Evaluation of Epirubicin in Thermogelling and Bioadhesive Liquid and Solid Suppository Formulations for Rectal Administration
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
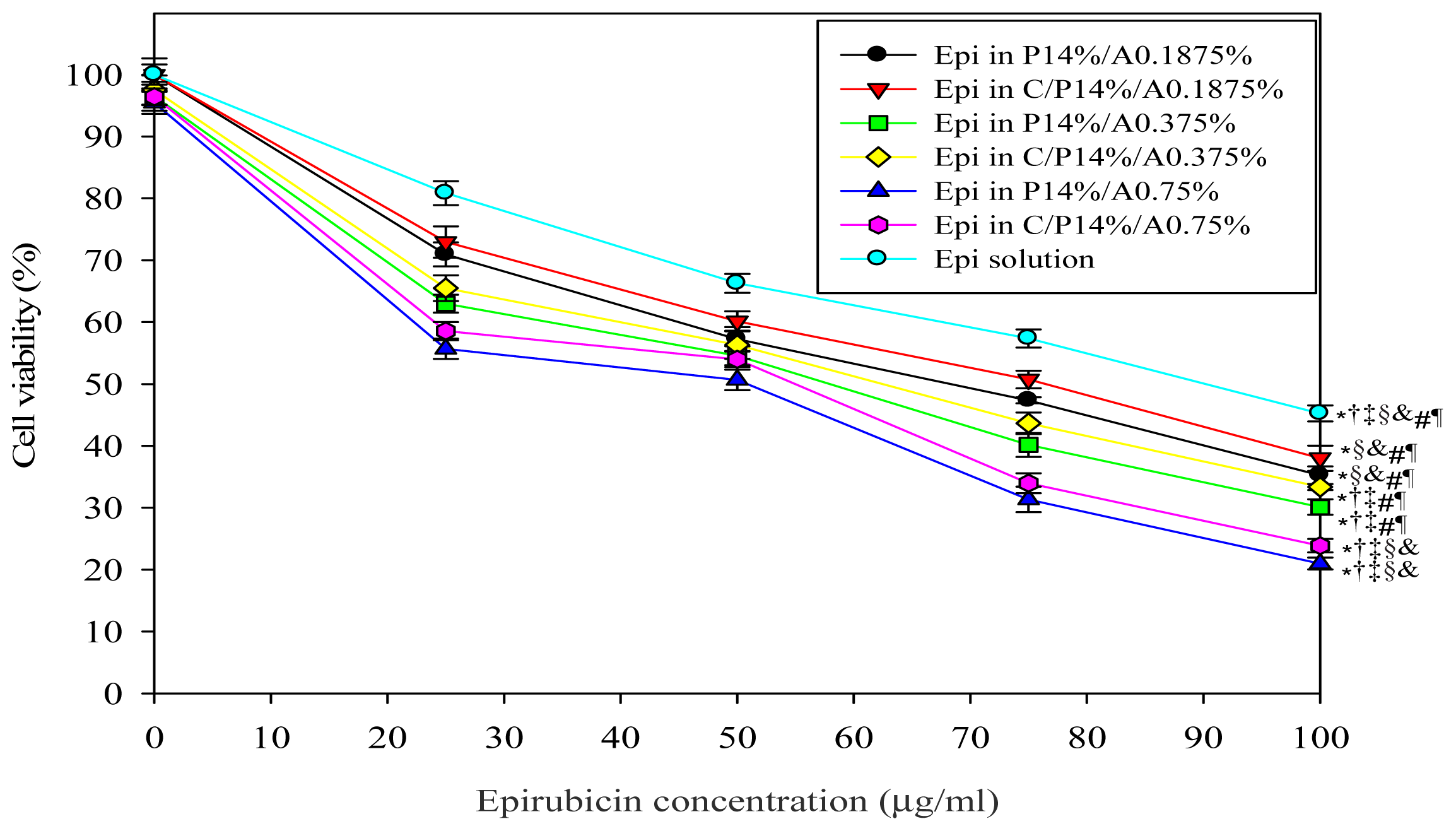
2.1.1. Cell Growth Inhibition Assay
2.1.2. Determination of Bioadhesive Force
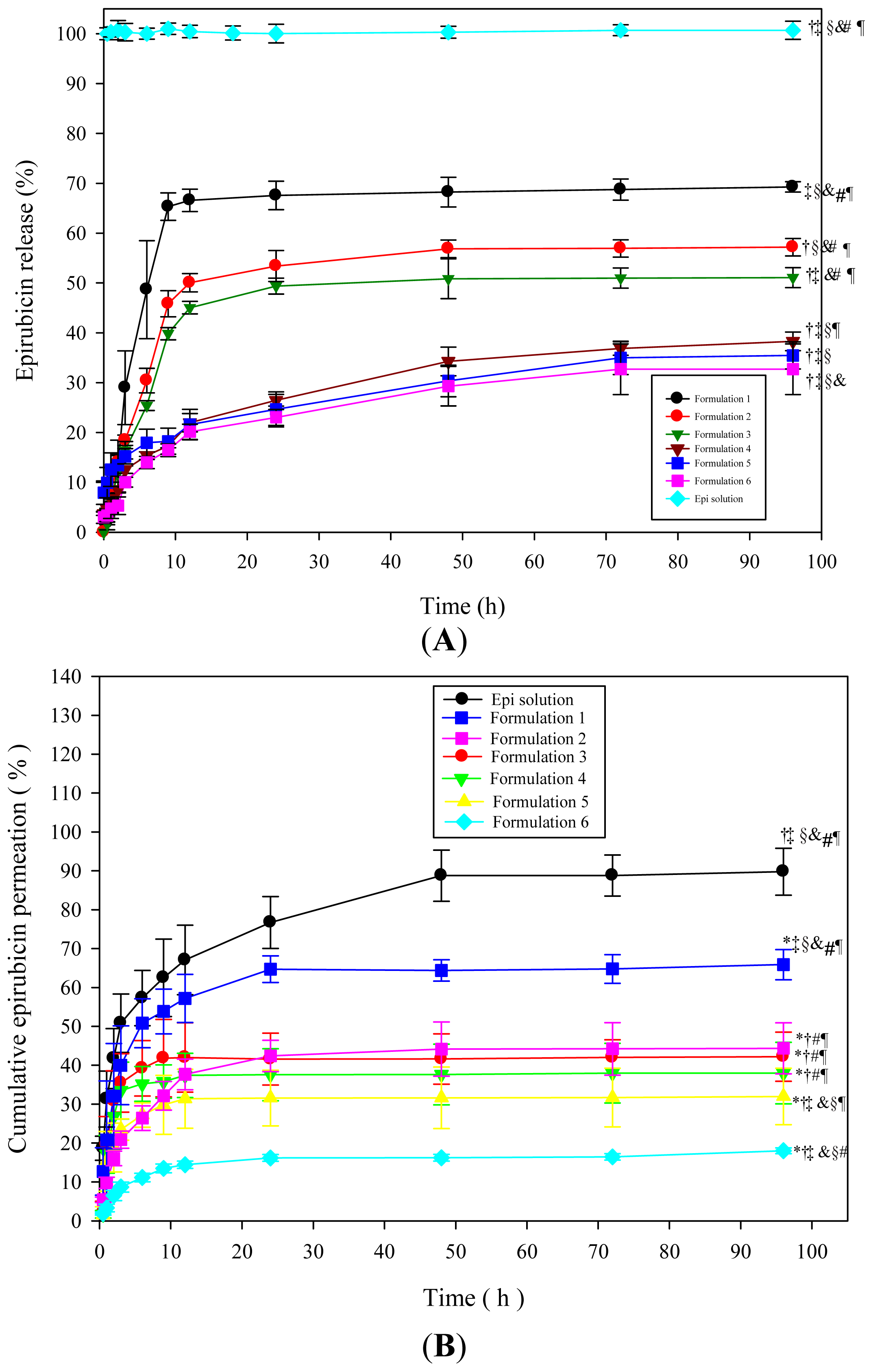
2.1.3. In Vitro Drug Release Study
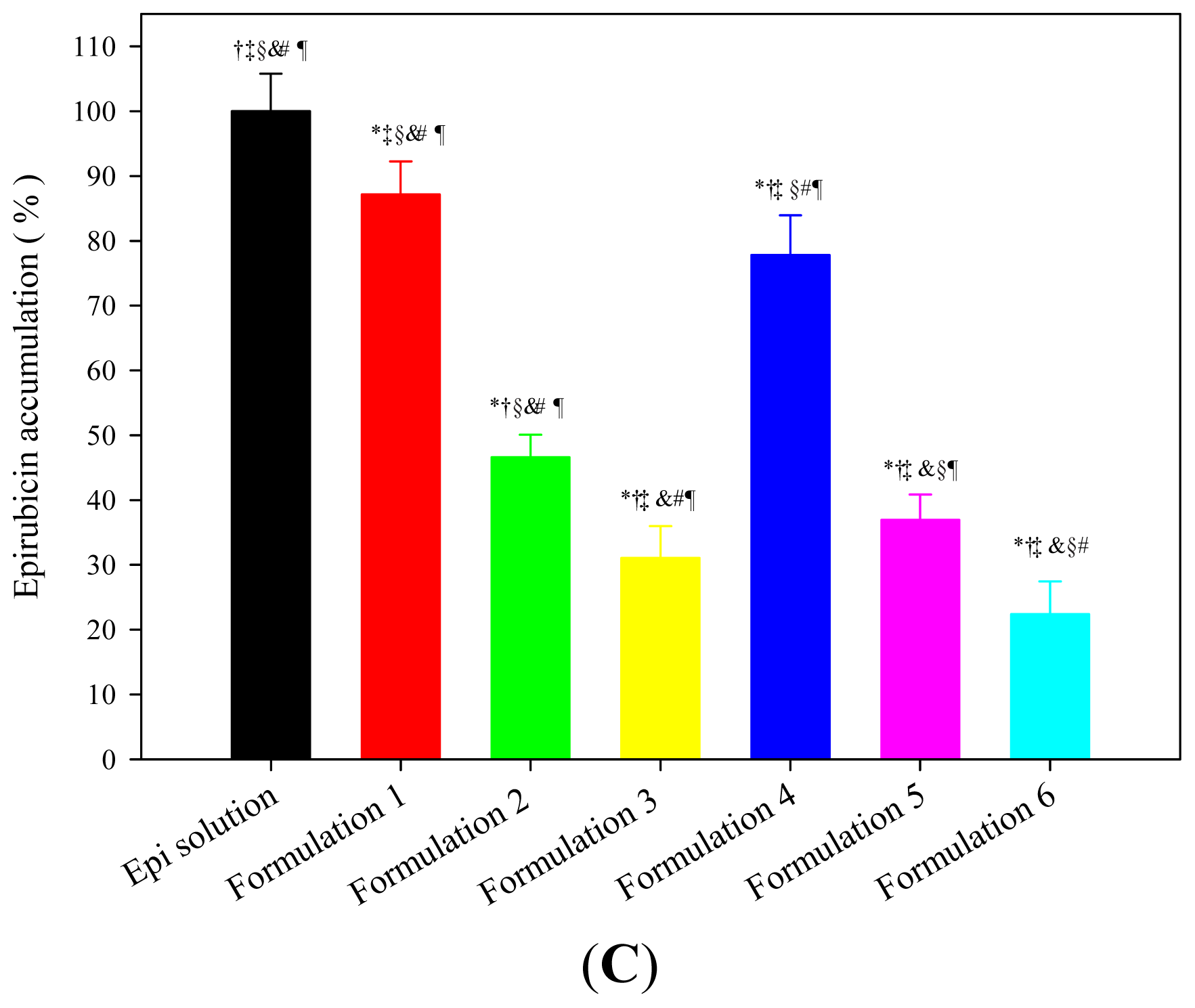
2.1.4. In Vitro Drug Permeation and Accumulation Tests in Rectal Mucosa
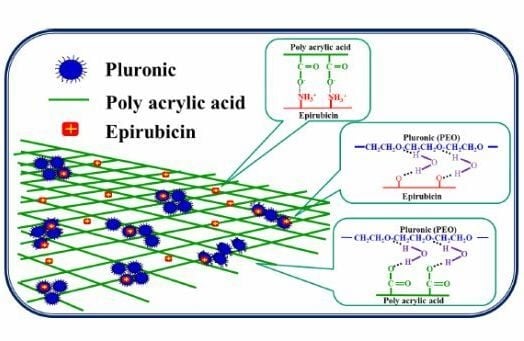
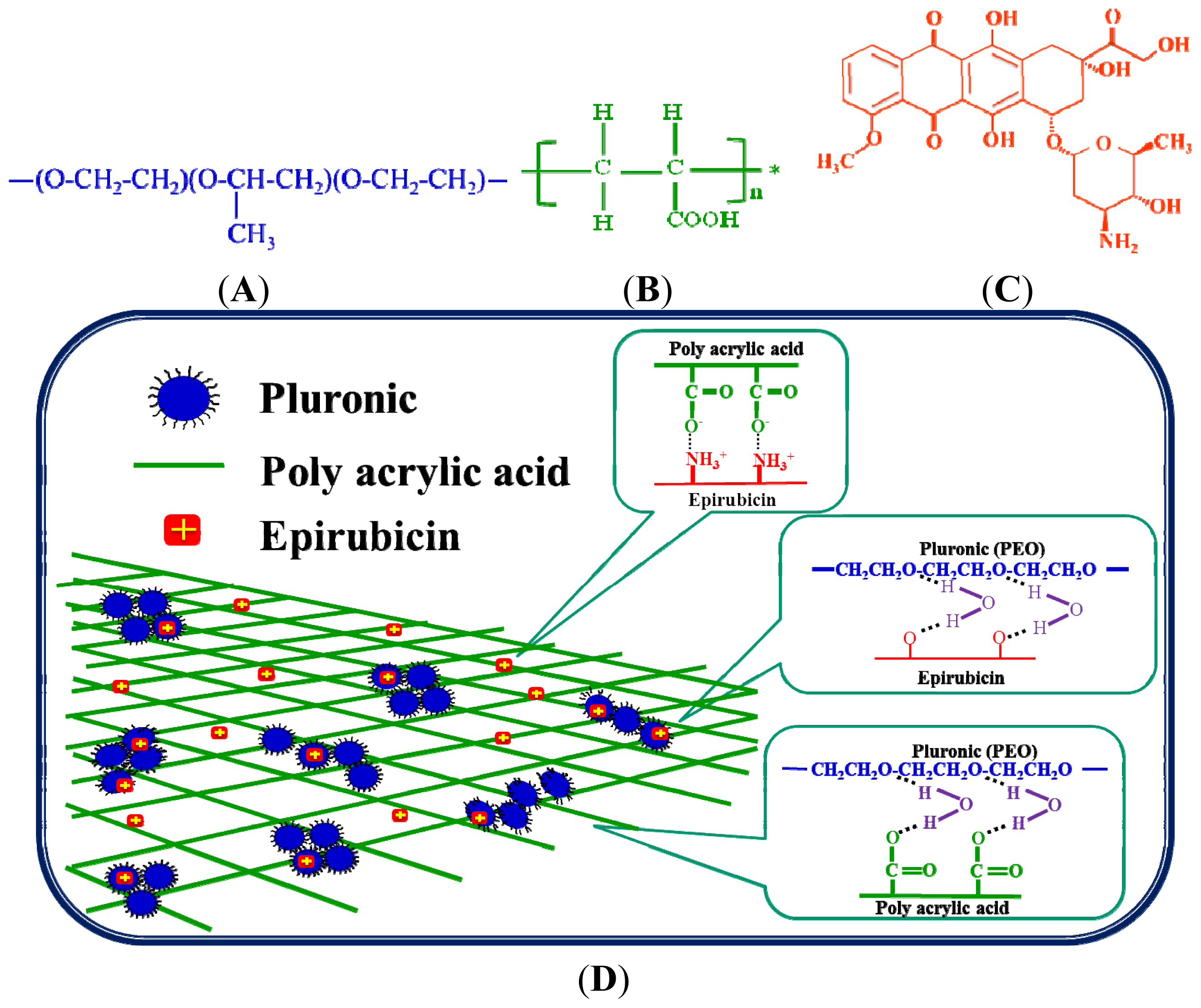
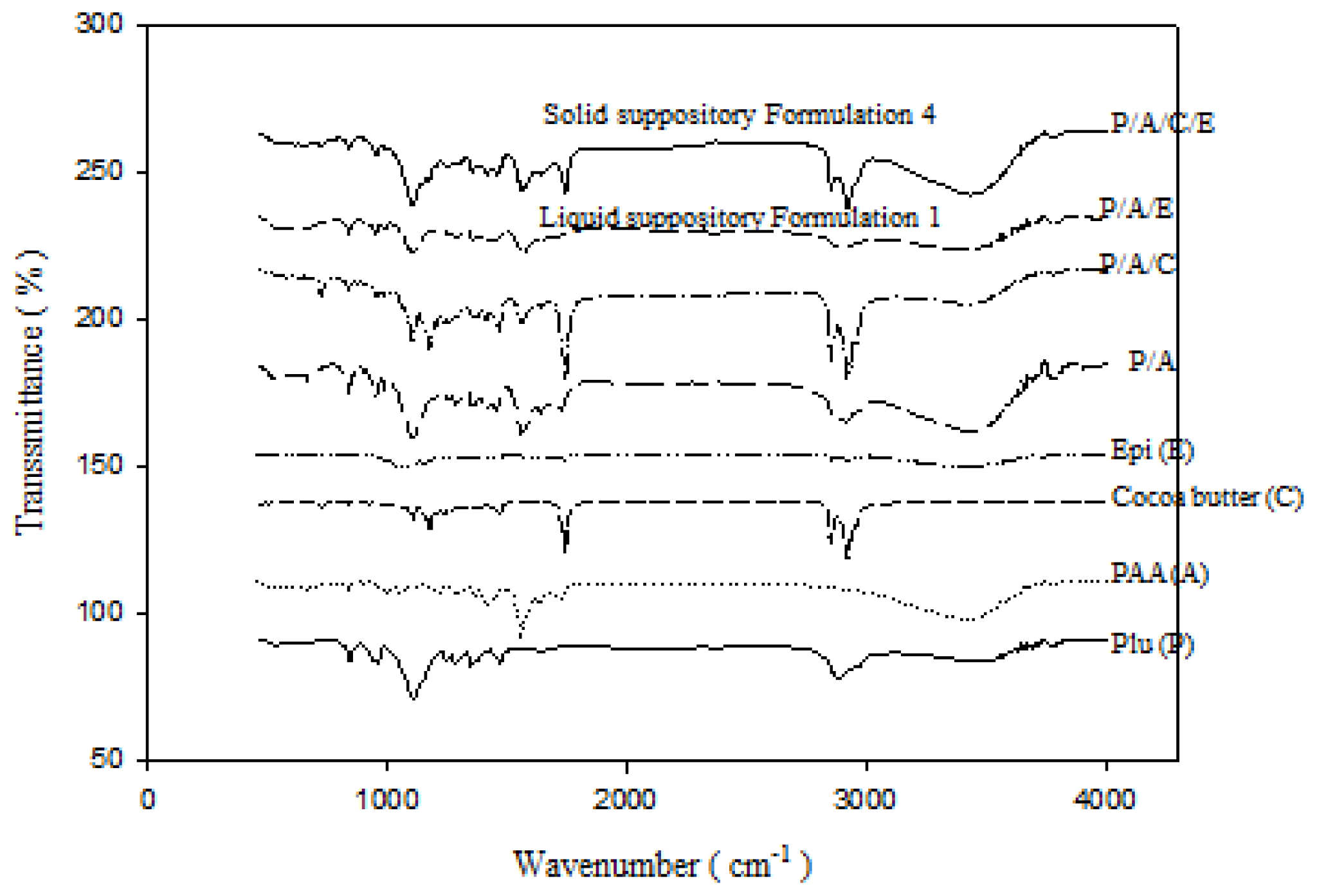
2.1.5. FTIR Analysis
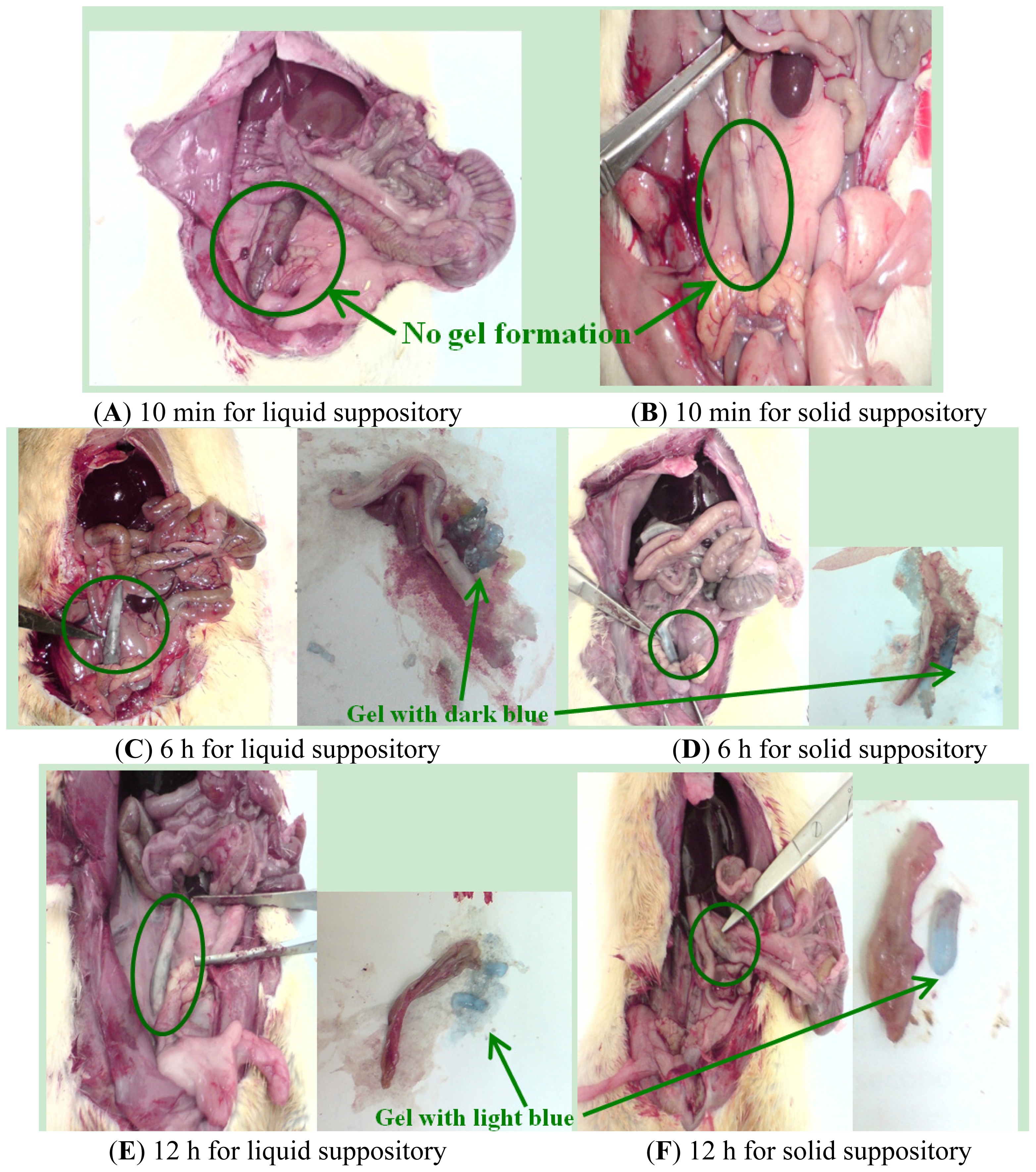
