14-3-3 Proteins Participate in Light Signaling through Association with PHYTOCHROME INTERACTING FACTORs
Abstract
:1. Introduction
2. Results
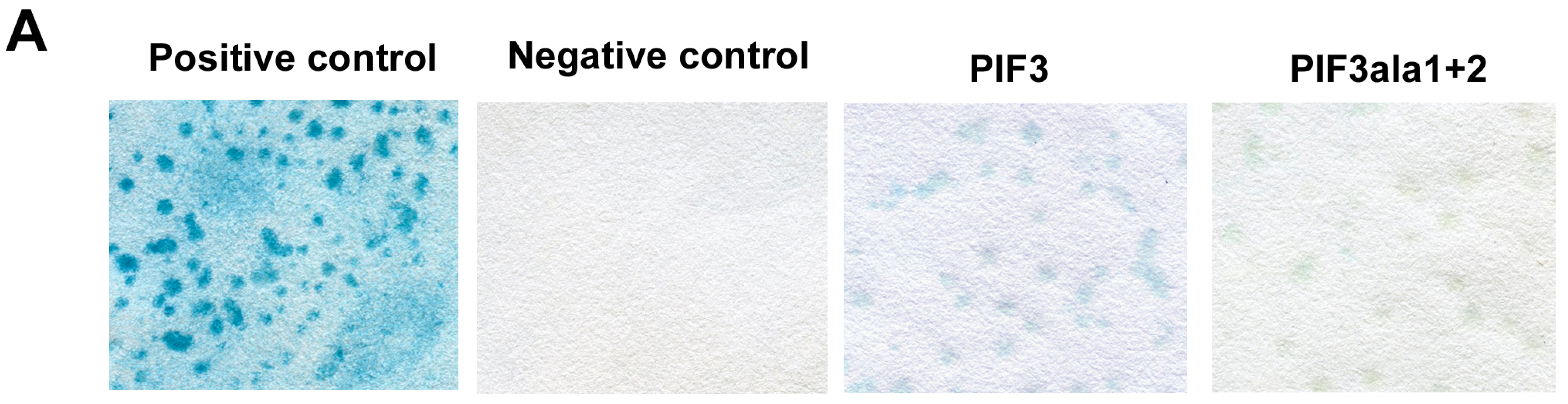
2.1. Yeast Two-Hybrid Analysis of 14-3-3 with Light-Related Proteins
| AGI | Gene Name | 14-3-3 Binding Motif |
|---|---|---|
| AT2G18790 | PHYTOCHROME B (PHYB) | yes |
| AT5G58140 | PHOTOTROPIN2 (PHOT2) | yes |
| AT2G30520 | ROOT PHOTOTROPISM2 (RPT2) | incomplete |
| AT2G32950 | CONSTITUTIVE PHOTOMORPHOGENIC1 (COP1) | yes |
| AT5G42970 | CONSTITUTIVE PHOTOMORPHOGENIC8 (COP8) | incomplete |
| AT1G09530 | PHYTOCHROME INTERACTING FACTOR 3 (PIF3) | yes |
| AT2G01570 | REPRESSOR OF GA1-3 1 (RGA1) | yes |
| AT3G03450 | RGA-LIKE2 (RGL2) | incomplete |
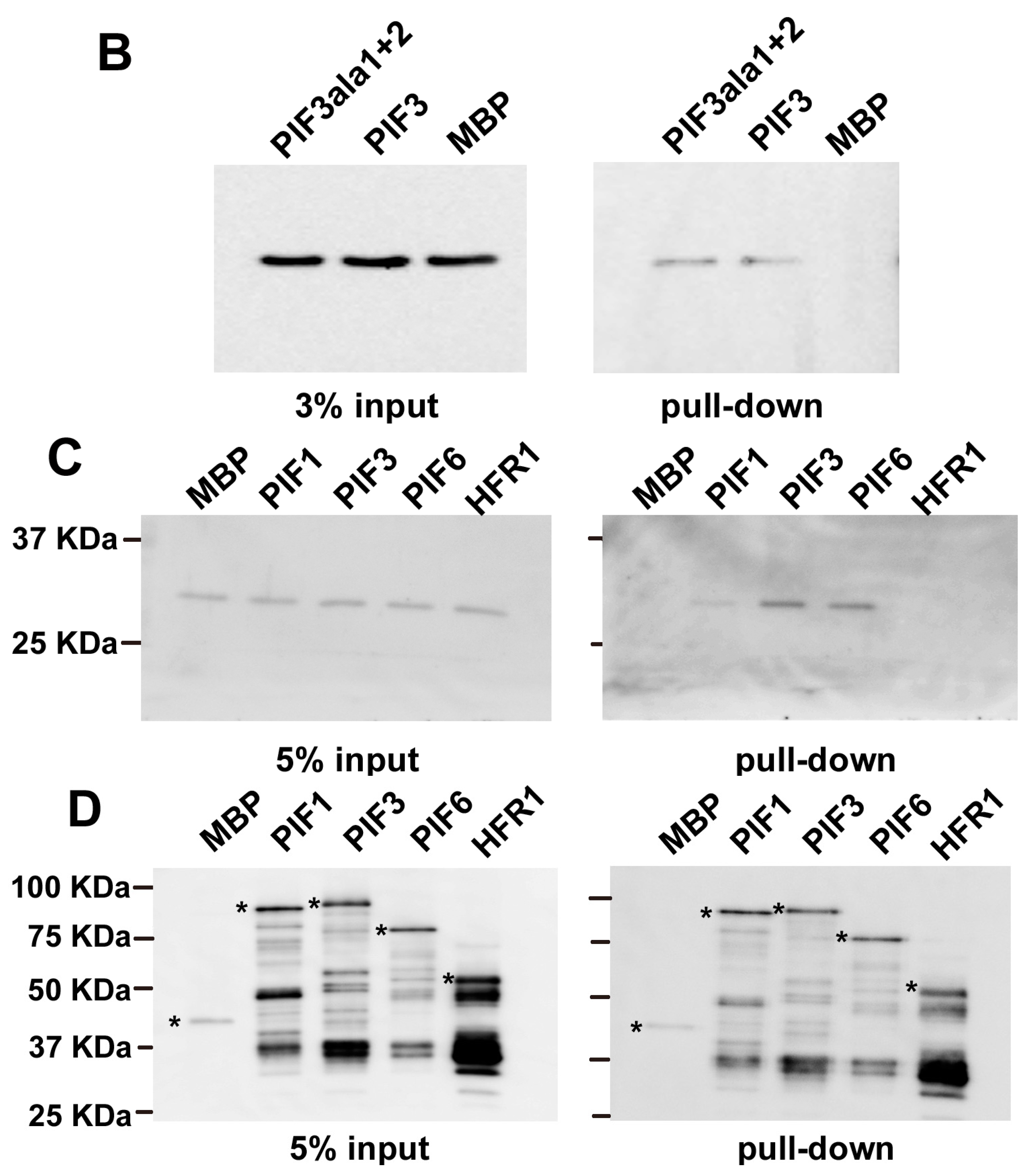
2.2. In Vitro Pull-Down of 14-3-3 with PIFs
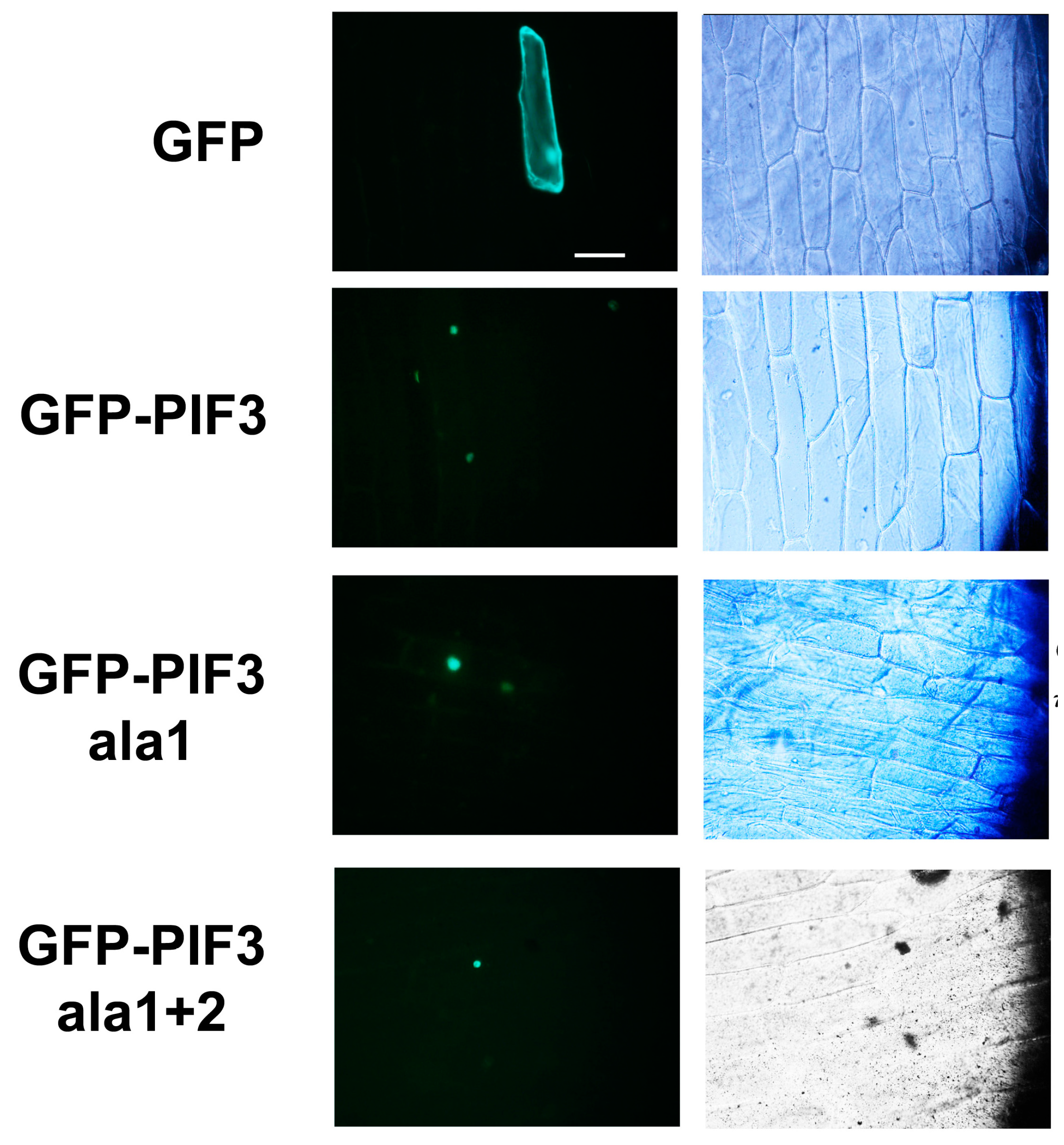
2.3. Subcellular Localization of GFP-PIF3ala1+2
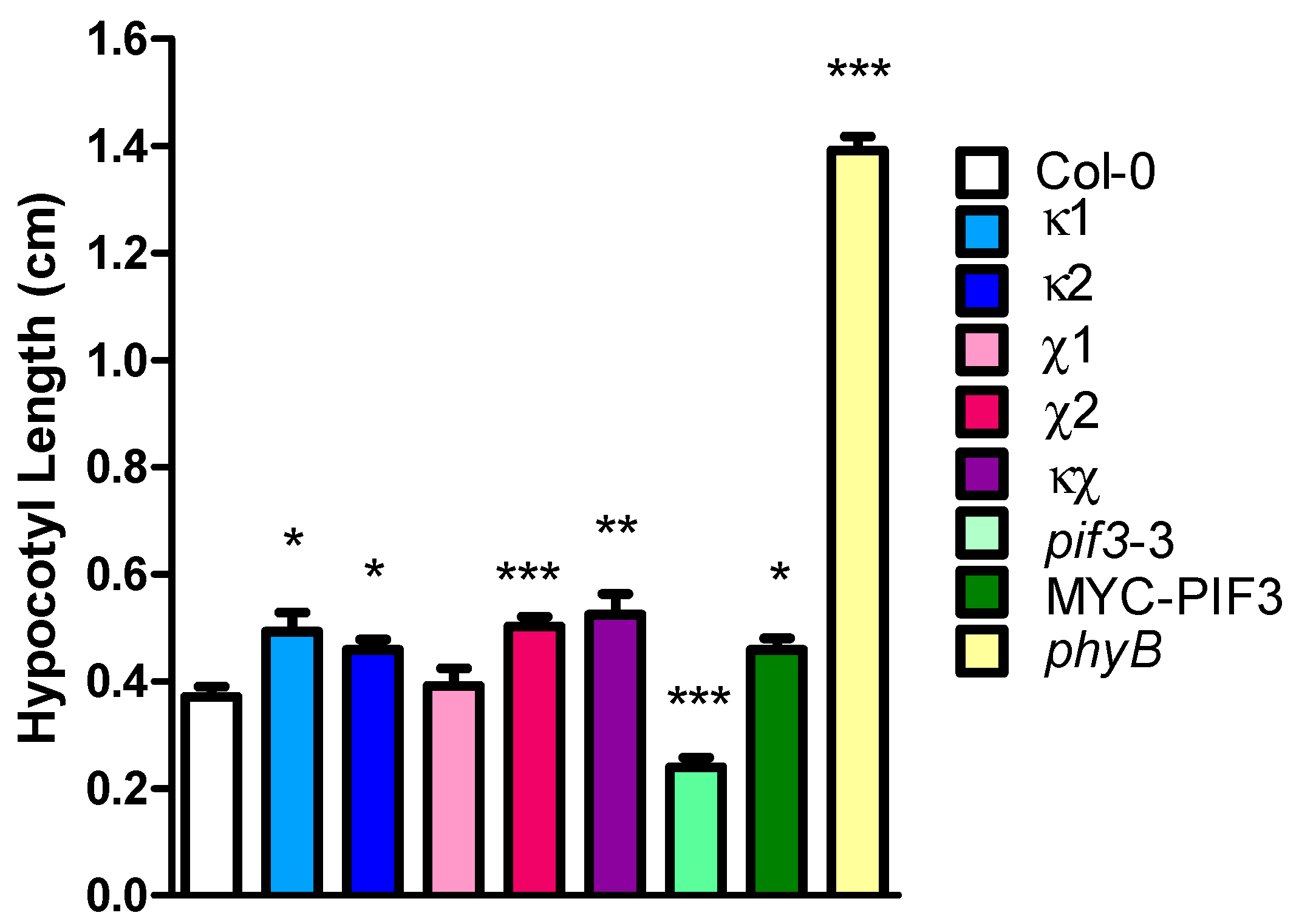
2.4. Light Phenotype of 14-3-3 Mutants
3. Discussion
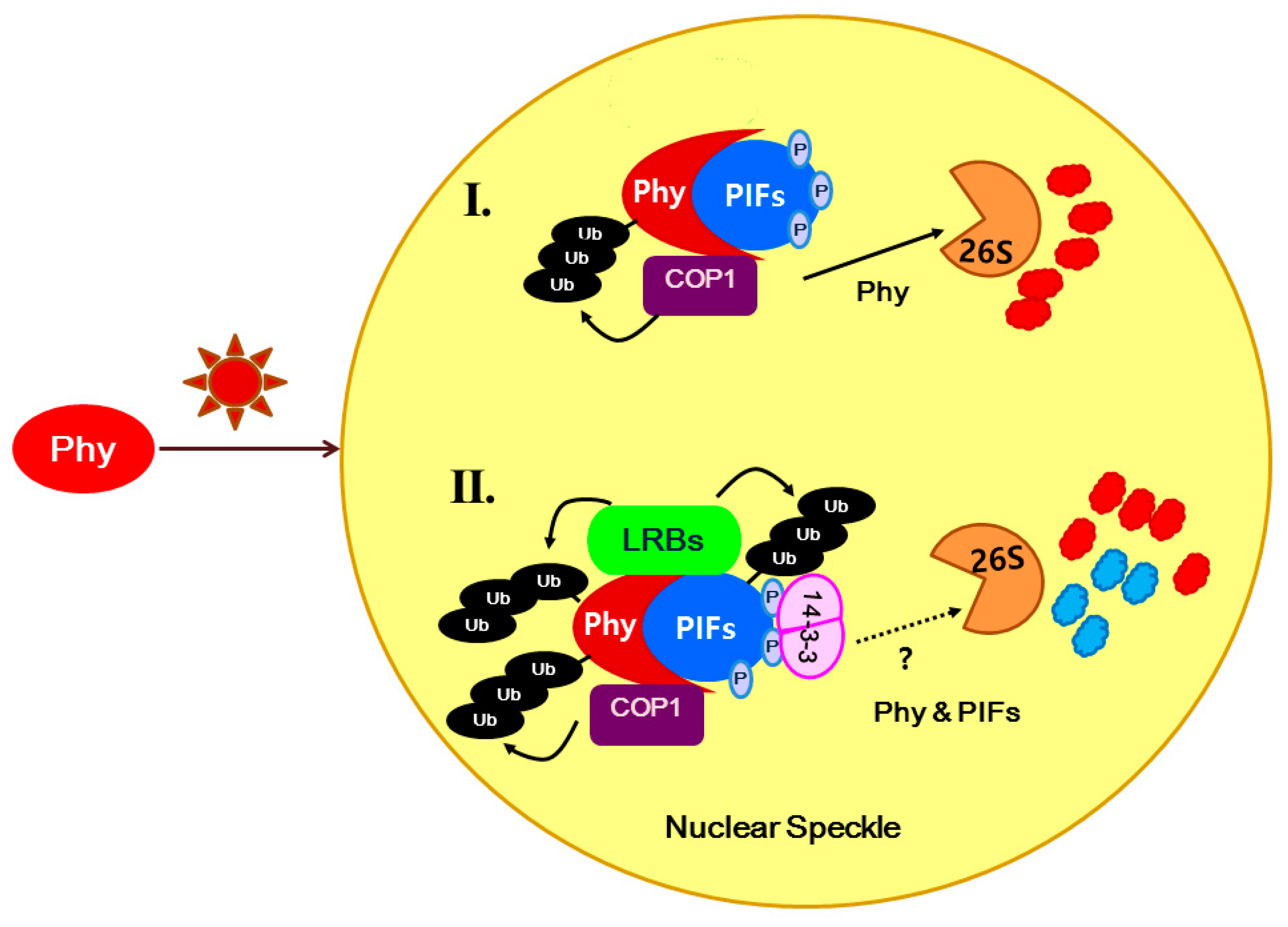
4. Experimental Section
4.1. Plant Material and Growth Conditions
4.2. Yeast Two-Hybrid
4.3. In Vitro Pull-Down
4.4. Subcellular Localization of PIF3 in Onion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aitken, A.; Collinge, D.B.; van Heusden, B.P.; Isobe, T.; Roseboom, P.H.; Rosenfeld, G.; Soll, J. 14-3-3 proteins: a highly conserved, widespread family of eukaryotic proteins. Trends Biochem. Sci. 1992, 17, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, D.; Morris, E.R.; Walker, J.C. 14-3-3 and FHA domains mediate phosphoprotein interactions. Annu. Rev. Plant Biol. 2009, 60, 67–91. [Google Scholar] [CrossRef] [PubMed]
