Evaluation of Osseointegration of Titanium Alloyed Implants Modified by Plasma Polymerization
Abstract
:1. Introduction
2. Results and Discussion
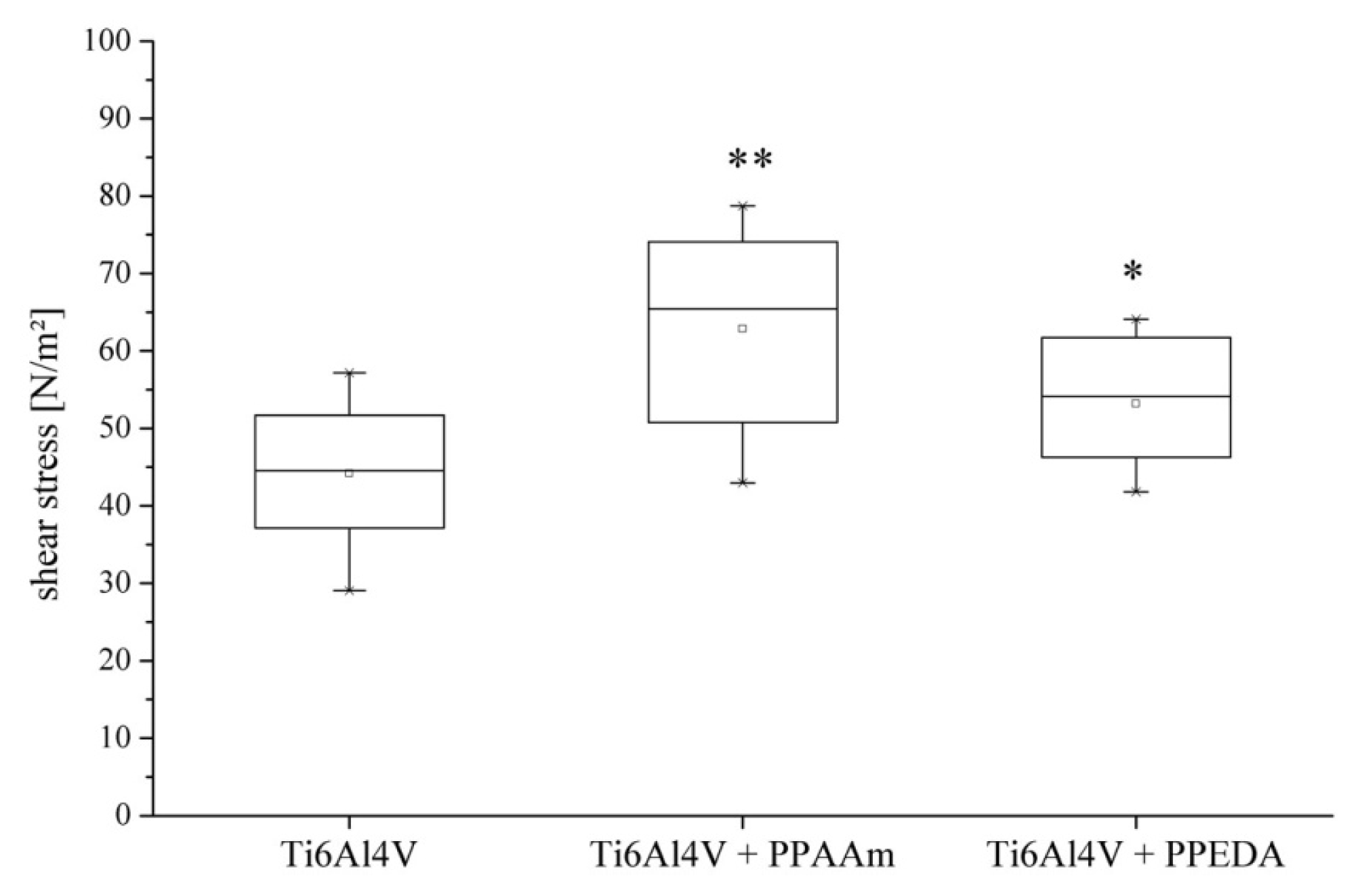
2.1. Cell Adhesion
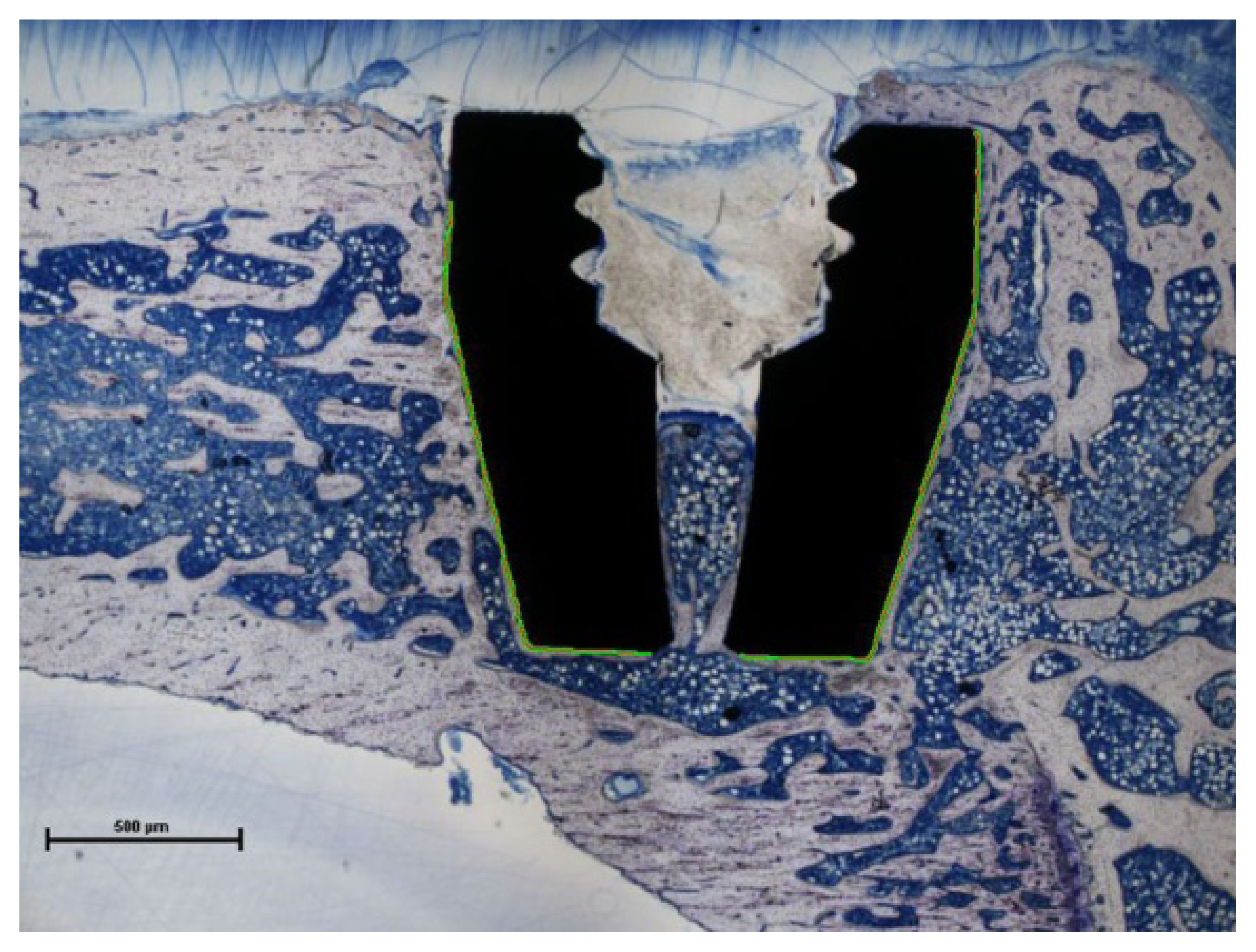
2.2. Bone-to-Implant Contact
2.3. Discussion
3. Experimental Section
3.1. Implant Material and Surface Coatings
3.2. Cell Adhesion
3.3. Animal Testing
3.4. Histomorphometric Analysis
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Jones, C.A.; Beaupre, L.A.; Johnston, D.W.C.; Suarez-Almazor, M.E. Total joint arthroplasties: Current concepts of patient outcomes after surgery. Rheum. Dis. Clin. N. Am 2007, 33, 71–86. [Google Scholar]
- Goodman, S.B. Wear particles, periprosthetic osteolysis and the immune system. Biomaterials 2007, 28, 5044–5048. [Google Scholar]
- Bauer, T.W.; Schils, J. The pathology of total joint arthroplasty. Skelet. Radiol 1999, 28, 483–497. [Google Scholar]
- Kurtz, S.M.; Lau, E.; Watson, H.; Schmier, J.K.; Parvizi, J. Economic burden of periprosthetic joint infection in the United States. J. Arthroplast 2012, 27. [Google Scholar] [CrossRef]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar]
- Gristina, A.G.; Naylor, P.; Myrvik, Q. Infections from biomaterials and implants: A race for the surface. Med. Prog. Technol 1988, 14, 205–224. [Google Scholar]
- Neoh, K.G.; Hu, X.; Zheng, D.; Kang, E.T. Balancing osteoblast functions and bacterial adhesion on functionalized titanium surfaces. Biomaterials 2012, 33, 2813–2822. [Google Scholar]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res 2009, 20, 172–184. [Google Scholar]
- Junker, R.; Dimakis, A.; Thoneick, M.; Jansen, J.A. Effects of implant surface coatings and composition on bone integration: A systematic review. Clin. Oral Implant. Res 2009, 20, 185–206. [Google Scholar]
- Finke, B.; Hempel, F.; Testrich, H.; Artemenko, A.; Rebl, H.; Kylián, O.; Meichsner, J.; Biederman, H.; Nebe, B.; Weltmann, K.D.; et al. Plasma processes for cell-adhesive titanium surfaces based on nitrogen-containing coatings. Surf. Coat. Technol 2011, 205, S520–S524. [Google Scholar]
