Expression of a Deschampsia antarctica Desv. Polypeptide with Lipase Activity in a Pichia pastoris Vector
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Analysis of the Lip3F9 Polypeptide
2.2. Expression of the Lip3F9 Polypeptide in Pichia pastoris
2.3. Determination of Range and Optimum Temperature
2.4. Determination of Tolerance Detergent
3. Experimental Section
3.1. Screening of the cDNA Library for D. antarctica
3.2. Sequence Analysis
3.3. Vector Construction and Transformation
3.4. Lipase Expression in Pichia pastoris
3.5. Induction of pPICZαB-Lip3F9 Expression
3.6. Measurement of Lipase Activity
3.7. Determination of Range and Optimum Temperature
3.8. Determination of Detergent Tolerance
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Rosa, L.; Vaz, A.; Caligiorne, R.; Campolina, S.; Rosa, C. Endophytic fungi associated with the Antarctic grass Deschampsia antarctica Desv. (Poaceae). Polar Biol 2009, 32, 161–167. [Google Scholar]
- Deming, J. Psychrophiles and polar regions. Curr. Opin. Microbiol 2002, 5, 301–309. [Google Scholar]
- Singh, J.; Dubey, A.; Singh, R. Antarctic terrestrial ecosystem and role of pigments in enhanced UV-B radiations. Rev. Environ. Sci. Biotechnol 2011, 10, 63–77. [Google Scholar]
- Barrientos-Díaz, L.; Gidekel, M.; Gutierréz-Moraga, A. Characterization of rhizospheric bacteria isolated from Deschampsia antarctica Desv. World J. Microb. Biotechnol 2008, 24, 2289–2296. [Google Scholar]
- Laws, R.M. Antarctic Ecology; British Antarctic Survey: Academic Press, London, UK, 1984. [Google Scholar]
- Mrdakovi, M.; Lazarevi, J.; Peric-Mataruga, V.; Ilijin, L.; Vlahovic, M. Partial characterization of a lipase from gypsy moth (Lymantria dispar L.) larval midgut. Folia Biol 2008, 56, 103–110. [Google Scholar]
- Joseph, B.; Ramteke, P.; Thomas, G.; Shrivastava, N. Standard review cold-active microbial lipases: A versatile tool for industrial applications. Microbiol. Mol. Biol. Rev 2007, 2, 39–48. [Google Scholar]
- Chepyshko, H.; Lai, C.-P.; Huang, L.-M.; Liu, J.-H.; Shaw, J.-F. Multifunctionality and diversity of GDSL esterase/lipase gene family in rice (Orysa sativa L. japonica) genome: New insights from bioinformatics analysis. BMC Genomics 2012, 13, 309. [Google Scholar]
- Ling, L. Sequence analysis of GDSL lipase gene family in Arabidopsis thaliana. Pak. J. Biol. Sci 2008, 11, 736–767. [Google Scholar]
- Sánchez-Venegas, J.; Dinamarca, J.; Gutiérrez-Moraga, A.; Gidekel, M. Molecular characterization of a cDNA encoding Cu/Zn superoxide dismutase from Deschampsia antarctica and its expression regulated by cold and UV stresses. BMC Res. Notes 2009, 2, 198–204. [Google Scholar]
- Sánchez-Venegas, J.; Navarrete, A.; Dinamarca, J.; Bravo, L.A.; Gutierrez, A.; Gidekel, M. Cloning and constitutive expression of Deschampsia antarctica Cu/Zn superoxide dismutase in Pichia pastoris. BMC Res. Notes 2009, 2, 207–215. [Google Scholar]
- González-Bacerio, J.; Moreno-Medina, V.R.; del Monte Martínez, A. Las lipasas: Enzimas con potencial para el desarrollo de biocatalizadores inmovilizados por adsorción interfacial. Rev. Colomb. Biotechnol 2010, 12, 113–140. [Google Scholar]
- Rotticci-Mulder, J.C.; Gustavsson, M.; Holmquist, M.; Hult, K.; Martinelle, M. Expression in Pichia pastoris of Candida antarctica Lipase A and Lipase B fused to a cellulose-binding domain. Protein Expr. Purif 2001, 21, 386–392. [Google Scholar]
