Beneficial Effects of Melatonin Combined with Exercise on Endogenous Neural Stem/Progenitor Cells Proliferation after Spinal Cord Injury
Abstract
:1. Introduction
2. Results
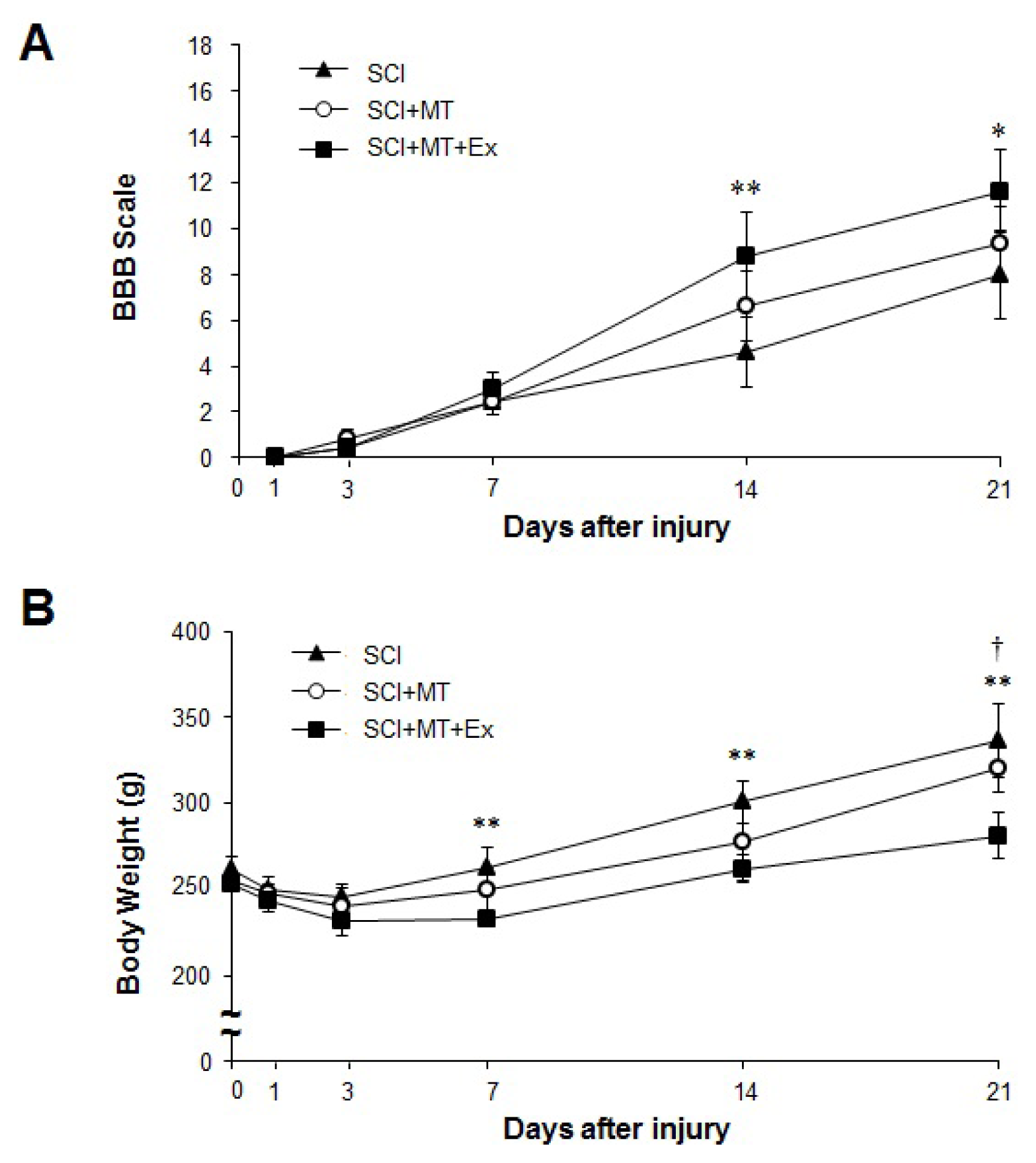
2.1. The Synergistic Effects of Melatonin and Treadmill Exercise Dual Treatment
2.2. Reconstruction of Spinal Cord Lesion through Melatonin and Treadmill Exercise Dual Treatment
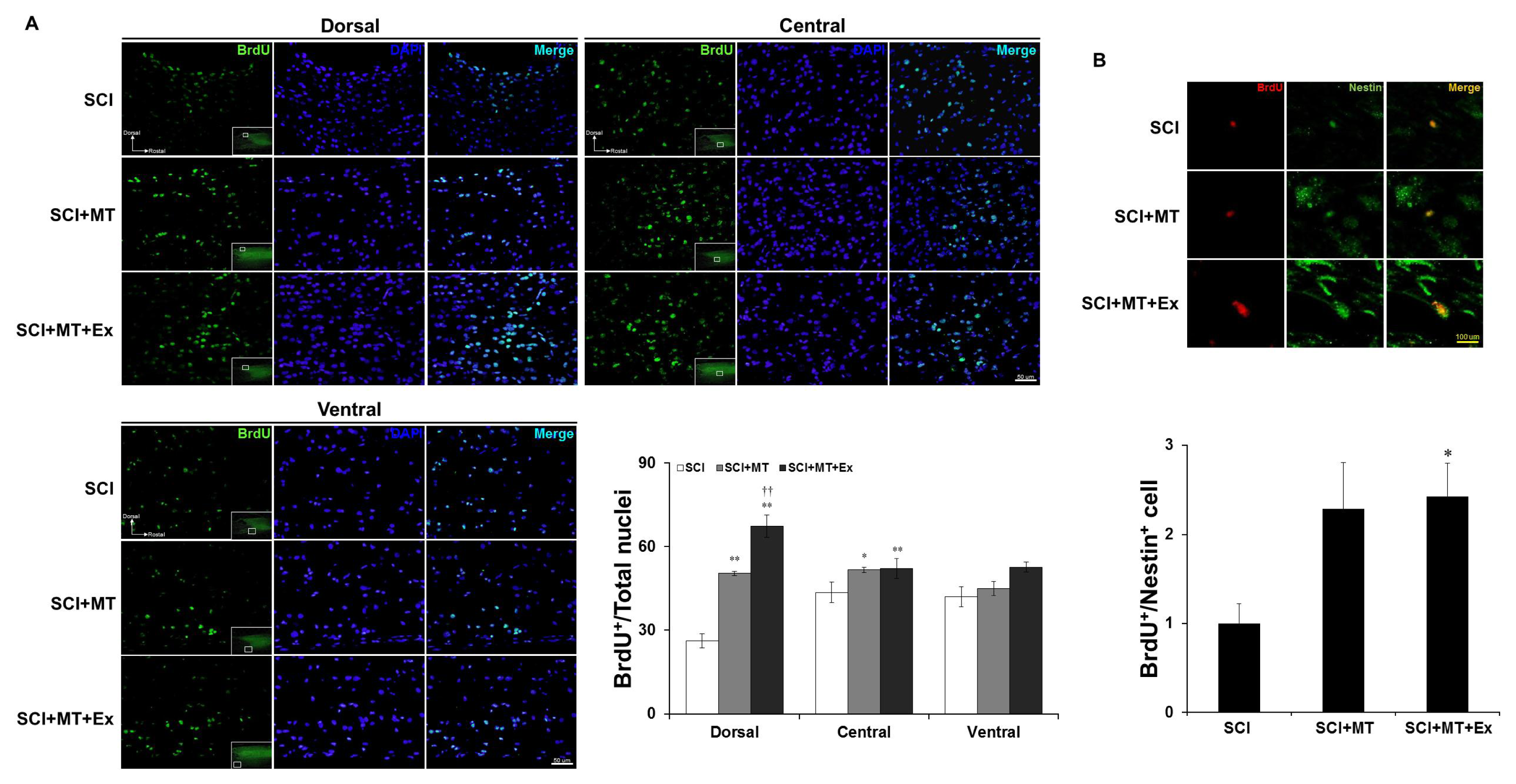
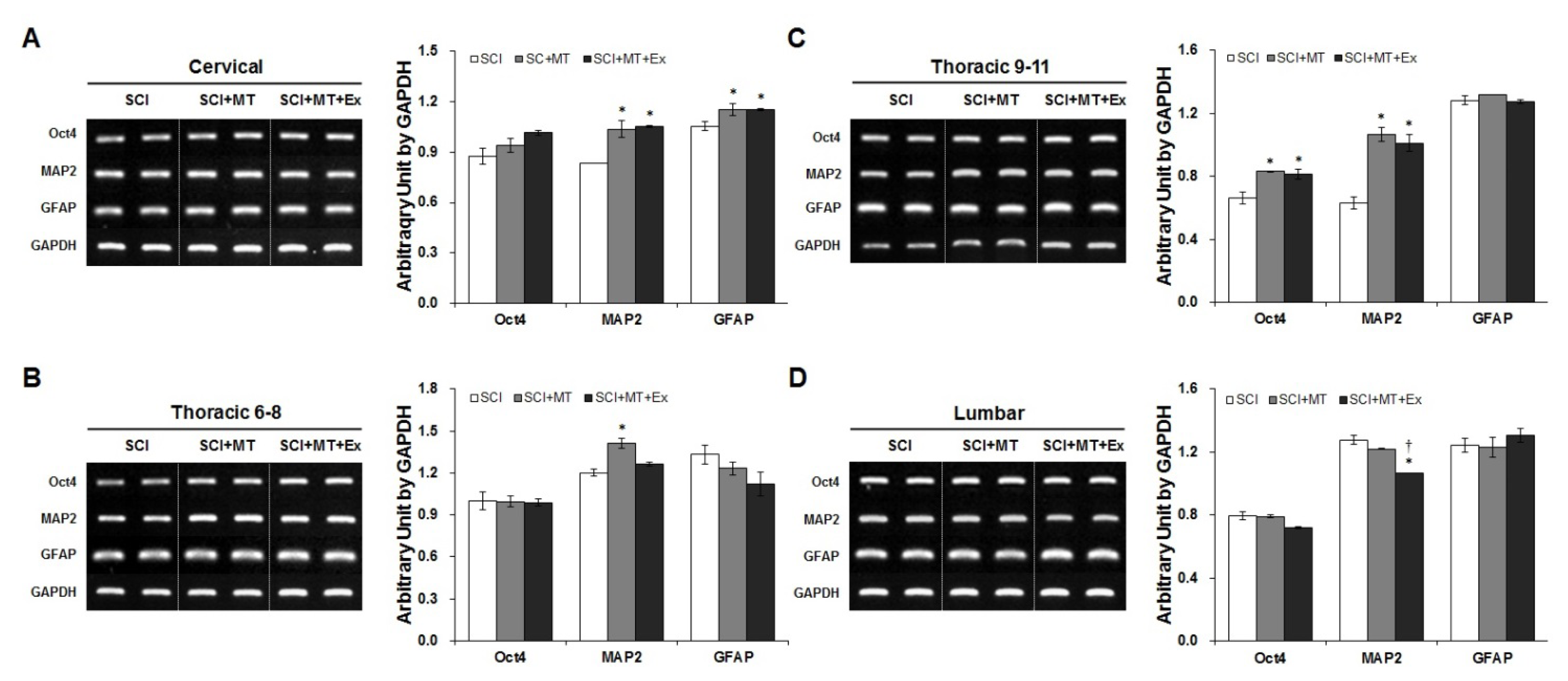
2.3. Melatonin Plus Treadmill Exercise-Induced New Differentiation for Reorganization of Spinal Cord Structure
3. Discussion
4. Experimental Section
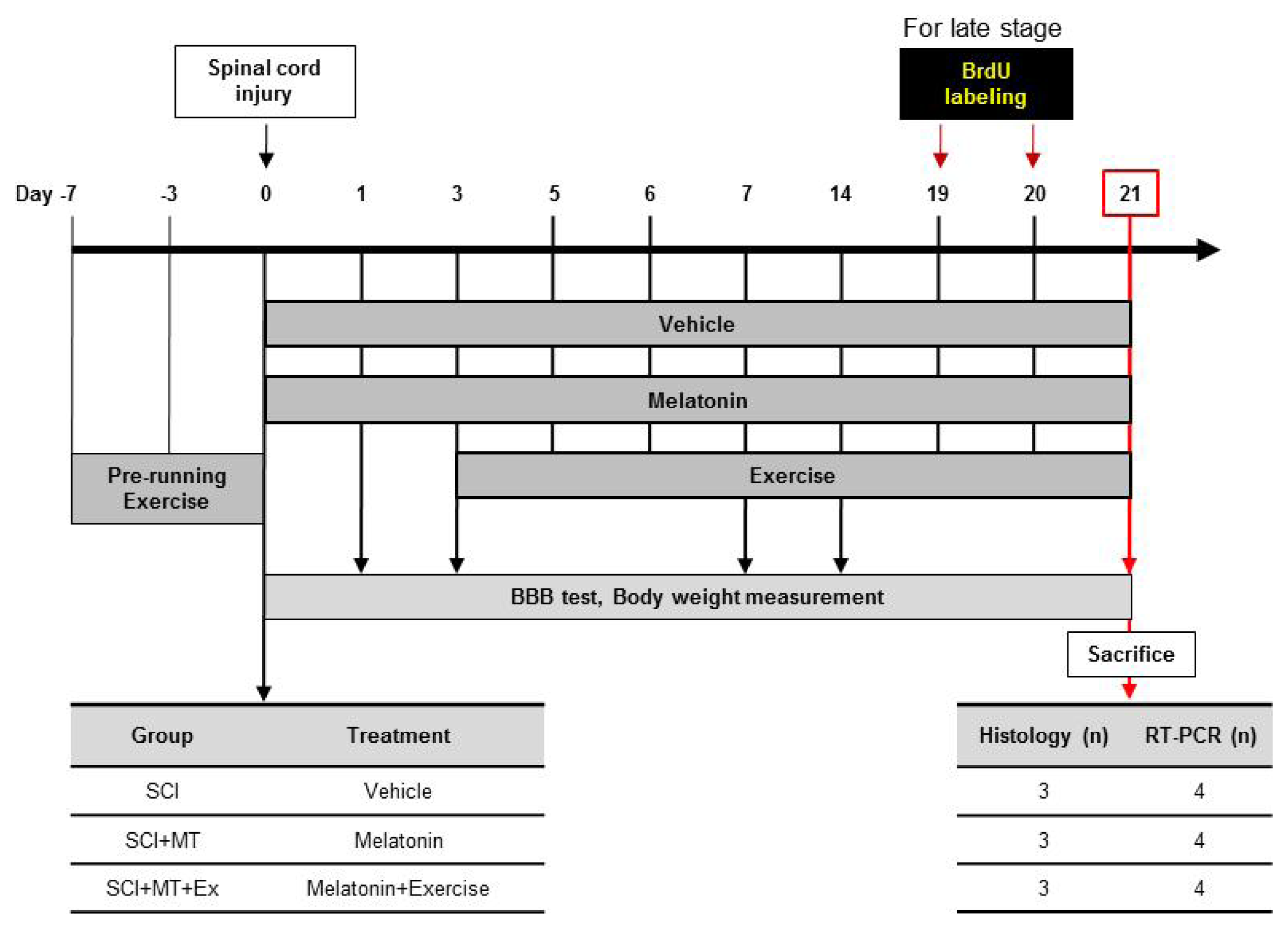
4.1. Experimental Animals
4.2. Spinal Cord Injury Animal Model
4.3. Melatonin Treatment
4.4. Treadmill Exercise
4.5. Bromodeoxyuridine (BrdU) Injection
4.6. Assessment of Motor Function (the BBB Open Field LocomotionTest)
4.7. RNA Isolation and RT-PCR Analysis
4.8. Tissue Processing and Immunohistochemistry
4.9. Golgi-Cox Staining
4.10. Statistics
5. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviation
| BBB | Basso, Beattie, and Bresnahan |
| BrdU | bromodeoxyuridine |
| GFAP | glial fibrillary acidic protein |
| GM-SCF | granulocyte-macrophage colony stimulating factor |
| LIF | leukemia inhibitory factor |
| MAP2 | microtubule-associated protein 2 |
| NG2 | neural-glial 2 |
| eNSPCs | endogenous neural stem/progenitor cells |
| Oct4 | octamer-binding transcription factor 4 |
| SCI | spinal cord injury |
References
- Snyder, E.Y.; Teng, Y.D. Stem cells and spinal cord repair. N. Engl. J. Med 2012, 366, 1940–1942. [Google Scholar]
- Hawryluk, G.W.J.; Fehlings, M.G. The center of the spinal cord may be central to its repair. Cell Stem Cell 2008, 3, 230–232. [Google Scholar]