- Finke, B.; Luethen, F.; Schroeder, K.; Mueller, P.D.; Bergemann, C.; Frant, M.; Ohl, A.; Nebe, B.J. The effect of positively charged plasma polymerization on initial osteoblastic focal adhesion on titanium surfaces. Biomaterials 2007, 28, 4521–4534. [Google Scholar]
- Rebl, H.; Finke, B.; Lange, R.; Weltmann, K.-D.; Nebe, J.B. Impact of plasma chemistry versus titanium surface topography on osteoblast orientation. Acta Biomater 2012, 8, 3840–3851. [Google Scholar]
- Nebe, B.; Finke, B.; Lüthen, F.; Bergemann, C.; Schröder, K.; Rychly, J.; Liefeith, K.; Ohl, A. Improved initial osteoblast functions on amino-functionalized titanium surfaces. Biomol. Eng 2007, 24, 447–454. [Google Scholar]
- Hoene, A.; Walschus, U.; Patrzyk, M.; Finke, B.; Lucke, S.; Nebe, B.; Schroeder, K.; Ohl, A.; Schlosser, M. In vivo investigation of the inflammatory response against allylamine plasma polymer coated titanium implants in a rat model. Acta Biomater 2010, 6, 676–683. [Google Scholar]
- Walschus, U.; Hoene, A.; Patrzyk, M.; Finke, B.; Polak, M.; Lucke, S.; Nebe, B.; Schroeder, K.; Podbielski, A.; Wilhelm, L.; et al. Serum profile of pro- and anti-inflammatory cytokines in rats following implantation of low-temperature plasma-modified titanium plates. J. Mater. Sci 2012, 23, 1299–1307. [Google Scholar]
- Hoene, A.; Patrzyk, M.; Walschus, U.; Straňák, V.; Hippler, R.; Testrich, H.; Meichsner, J.; Finke, B.; Rebl, H.; Nebe, B.; et al. In vivo examination of the local inflammatory response after implantation of Ti6Al4V samples with a combined low-temperature plasma treatment using pulsed magnetron sputtering of copper and plasma-polymerized ethylenediamine. J. Mater. Sci 2013, 24, 761–771. [Google Scholar]
- De Groot, K.; Wolke, J.G.C.; Jansen, J.A. Calcium phosphate coatings for medical implants. Proc. Inst. Mech. Eng. H 1998, 212, 137–147. [Google Scholar]
- Daculsi, G. Biphasic calcium phosphate concept applied to artificial bone, implant coating and injectable bone substitute. Biomaterials 1998, 19, 1473–1478. [Google Scholar]
- Nguyen, H.Q.; Deporter, D.A.; Pilliar, R.M.; Valiquette, N.; Yakubovich, R. The effect of sol-gel-formed calcium phosphate coatings on bone ingrowth and osteoconductivity of porous-surfaced Ti alloy implants. Biomaterials 2004, 25, 865–876. [Google Scholar]