2.1.6. In Vivo Localization of Suppository in the SD Rats
2.1.7. In Vivo Pharmacokinetic Study
2.1.8. Antitumor Efficacy
2.2. Discussion
3. Experimental Section
3.1. Materials
3.2. Preparation of Epi in Plu/PAA Formulation
3.2.1. Preparation of Plu/PAA Mixture
3.2.2. Preparation of Epi in Plu/PAA Liquid Suppository
3.2.3. Preparation of Epi in Plu/PAA Solid Suppository
3.3. Cell Culture
3.4. Cell Growth Inhibition Assay
3.5. Determination of Bioadhesive Force
3.6. In Vitro Drug Release Study
3.7. Epi Concentration Analyzed Using HPLC
3.8. In Vitro Drug Penetration Test
3.9. In Vitro Drug Accumulation Study
3.10. FT-IR Analysis
3.11. Identification of Suppository Localization in Vivo
3.12. In Vivo Pharmacokinetic Study
3.13. Establishment of in Vivo Model of Mouse Tumor Xenografts
3.14. In Vivo Antitumor Efficacy
3.15. Statistical Analysis
4. Conclusions
Supplementary Information
ijms-15-00342-s001.pdfAcknowledgments
Conflicts of Interest
References
- Bromberg, L. Polymeric micelles in oral chemotherapy. J. Control. Release 2008, 128, 99–112. [Google Scholar]
- Plosker, G.L.; Faulds, D. Epirubicin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in cancer chemotherapy. Drugs 1993, 45, 788–856. [Google Scholar]
- Ghorab, D.; Refai, H.; Tag, R. Preparation and evaluation of fenoterol hydrobromide suppositories. Drug Discov. Ther 2011, 5, 311–318. [Google Scholar]