- Barnabé-Heider, F.; Göritz, C.; Sabelström, H.; Takebayashi, H.; Pfrieger, F.W.; Meletis, K.; Frisén, J. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell 2010, 7, 470–482. [Google Scholar]
- Mothe, A.J.; Tator, C.H. Proliferation, migration, and differentiation of endogenous ependymal region stem/progenitor cells following minimal spinal cord injury in the adult rat. Neuroscience 2005, 131, 177–187. [Google Scholar]
- Ruff, C.A.; Wilcox, J.T.; Fehlings, M.G. Cell-based transplantation strategies to promote plasticity following spinal cord injury. Exp. Neurol 2012, 235, 78–90. [Google Scholar]
- Obermair, F.J.; Schroter, A.; Thallmair, M. Endogenous neural progenitor cells as therapeutic target after spinal cord injury. Physiology (Bethesda) 2008, 23, 296–304. [Google Scholar]
- Hayashi, K.; Ohta, S.; Kawakami, Y.; Toda, M. Activation of dendritic-like cells and neural stem/progenitor cells in injured spinal cord by GM-CSF. Neurosci. Res 2009, 64, 96–103. [Google Scholar]
- Azari, M.F.; Profyris, C.; Zang, D.W.; Petratos, S.; Cheema, S.S. Induction of endogenous neural precursors in mouse models of spinal cord injury and disease. Eur. J. Neurol 2005, 12, 638–648. [Google Scholar]
- Ohori, Y.; Yamamoto, S.; Nagao, M.; Sugimori, M.; Yamamoto, N.; Nakamura, K.; Nakafuku, M. Growth factor treatment and genetic manipulation stimulate neurogenesis and oligodendrogenesis by endogenous neural progenitors in the injured adult spinal cord. J. Neurosci 2006, 26, 11948–11960. [Google Scholar]
- Foret, A.; Quertainmont, R.; Botman, O.; Bouhy, D.; Amabili, P.; Brook, G.; Schoenen, J.; Franzen, R. Stem cells in the adult rat spinal cord: Plasticity after injury and treadmill training exercise. J. Neurochem 2010, 112, 762–772. [Google Scholar]