- Moorhead, G.; Douglas, P.; Cotelle, V.; Harthill, J.; Morrice, N.; Meek, S.; Deiting, U.; Stitt, M.; Scarabel, M.; Aitken, A.; et al. Phosphorylation-dependent interactions between enzymes of plant metabolism and 14-3-3 proteins. Plant J. 1999, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jaspert, N.; Throm, C.; Oecking, C. Arabidopsis 14-3-3 proteins: fascinating and less fascinating aspects. Front. Plant Sci. 2011, 2. [Google Scholar] [CrossRef]
- Oecking, C.; Jaspert, N. Plant 14-3-3 proteins catch up with their mammalian orthologs. Curr. Opin. Plant Biol. 2009, 12, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.F.; Curran, A.; Woolsey, R.; Quilici, D.; Cushman, J.C.; Mittler, R.; Harmon, A.; Harper, J.F. Proteomic profiling of tandem affinity purified 14-3-3 protein complexes in Arabidopsis thaliana. Proteomics 2009, 9, 2967–2985. [Google Scholar] [CrossRef] [PubMed]
- Shin, R.; Jez, J.M.; Basra, A.; Zhang, B.; Schachtman, D.P. 14-3-3 proteins fine-tune plant nutrient metabolism. FEBS Lett. 2011, 585, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Sehnke, P.C.; Laughner, B.; Cardasis, H.; Powell, D.; Ferl, R.J. Exposed loop domains of complexed 14-3-3 proteins contribute to structural diversity and functional specificity. Plant Physiol. 2006, 140, 647–660. [Google Scholar] [CrossRef] [PubMed]
- Shin, R.; Alvarez, S.; Burch, A.Y.; Jez, J.M.; Schachtman, D.P. Phosphoproteomic identification of targets of the Arabidopsis sucrose nonfermenting-like kinase SnRK2.8 reveals a connection to metabolic processes. Proc. Natl. Acad. Sci. USA 2007, 104, 6460–6465. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Emi, T.; Tominaga, M.; Sakamoto, K.; Shigenaga, A.; Doi, M.; Shimazaki, K. Blue-light- and phosphorylation-dependent binding of a 14-3-3 protein to phototropins in stomatal guard cells of broad bean. Plant Physiol. 