; Otsuka, T.; Zhang, Z.; Koehler, R.C.; et al. Poly(ADP-ribose) (PAR) polymer is a death signal. Proc. Natl. Acad. Sci. USA 2006, 103, 18308–18313. [Google Scholar]
- Yu, S.W.; Andrabi, S.A.; Wang, H.; Kim, N.S.; Poirier, G.G.; Dawson, T.M.; Dawson, V.L. Apoptosis-inducing factor mediates poly(ADP-ribose) (PAR) polymer-induced cell death. Proc. Natl. Acad. Sci. USA 2006, 103, 18314–18319. [Google Scholar]
- Huerta, S.; Goulet, E.J.; Huerta-Yepez, S.; Livingston, E.H. Screening and detection of apoptosis. J. Surg. Res 2007, 139, 143–156. [Google Scholar]
- Boise, L.H.; Gonzalez-Garcia, M.; Postema, C.E.; Ding, L.; Lindsten, T.; Turka, L.A.; Mao, X.; Nunez, G.; Thompson, C.B. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 1993, 74, 597–608. [Google Scholar]
- Sen, P.; Mukherjee, S.; Ray, D.; Raha, S. Involvement of the Akt/PKB signaling pathway with disease processes. Mol. Cell. Biochem 2003, 253, 241–246. [Google Scholar]
- Kim, E.J.; Kang, I.J.; Cho, H.J.; Kim, W.K.; Ha, Y.L.; Park, J.H. Conjugated linoleic acid downregulates insulin-like growth factor-I receptor levels in HT-29 human colon cancer cells. J. Nutr 2003, 133, 2675–2681. [Google Scholar]
- Cho, H.J.; Kim, W.K.; Kim, E.J.; Jung, K.C.; Park, S.; Lee, H.S.; Tyner, A.L.; Park, J.H. Conjugated linoleic acid inhibits cell proliferation and ErbB3 signaling in HT-29 human colon cell line. Am. J. Physiol. Gastrointest. Liver Physiol 2003, 284, G996–G1005. [Google Scholar]
- Cheng, E.H.; Wei, M.C.; Weiler, S.; Flavell, R.A.; Mak, T.W.; Lindsten, T.; Korsmeyer, S.J. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol. Cell 2001, 8, 705–711. [Google Scholar]
- Wei, M.C.; Zong, W.X.; Cheng, E.H.; Lindsten, T.; Panoutsakopoulou, V.; Ross, A.J.; Roth, K.A.; MacGregor, G.R.; Thompson, C.B.; Korsmeyer, S.J. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science 2001, 292, 727–730. [Google Scholar]
- Garrido, C.; Galluzzi, L.; Brunet, M.; Puig, P.E.; Didelot, C.; Kroemer, G. Mechanisms of cytochrome c release from mitochondria. Cell. Death Differ 2006, 13, 1423–1433. [Google Scholar]
- Ozoren, N.; El-Deiry, W.S. Cell surface death receptor signaling in normal and cancer cells. Semin. Cancer Biol 2003, 13, 135–147. [Google Scholar]
- Jin, Z.; El-Deiry, W.S. Overview of cell death signaling pathways. Cancer Biol. Ther 2005, 4, 139–163. [Google Scholar]
- Jung, J.I.; Lim, S.S.; Choi, H.J.; Cho, H.J.; Shin, H.K.; Kim, E.J.; Chung, W.Y.; Park, K.K.; Park, J.H. Isoliquiritigenin induces apoptosis by depolarizing mitochondrial membranes in prostate cancer cells. J. Nutr. Biochem 2006, 17, 689–696. [Google Scholar]
- De Proost, I.; Pintelon, I.; Brouns, I.; Kroese, A.B.; Riccardi, D.; Kemp, P.J.; Timmermans, J.P.; Adriaensen, D. Functional live cell imaging of the pulmonary neuroepithelial body microenvironment. Am. J. Respir. Cell Mol. Biol 2008, 39, 180–189. [Google Scholar]
- Cho, H.J.; Kwon, G.T.; Park, J.H. trans-10,cis-12 Conjugated linoleic acid induces depolarization of mitochondrial membranes in HT-29 human colon cancer cells: A possible mechanism for induction of apoptosis. J. Med. Food 2009, 12, 952–958. [Google Scholar]
- DuPont, M.S.; Day, A.J.; Bennett, R.N.; Mellon, F.A.; Kroon, P.A. Absorption of kaempferol from endive, a source of kaempferol-3-glucuronide, in humans. Eur. J. Clin. Nutr 2004, 58, 947–954. [Google Scholar]
- Barve, A.; Chen, C.; Hebbar, V.; Desiderio, J.; Saw, C.L.; Kong, A.N. Metabolism, oral bioavailability and pharmacokinetics of chemopreventive kaempferol in rats. Biopharm. Drug Dispos 2009, 30, 356–365. [Google Scholar]



© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lee, H.S.; Cho, H.J.; Yu, R.; Lee, K.W.; Chun, H.S.; Park, J.H.Y. Mechanisms Underlying Apoptosis-Inducing Effects of Kaempferol in HT-29 Human Colon Cancer Cells. Int. J. Mol. Sci. 2014, 15, 2722-2737. https://doi.org/10.3390/ijms15022722
Lee HS, Cho HJ, Yu R, Lee KW, Chun HS, Park JHY. Mechanisms Underlying Apoptosis-Inducing Effects of Kaempferol in HT-29 Human Colon Cancer Cells. International Journal of Molecular Sciences. 2014; 15(2):2722-2737. https://doi.org/10.3390/ijms15022722
Chicago/Turabian StyleLee, Hyun Sook, Han Jin Cho, Rina Yu, Ki Won Lee, Hyang Sook Chun, and Jung Han Yoon Park. 2014. "Mechanisms Underlying Apoptosis-Inducing Effects of Kaempferol in HT-29 Human Colon Cancer Cells" International Journal of Molecular Sciences 15, no. 2: 2722-2737. https://doi.org/10.3390/ijms15022722