- Schliephake, H.; Scharnweber, D.; Dard, M.; Röβler, S.; Sewing, A.; Hüttmann, C. Biological performance of biomimetic calcium phosphate coating of titanium implants in the dog mandible. J. Biomed. Mater. Res. A 2003, 64A, 225–234. [Google Scholar]
- Xu, L.; Pan, F.; Yu, G.; Yang, L.; Zhang, E.; Yang, K. In vitro and in vivo evaluation of the surface bioactivity of a calcium phosphate coated magnesium alloy. Biomaterials 2009, 30, 1512–1523. [Google Scholar]
- Manjubala, I.; Sivakumar, M.; Sureshkumar, R.V.; Sastry, T.P. Bioactivity and osseointegration study of calcium phosphate ceramic of different chemical composition. J. Biomed. Mater. Res 2002, 63, 200–208. [Google Scholar]
- Morris, H.F.; Ochi, S.; Spray, J.R.; Olson, J.W. Periodontal-type measurements associated with hydroxyapatite-coated and non-HA-coated implants: Uncovering to 36 months. Ann. Periodontol 2000, 5, 56–67. [Google Scholar]
- Lee, J.J.; Rouhfar, L.; Beirne, O.R. Survival of hydroxyapatite-coated implants: A meta-analytic review. J. Oral Maxillofac. Surg 2000, 58, 1372–1379. [Google Scholar]
- Yu-Liang, C.; Lew, D.; Park, J.B.; Keller, J.C. Biomechanical and morphometric analysis of hydroxyapatite-coated implants with varying crystallinity. J. Oral Maxillofac. Surg 1999, 57, 1096–1108. [Google Scholar]
- Chua, P.-H.; Neoh, K.-G.; Kang, E.-T.; Wang, W. Surface functionalization of titanium with hyaluronic acid/chitosan polyelectrolyte multilayers and RGD for promoting osteoblast functions and inhibiting bacterial adhesion. Biomaterials 2008, 29, 1412–1421. [Google Scholar]
- Shi, Z.; Neoh, K.G.; Kang, E.T.; Poh, C.; Wang, W. Bacterial adhesion and osteoblast function on titanium with surface-grafted chitosan and immobilized RGD peptide. J. Biomed. Mater. Res. A 2008, 86A, 865–872. [Google Scholar]
- Maddikeri, R.R.; Tosatti, S.; Schuler, M.; Chessari, S.; Textor, M.; Richards, R.G.; Harris, L.G. Reduced medical infection related bacterial strains adhesion on bioactive RGD modified titanium surfaces: A first step toward cell selective surfaces. J. Biomed. Mater. Res. A 2008, 84A, 425–435. [Google Scholar]