2003, 133, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.; Thomson, C.E.; Kaiserli, E.; Christie, J.M. Interaction specificity of Arabidopsis 14-3-3 proteins with phototropin receptor kinases. FEBS Lett. 2009, 583, 2187–2193. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.S.; Whippo, C.; Hangarter, R.P.; Briggs, W.R. The role of a 14-3-3 protein in stomatal opening mediated by PHOT2 in Arabidopsis. Plant Cell 2012, 24, 1114–1126. [Google Scholar] [CrossRef] [PubMed]
- Mayfield, J.D.; Folta, K.M.; Paul, A.L.; Ferl, R.J. The 14-3-3 Proteins µ and υ influence transition to flowering and early phytochrome response. Plant Physiol. 2007, 145, 1692–1702. [Google Scholar] [CrossRef] [PubMed]
- Mayfield, J.D.; Paul, A.L.; Ferl, R.J. The 14-3-3 proteins of Arabidopsis regulate root growth and chloroplast development as components of the photosensory system. J. Exp. Bot. 2012, 63, 3061–3070. [Google Scholar] [CrossRef] [PubMed]
- Pnueli, L.; Gutfinger, T.; Hareven, D.; Ben-Naim, O.; Ron, N.; Adir, N.; Lifschitz, E. Tomato SP-interacting proteins define a conserved signaling system that regulates shoot architecture and flowering. Plant Cell 2001, 13, 2687–2702. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, J.F.; Huq, E.; Quail, P.H. Direct targeting of light signals to a promoter element-bound transcription factor. Science 2000, 288, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Viczian, A.; Kircher, S.; Fejes, E.; Millar, A.J.; Schafer, E.; Kozma-Bognar, L.; Nagy, F. Functional characterization of phytochrome interacting factor 3 for the Arabidopsis thaliana circadian clockwork. Plant Cell Physiol. 