- Weiss, S.; Dunne, C.; Hewson, J.; Wohl, C.; Wheatley, M.; Peterson, A.C.; Reynolds, B.A. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J. Neurosci 1996, 16, 7599–7609. [Google Scholar]
- Mayer-Proschel, M.; Kalyani, A.J.; Mujtaba, T.; Rao, M.S. Isolation of lineage-restricted neuronal precursors from multipotent neuroepithelial stem cells. Neuron 1997, 19, 773–785. [Google Scholar]
- Parr, A.M.; Kulbatski, I.; Tator, C.H. Transplantation of adult rat spinal cord stem/progenitor cells for spinal cord injury. J. Neurotrauma 2007, 24, 835–845. [Google Scholar]
- Okano, H.; Sakaguchi, M.; Ohki, K.; Suzuki, N.; Sawanoto, K. Regeneration of the central nervous system using endogenous repair mechanisms. J. Neurochem 2007, 102, 1459–1465. [Google Scholar]
- Ke, Y.; Chi, L.; Xu, R.; Luo, C.; Gozal, D.; Liu, R. Early response of endogenous adult neural progenitor cells to acute spinal cord injury in mice. Stem Cells 2006, 24, 1011–1019. [Google Scholar]
- Johansson, C.B.; Momma, S.; Clarke, D.L.; Risling, M.; Lendahl, U.; Frisén, J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell 1999, 96, 25–34. [Google Scholar]
- Vanecek, J. Cellular mechanisms of melatonin action. Physiol. Rev 1998, 78, 687–721. [Google Scholar]
- Radio, N.M.; Doctor, J.S.; Witt-Enderby, P.A. Melatonin enhances alkaline phosphatase activity in differentiating human adult mesenchymal stem cells grown in osteogenic medium via MT2 melatonin receptors and the MEK/ERK (1/2) signaling cascade. J. Pineal Res 2006, 40, 332–342. [Google Scholar]