- Ferris, D.M.; Moodie, G.D.; Dimond, P.M.; Giorani, C.W.D.; Ehrlich, M.G.; Valentini, R.F. RGD-coated titanium implants stimulate increased bone formation in vivo. Biomaterials 1999, 20, 2323–2331. [Google Scholar]
- Schliephake, H.; Scharnweber, D.; Dard, M.; Rößler, S.; Sewing, A.; Meyer, J.; Hoogestraat, D. Effect of RGD peptide coating of titanium implants on periimplant bone formation in the alveolar crest. Clin. Oral Implant. Res 2002, 13, 312–319. [Google Scholar]
- Kroese-Deutman, H.; den Dolder, J.V.; Spauwen, P.; Jansen, J. Influence of RGD-loaded titanium implants on bone formation in vivo. Tissue Eng. 2005, 11, 1867–1875. [Google Scholar]
- Elmengaard, B.; Bechtold, J.E.; Søballe, K. In vivo study of the effect of RGD treatment on bone ongrowth on press-fit titanium alloy implants. Biomaterials 2005, 26, 3521–3526. [Google Scholar]
- Morra, M. Biochemical modification of titanium surfaces: Peptides and ECM proteins. Eur. Cells Mater 2006, 12, 1–15. [Google Scholar]
- Li, R.H.; Wozney, J.M. Delivering on the promise of bone morphogenetic proteins. Trends Biotechnol 2001, 19, 255–265. [Google Scholar]
- Harwood, P.J.; Giannoudis, P.V. Application of bone morphogenetic proteins in orthopaedic practice: Their efficacy and side effects. Expert Opin. Drug Saf 2005, 4, 75–89. [Google Scholar]
- Shi, Z.; Neoh, K.G.; Kang, E.T.; Poh, C.K.; Wang, W. Surface functionalization of titanium with carboxymethyl chitosan and immobilized bone morphogenetic protein-2 for enhanced osseointegration. Biomacromolecules 2009, 10, 1603–1611. [Google Scholar]
- Shi, Z.; Neoh, K.G.; Kang, E.-T.; Poh, C.; Wang, W. Titanium with surface-grafted dextran and immobilized bone morphogenetic protein-2 for inhibition of bacterial adhesion and enhancement of osteoblast functions. Tissue Eng. A 2008, 15, 417–426. [Google Scholar]
- Testrich, H.; Rebl, H.; Finke, B.; Hempel, F.; Nebe, B.; Meichsner, J. Aging effects of plasma polymerized ethylenediamine (PPEDA) thin films on cell-adhesive implant coatings. Mater. Sci. Eng. C 2013, 33, 3875–3880. [Google Scholar]