2005, 46, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, C.Y.; Wang, K.C.; Luo, M.; Tai, R.; Yuan, L.; Zhao, M.; Yang, S.; Tian, G.; Cui, Y.; et al. PHYTOCHROME INTERACTING FACTOR3 associates with the histone deacetylase HDA15 in repression of chlorophyll biosynthesis and photosynthesis in etiolated Arabidopsis seedlings. Plant Cell 2013, 25, 1258–1273. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, X.; Lin, F.; He, G.; Terzaghi, W.; Zhu, D.; Deng, X.W. Arabidopsis noncoding RNA mediates control of photomorphogenesis by red light. Proc. Natl. Acad. Sci. USA 2014, 111, 10359–10364. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Tepperman, J.M.; Quail, P.H. PIF3, a phytochrome-interacting factor necessary for normal photoinduced signal transduction, is a novel basic helix-loop-helix protein. Cell 1998, 95, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Tepperman, J.M.; Quail, P.H. Binding of phytochrome B to its nuclear signalling partner PIF3 is reversibly induced by light. Nature 1999, 400, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tepperman, J.M.; Fairchild, C.D.; Quail, P.H. Phytochrome B binds with greater apparent affinity than phytochrome A to the basic helix-loop-helix factor PIF3 in a reaction requiring the PAS domain of PIF3. Proc. Natl. Acad. Sci. USA 2000, 97, 13419–13424. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, A.; Nagel, M.K.; Popp, C.; Wust, F.; Bindics, J.; Viczian, A.; Hiltbrunner, A.; Nagy, F.; Kunkel, T.; Schafer, E. Interaction with plant transcription factors can mediate nuclear import of phytochrome B. Proc. Natl. Acad. Sci. USA 2012, 109, 5892–5897. [Google Scholar] [CrossRef] [PubMed]