- Sethi, S.; Radio, N.M.; Kotlarczyk, M.P.; Chen, C.T.; Wei, Y.H.; Jockers, R.; Witt-Enderby, P.A. Determination of the minimal melatonin exposure required to induce osteoblast differentiation from human mesenchymal stem cells and these effects on downstream signaling pathways. J. Pineal Res 2010, 49, 222–238. [Google Scholar]
- Kong, X.; Li, X.; Cai, Z.; Yang, N.; Liu, Y.; Shu, J.; Pan, L.; Zuo, P. Melatonin regulates the viability and differentiation of rat midbrain neural stem cells. Cell. Mol. Neurobiol 2008, 28, 569–579. [Google Scholar]
- Rennie, K.; de Butte, M.; Pappas, B.A. Melatonin promotes neurogenesis in dentate gyrus in the pinealectomized rat. J. Pineal Res 2009, 47, 313–317. [Google Scholar]
- Ramírez-Rodríguez, G.; Klempin, F.; Babu, H.; Benítez-King, G.; Kempermann, G. Melatonin modulates cell survival of new neurons in the hippocampus of adult mice. Neuropsychopharmacology 2009, 34, 2180–2191. [Google Scholar]
- Manda, K.; Reiter, R.J. Melatonin maintains adult hippocampal neurogenesis and cognitive functions after irradiation. Prog. Neurobiol 2010, 90, 60–68. [Google Scholar]
- Fu, J.; Zhao, S.D.; Liu, H.J.; Yuan, Q.H.; Liu, S.M.; Zhang, Y.M.; Ling, E.A.; Hao, A.J. Melatonin promotes proliferation and differentiation of neural stem cells subjected to hypoxia in vitro. J. Pineal Res. 2011, 51, 104–112. [Google Scholar]
- Xu, W.P.; Shan, L.D.; Gong, S.; Chen, L.; Zhang, Y.J.; Yin, Q.Z.; Hisamitsu, T.; Jiang, X.H.; Guo, S.Y. Forced running enhances neurogenesis in the hippocampal dentate gyrus of adult rats and improves learning ability. Sheng Li Xue Bao 2006, 58, 415–420. [Google Scholar]
- Ra, S.M.; Kim, H.; Jang, M.H.; Shin, M.C.; Lee, T.H.; Lim, B.V.; Kim, C.J.; Kim, E.H.; Kim, K.M.; Kim, S.S. Treadmill running and swimming increase cell proliferation in the hippocampal dentate gyrus of rats. Neurosci. Lett 2002, 333, 123–126. [Google Scholar]