- Pásztor, E.; Makó, Á.; Csóka, G.; Fenyvesi, Z.; Benko, R.; Prosszer, M.; Marton, S.; Antal, I.; Klebovich, I. New formulation of in situ gelling Metolose-based liquid suppository. Drug Dev. Ind. Pharm 2011, 37, 1–7. [Google Scholar]
- Koffi, A.A.; Agnely, F.; Besnard, M.; Kablan Brou, J.; Grossiord, J.L.; Ponchel, G. In vitro and in vivo characteristics of a thermogelling and bioadhesive delivery system intended for rectal administration of quinine in children. Eur. J. Pharm. Biopharm 2008, 69, 167–175. [Google Scholar]
- Paek, S.H.; Xuan, J.J.; Choi, H.G.; Park, B.C.; Lee, Y.S.; Jeong, T.C.; Jin, C.H.; Oh, Y.K.; Kim, J.A. Poloxamer 188 and propylene glycol-based rectal suppository enhances anticancer effect of 5-fluorouracil in mice. Biol. Pharm. Bull 2006, 29, 1060–1063. [Google Scholar]
- Lin, H.-R.; Tseng, C.C.; Lin, Y.J.; Ling, M.H. A novel in situ-gelling liquid suppository for site-targeting delivery of anti-colorectal cancer drugs. J. Biomater. Sci. Polym. Ed 2012, 23, 807–822. [Google Scholar]
- Kundu, J.; Poole-Warren, L.A.; Martens, P.; Kundu, S.C. Silk fibroin/poly(vinyl alcohol) photocrosslinked hydrogels for delivery of macromolecular drugs. Acta. Biomater 2012, 8, 1720–1729. [Google Scholar]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm 2000, 50, 27–46. [Google Scholar]
- Park, K.M.; Bae, J.W.; Joung, Y.K.; Shin, J.W.; Park, K.D. Nanoaggregate of thermosensitive chitosan-Pluronic for sustained release of hydrophobic drug. Colloids Surf. B Biointerfaces 2008, 63, 1–6. [Google Scholar]