- Hiltbrunner, A.; Tscheuschler, A.; Viczian, A.; Kunkel, T.; Kircher, S.; Schafer, E. FHY1 and FHL act together to mediate nuclear accumulation of the phytochrome A photoreceptor. Plant Cell Physiol. 2006, 47, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Hiltbrunner, A.; Viczian, A.; Bury, E.; Tscheuschler, A.; Kircher, S.; Toth, R.; Honsberger, A.; Nagy, F.; Fankhauser, C.; Schafer, E. Nuclear accumulation of the phytochrome A photoreceptor requires FHY1. Curr. Biol. 2005, 15, 2125–2130. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Kim, J.; Lee, Y.; Shin, J.; Oh, E.; Chung, W.I.; Liu, J.R.; Choi, G. Degradation of phytochrome interacting factor 3 in phytochrome-mediated light signaling. Plant Cell Physiol. 2004, 45, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Al-Sady, B.; Ni, W.; Kircher, S.; Schafer, E.; Quail, P.H. Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome-mediated degradation. Mol. Cell 2006, 23, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Xu, S.L.; Tepperman, J.M.; Stanley, D.J.; Maltby, D.A.; Gross, J.D.; Burlingame, A.L.; Wang, Z.Y.; Quail, P.H. A mutually assured destruction mechanism attenuates light signaling in Arabidopsis. Science 2014, 344, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Park, J.; Kim, J.; Nagatani, A.; Lagarias, J.C.; Choi, G. Phytochrome B inhibits binding of phytochrome-interacting factors to their target promoters. Plant J. 2012, 72, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Van Buskirk, E.K.; Reddy, A.K.; Nagatani, A.; Chen, M. Photobody localization of phytochrome B is tightly correlated with prolonged and light-dependent inhibition of hypocotyl elongation in the dark. Plant Physiol. 2014, 165, 595–607. [Google Scholar]