- Krityakiarana, W.; Espinosa-Jeffrey, A.; Ghiani, C.A.; Zhao, P.M.; Topaldjikian, N.; Gomez-Pinilla, F.; Yamaguchi, M.; Kotchabhakdi, N.; de Vellis, J. Voluntary exercise increases oligodendrogenesis in spinal cord. Int. J. Neurosci 2010, 120, 280–290. [Google Scholar]
- Park, K.; Lee, Y.; Park, S.; Lee, S.; Hong, Y.; Lee, S.K.; Hong, Y. Synergistic effect of melatonin on exercise-induced neuronal reconstruction and functional recovery in a spinal cord injury animal model. J. Pineal Res 2010, 48, 270–281. [Google Scholar]
- Fouad, K.; Metz, G.A.; Merkler, D.; Dietz, V.; Schwab, M.E. Treadmill training in incomplete spinal cord injured rats. Behav. Brain Res 2000, 115, 107–113. [Google Scholar]
- Hong, Y.; Palaksha, K.J.; Park, K.; Park, S.; Kim, H.D.; Reiter, R.J.; Chang, K.T. Melatonin plus exercise-based neurorehabilitative therapy for spinal cord injury. J. Pineal Res 2010, 49, 201–209. [Google Scholar]
- Darian-Smith, C. Synaptic plasticity, neurogenesis, and functional recovery after spinal cord injury. Neuroscientist 2009, 15, 149–165. [Google Scholar]
- Tan, A.M.; Waxman, S.G. Spinal cord injury, dendritic spine remodeling, and spinal memory mechanisms. Exp. Neurol 2012, 234, 142–151. [Google Scholar]
- Okano, H.; Okada, S.; Nakamura, M.; Toyama, Y. Neural stem cells and regeneration of injured spinal cord. Kidney Int 2005, 68, 1927–1931. [Google Scholar]
- Pierpaoli, W.; Maestroni, G.J. Melatonin: A principal neuroimmuno-regulatory and anti-stress hormone: Its anti-aging effects. Immunol. Lett 1987, 16, 355–361. [Google Scholar]
- Jan, J.E.; Espezel, H.; Applenton, R.E. The treatment of sleep disorders with melatonin. Dev. Med. Child. Neurol 1994, 36, 97–107. [Google Scholar]