- Tang, S.-J.; Shaw, J.-F.; Sun, K.-H.; Sun, G.-H.; Chang, T.-Y.; Lin, C.-K.; Lo, Y.-C.; Lee, G.-C. Recombinant expression and characterization of the Candida rugosa lip4 lipase in Pichia pastoris: Comparison of glycosylation, activity, and stability. Arch. Biochem. Biophys 2001, 387, 93–98. [Google Scholar]
- Yu, M.; Wen, S.; Tan, T. Enhancing production of Yarrowia lipolytica lipase Lip2 in Pichia pastoris. Eng. Life Sci 2010, 10, 458–464. [Google Scholar]
- Belguith, H.; Fattouch, S.; Jridi, T.; Hamida, B. Immunopurification of a rape (Brassica napus L.) seedling lipase. Afr. J. Biotechnol 2009, 3, 356–365. [Google Scholar]
- Bhumibhamon, O.; Jinda, J.; Fungthong, S. Isolation and characterization of Pseudomonas sp. KLB1 lipase from high fat wastewater. Kasetsart J. (Nat. Sci.) 2003, 37, 176–185. [Google Scholar]
- De Caro, J.; Rouimi, P.; Rovery, M. Hydrolysis of p-nitrophenyl acetate by the peptide chain fragment 336–449 of porcine pancreatic lipase. Eur. J. Biochem 1986, 158, 601–607. [Google Scholar]
- Ejedegba, B.O.; Onyeneke, E.C.; Oviasogie, P.O. Characteristics of lipase isolated from coconut (Cocos nucifera linn) seed under different nutrient treatments. Afr. J. Biotechnol 2007, 6, 723–727. [Google Scholar]
- Sonesson, A.W.; Elofsson, U.M.; Brismar, H.; Callisen, T.H. Adsorption and mobility of a lipase at a hydrophobic surface in the presence of surfactants. Langmuir 2006, 22, 5810–5817. [Google Scholar]
- Bhardwaj, K.; Raju, A.; Rajasekharan, R. Identification, purification, and characterization of thermally stable lipase from rice bran. A new member of the (phospho) lipase family. Plant Physiol 2001, 127, 1728–1738. [Google Scholar]
- Aloulou, A.; Rodríguez, J.A.; Fernández, S.; van Oosterhout, D.; Puccinelli, D.; Carrière, F. Exploring the specific features of interfacial enzymology based on lipase studies. Biochim. Biophys. Acta 2006, 1761, 995–1013. [Google Scholar]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001. [Google Scholar]
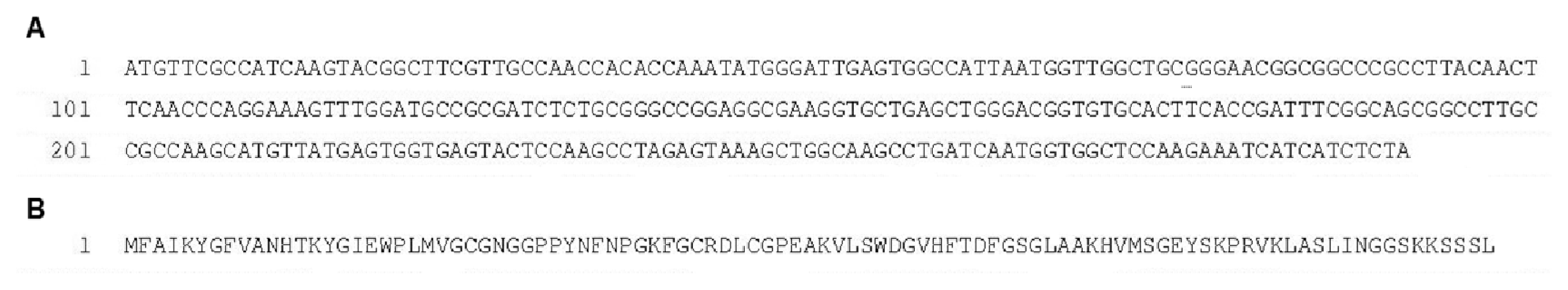


© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rabert, C.; Gutiérrez-Moraga, A.; Navarrete-Gallegos, A.; Navarrete-Campos, D.; Bravo, L.A.; Gidekel, M. Expression of a Deschampsia antarctica Desv. Polypeptide with Lipase Activity in a Pichia pastoris Vector. Int. J. Mol. Sci. 2014, 15, 2359-2367. https://doi.org/10.3390/ijms15022359
Rabert C, Gutiérrez-Moraga A, Navarrete-Gallegos A, Navarrete-Campos D, Bravo LA, Gidekel M. Expression of a Deschampsia antarctica Desv. Polypeptide with Lipase Activity in a Pichia pastoris Vector. International Journal of Molecular Sciences. 2014; 15(2):2359-2367. https://doi.org/10.3390/ijms15022359
Chicago/Turabian StyleRabert, Claudia, Ana Gutiérrez-Moraga, Alejandro Navarrete-Gallegos, Darío Navarrete-Campos, León A. Bravo, and Manuel Gidekel. 2014. "Expression of a Deschampsia antarctica Desv. Polypeptide with Lipase Activity in a Pichia pastoris Vector" International Journal of Molecular Sciences 15, no. 2: 2359-2367. https://doi.org/10.3390/ijms15022359
APA StyleRabert, C., Gutiérrez-Moraga, A., Navarrete-Gallegos, A., Navarrete-Campos, D., Bravo, L. A., & Gidekel, M. (2014). Expression of a Deschampsia antarctica Desv. Polypeptide with Lipase Activity in a Pichia pastoris Vector. International Journal of Molecular Sciences, 15(2), 2359-2367. https://doi.org/10.3390/ijms15022359