- Jang, I.C.; Henriques, R.; Seo, H.S.; Nagatani, A.; Chua, N.H. Arabidopsis PHYTOCHROME INTERACTING FACTOR proteins promote phytochrome B polyubiquitination by COP1 E3 ligase in the nucleus. Plant Cell 2010, 22, 2370–2383. [Google Scholar] [CrossRef] [PubMed]
- Bauer, D.; Viczian, A.; Kircher, S.; Nobis, T.; Nitschke, R.; Kunkel, T.; Panigrahi, K.C.; Adam, E.; Fejes, E.; Schafer, E.; et al. Constitutive photomorphogenesis 1 and multiple photoreceptors control degradation of phytochrome interacting factor 3, a transcription factor required for light signaling in Arabidopsis. Plant Cell 2004, 16, 1433–1445. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Xu, S.L.; Chalkley, R.J.; Pham, T.N.; Guan, S.; Maltby, D.A.; Burlingame, A.L.; Wang, Z.Y.; Quail, P.H. Multisite light-induced phosphorylation of the transcription factor PIF3 is necessary for both its rapid degradation and concomitant negative feedback modulation of photoreceptor phyB levels in Arabidopsis. Plant Cell 2013, 25, 2679–2698. [Google Scholar] [CrossRef] [PubMed]
- Sharrock, R.A.; Quail, P.H. Novel phytochrome sequences in Arabidopsis thaliana: structure, evolution, and differential expression of a plant regulatory photoreceptor family. Genes Dev. 1989, 3, 1745–1757. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, T.; Sakai, T.; Suetsugu, N.; Oikawa, K.; Ishiguro, S.; Kato, T.; Tabata, S.; Okada, K.; Wada, M. Arabidopsis NPL1: A phototropin homolog controlling the chloroplast high-light avoidance response. Science 2001, 291, 2138–2141. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Kurose, T.; Hino, T.; Tanaka, K.; Kawamukai, M.; Niwa, Y.; Toyooka, K.; Matsuoka, K.; Jinbo, T.; Kimura, T. Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 2007, 104, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Martinez, C.; Gusmaroli, G.; Wang, Y.; Zhou, J.; Wang, F.; Chen, L.; Yu, L.; Iglesias-Pedraz, J.M.; Kircher, S.; et al. Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 2008, 451, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhu, L.; Castillon, A.; Majee, M.; Downie, B.; Huq, E. Light-induced phosphorylation and degradation of the negative regulator PHYTOCHROME-INTERACTING FACTOR1 from Arabidopsis depend upon its direct physical interactions with photoactivated phytochromes. Plant Cell 2008, 20, 1586–1602. [Google Scholar] [CrossRef] [PubMed]
- Khanna, R.; Huq, E.; Kikis, E.A.; Al-Sady, B.; Lanzatella, C.; Quail, P.H. A novel molecular recognition motif necessary for targeting photoactivated phytochrome signaling to specific basic helix-loop-helix transcription factors. Plant Cell 2004, 16, 3033–3044. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.; Castillon, A.; Chen, F.; Zhu, L.; Huq, E. Dimerization and blue light regulation of PIF1 interacting bHLH proteins in Arabidopsis. Plant Mol. Biol. 2011, 77, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Leivar, P.; Monte, E.; Oka, Y.; Liu, T.; Carle, C.; Castillon, A.; Huq, E.; Quail, P.H. Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Curr. Biol. 2008, 18, 1815–1823. [Google Scholar] [CrossRef] [PubMed]
- Fairchild, C.D.; Schumaker, M.A.; Quail, P.H. HFR1 encodes an atypical bHLH protein that acts in phytochrome A signal transduction. Genes Dev. 2000, 14, 2377–2391. [Google Scholar] [PubMed]
- Luo, Q.; Lian, H.L.; He, S.B.; Li, L.; Jia, K.P.; Yang, H.Q. COP1 and phyB physically interact with PIL1 to regulate its stability and photomorphogenic development in Arabidopsis. Plant Cell 2014, 26, 2441–2456. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yi, H.; Choi, G.; Shin, B.; Song, P.S.; Choi, G. Functional characterization of phytochrome interacting factor 3 in phytochrome-mediated light signal transduction. Plant Cell 2003, 15, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda-Garcia, E.; Rocha-Sosa, M. The Arabidopsis F-box protein AtFBS1 interacts with 14-3-3 proteins. Plant Sci. 2012, 195, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Yoon, G.M.; Kieber, J.J. 14-3-3 regulates 1-aminocyclopropane-1-carboxylate synthase protein turnover in Arabidopsis. Plant Cell 2013, 25, 1016–1028. [Google Scholar] [CrossRef] [PubMed]
- Monte, E.; Tepperman, J.M.; Al-Sady, B.; Kaczorowski, K.A.; Alonso, J.M.; Ecker, J.R.; Li, X.; Zhang, Y.; Quail, P.H. The phytochrome-interacting transcription factor, PIF3, acts early, selectively, and positively in light-induced chloroplast development. Proc. Natl. Acad. Sci. USA 2004, 101, 16091–16098. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adams, E.; Diaz, C.; Hong, J.-P.; Shin, R. 14-3-3 Proteins Participate in Light Signaling through Association with PHYTOCHROME INTERACTING FACTORs. Int. J. Mol. Sci. 2014, 15, 22801-22814. https://doi.org/10.3390/ijms151222801
Adams E, Diaz C, Hong J-P, Shin R. 14-3-3 Proteins Participate in Light Signaling through Association with PHYTOCHROME INTERACTING FACTORs. International Journal of Molecular Sciences. 2014; 15(12):22801-22814. https://doi.org/10.3390/ijms151222801
Chicago/Turabian StyleAdams, Eri, Celine Diaz, Jong-Pil Hong, and Ryoung Shin. 2014. "14-3-3 Proteins Participate in Light Signaling through Association with PHYTOCHROME INTERACTING FACTORs" International Journal of Molecular Sciences 15, no. 12: 22801-22814. https://doi.org/10.3390/ijms151222801