- Zahn, P.K.; Lansmann, T.; Berger, E.; Speckmann, E.J.; Musshoff, U. Gene expression and functional characterization of melatonin receptors in the spinal cord of the rat: Implications for pain modulation. J. Pineal Res 2003, 35, 24–31. [Google Scholar]
- Picinato, M.C.; Hirata, A.E.; Cipolla-Neto, J.; Curi, R.; Carvalho, C.R.; Anhê, G.F.; Carpinelli, A.R. Activation of insulin and IGF-1 signaling pathways by melatonin through MT1 receptor in isolated rat pancreatic islets. J. Pineal Res 2008, 44, 88–94. [Google Scholar]
- Du, B.L.; Xiong, Y.; Zeng, C.G.; He, L.M.; Zhang, W.; Quan, D.P.; Wu, J.L.; Li, Y.; Zeng, Y.S. Transplantation of artificial neural construct partly improved spinal tissue repair and functional recovery in rats with spinal cord transection. Brain Res 2011, 1400, 87–98. [Google Scholar]
- Kuhn, H.G.; Winkler, J.; Kempermann, G.; Thal, L.J.; Gage, F.H. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J. Neurosci 1997, 17, 5820–5829. [Google Scholar]
- Neeper, S.A.; Gómez-Pinilla, F.; Choi, J.; Cotaman, C. Exercise and brain neurotrophins. Nature 1995, 373, 109. [Google Scholar]
- Zhang, P.; Zhang, Y.; Zhang, J.; Wu, Y.; Jia, J.; Wu, J.; Hu, Y. Early exercise protects against cerebral ischemic injury through inhibiting neuron apoptosis in cortex in rats. Int. J. Mol. Sci 2013, 14, 6074–6089. [Google Scholar]
- Lu, P.; Jones, L.L.; Snyder, E.Y.; Tuszynski, M.H. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp. Neurol 2003, 181, 115–129. [Google Scholar]
- Dolbeare, F. Bromodeoxyuridine: A diagnostic tool in biology and medicine, Part III. Proliferation in normal, injured and diseased tissue, growth factors, differentiation, DNA replication sites and in situ hybridization. Histochem. J 1996, 28, 531–575. [Google Scholar]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol 1996, 139, 244–256. [Google Scholar]