- Thermal Spraying; Determination of Tensile Adhesive Strength; German version EN 582:1993. Available online: http://www.beuth.de/de/norm/din-en-582/2228453 (accessed on 10 February 2014).
- Standard Test Method for Tension Testing of Calcium Phosphate and Metallic Coatings. Available online: http://www.astm.org/Standards/F1147.htm (accessed on 28 January 2014).
- Fritsche, A.; Luethen, F.; Lembke, U.; Finke, B.; Zietz, C.; Rychly, J.; Mittelmeier, W.; Bader, R. Measuring bone cell adhesion on implant surfaces using a spinning disc device. Materialwissenschaft und Werkstofftechnik 2010, 41, 83–88. [Google Scholar]
- Fritsche, A.; Luethen, F.; Lembke, U.; Zietz, C.; Rychly, J.; Mittelmeier, W.; Bader, R. Time-dependent adhesive interaction of osteoblastic cells with polished titanium alloyed implant surfaces. J. Appl. Biomater. Funct. Mater 2013, 11, 1–8. [Google Scholar]
- Haenle, M.; Zietz, C.; Lindner, T.; Arndt, K.; Vetter, A.; Mittelmeier, W.; Podbielski, A.; Bader, R. A model of implant-associated infection in the tibial metaphysis of rats. Sci. World J 2013, 2013, 8. [Google Scholar]


© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gabler, C.; Zietz, C.; Göhler, R.; Fritsche, A.; Lindner, T.; Haenle, M.; Finke, B.; Meichsner, J.; Lenz, S.; Frerich, B.; et al. Evaluation of Osseointegration of Titanium Alloyed Implants Modified by Plasma Polymerization. Int. J. Mol. Sci. 2014, 15, 2454-2464. https://doi.org/10.3390/ijms15022454
Gabler C, Zietz C, Göhler R, Fritsche A, Lindner T, Haenle M, Finke B, Meichsner J, Lenz S, Frerich B, et al. Evaluation of Osseointegration of Titanium Alloyed Implants Modified by Plasma Polymerization. International Journal of Molecular Sciences. 2014; 15(2):2454-2464. https://doi.org/10.3390/ijms15022454
Chicago/Turabian StyleGabler, Carolin, Carmen Zietz, Rebecca Göhler, Andreas Fritsche, Tobias Lindner, Maximilian Haenle, Birgit Finke, Jürgen Meichsner, Solvig Lenz, Bernhard Frerich, and et al. 2014. "Evaluation of Osseointegration of Titanium Alloyed Implants Modified by Plasma Polymerization" International Journal of Molecular Sciences 15, no. 2: 2454-2464. https://doi.org/10.3390/ijms15022454