| Gene | Promer sequence (5′ to 3′) | Product length (bp) | Gen bank accession No. |
|---|---|---|---|
| GFAP | F: TGG CCA CCA GTA ACA TGC AA | 134 | NM_017009 |
| R: TCA AGT CAG CAA CGT GGA AG | |||
| MAP2 | F: CAA AGA GAA GGT GGC AAA GC | 200 | NM_013066 |
| R: GTG GGC AAG GGA TTT CTA CA | |||
| R: TCA AGC TGC AGC ATC CAT AC | |||
| Oct4 | F: GAG GGA TGG CAT ACT GTG GAC | 272 | NM_013633 |
| R: GGT GTA CCC CAA GGT GAT CC | |||
| GAPDH | F: GTA TGA CTC CAC TCA CGG CAA A | 100 | BC094037 |
| R: GGT CTC GCT CCT GGA AGA TG |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lee, Y.; Lee, S.; Lee, S.-R.; Park, K.; Hong, Y.; Lee, M.; Park, S.; Jin, Y.; Chang, K.-T.; Hong, Y. Beneficial Effects of Melatonin Combined with Exercise on Endogenous Neural Stem/Progenitor Cells Proliferation after Spinal Cord Injury. Int. J. Mol. Sci. 2014, 15, 2207-2222. https://doi.org/10.3390/ijms15022207
Lee Y, Lee S, Lee S-R, Park K, Hong Y, Lee M, Park S, Jin Y, Chang K-T, Hong Y. Beneficial Effects of Melatonin Combined with Exercise on Endogenous Neural Stem/Progenitor Cells Proliferation after Spinal Cord Injury. International Journal of Molecular Sciences. 2014; 15(2):2207-2222. https://doi.org/10.3390/ijms15022207
Chicago/Turabian StyleLee, Youngjeon, Seunghoon Lee, Sang-Rae Lee, Kanghui Park, Yunkyung Hong, Minkyung Lee, Sookyoung Park, Yunho Jin, Kyu-Tae Chang, and Yonggeun Hong. 2014. "Beneficial Effects of Melatonin Combined with Exercise on Endogenous Neural Stem/Progenitor Cells Proliferation after Spinal Cord Injury" International Journal of Molecular Sciences 15, no. 2: 2207-2222. https://doi.org/10.3390/ijms15022207