- Nie, S.; Hsiao, W.L.; Pan, W.; Yang, Z. Thermoreversible Pluronic F127-based hydrogel containing liposomes for the controlled delivery of paclitaxel: In vitro drug release, cell cytotoxicity, and uptake studies. Int. J. Nanomedicine 2011, 6, 151–166. [Google Scholar]
- Sharma, P.K.; Reilly, M.J.; Jones, D.N.; Robinson, P.M.; Bhatia, S.R. The effect of pharmaceuticals on the nanoscale structure of PEO-PPO-PEO micelles. Colloids. Surf. B Biointerfaces 2008, 61, 53–60. [Google Scholar]
- Bajaj, G.; Kim, M.R.; Mohammed, S.I.; Yeo, Y. Hyaluronic acid-based hydrogel for regional delivery of paclitaxel to intraperitoneal tumors. J. Control. Release 2012, 158, 386–392. [Google Scholar]
- Ma, W.D.; Xu, H.; Wang, C.; Nie, S.F.; Pan, W.S. Pluronic F127-g-poly(acrylic acid) copolymers as in situ gelling vehicle for ophthalmic drug delivery system. Int. J. Pharm 2008, 350, 247–256. [Google Scholar]
- Davies, N.M.; Farr, S.J.; Hadgraft, J.; Kellaway, I.W. Evaluation of mucoadhesive polymers in ocular drug delivery. I. Viscous solutions. Pharm. Res 1991, 8, 1039–1043. [Google Scholar]
- Choo, E.S.; Yu, B.; Xue, J. Synthesis of poly(acrylic acid) (PAA) modified Pluronic P123 copolymers for pH-stimulated release of doxorubicin. J. Colloid Interface Sci 2011, 358, 462–470. [Google Scholar]
- He, C.; Kim, S.W.; Lee, D.S. In situ gelling stimuli-sensitive block copolymer hydrogels for drug delivery. J Control. Release 2008, 127, 189–207. [Google Scholar]
- Ahn, J.S.; Choi, H.K.; Cho, C.S. A novel mucoadhesive polymer prepared by template polymerization of acrylic acid in the presence of chitosan. Biomaterials 2001, 22, 923–928. [Google Scholar]
- Lo, Y.L.; Ho, C.T.; Tsai, F.L. Inhibit multidrug resistance and induce apoptosis by using glycocholic acid and epirubicin. Eur. J. Pharm. Sci 2008, 35, 52–67. [Google Scholar]
- Falkson, G.; Vorobiof, D.A. Epirubicin in Colorectal Cancer. In Advances in Anthracycline Chemotherapy: Epirubicin; Bonadonna, G., Ed.; Masson Italia Editori: Roma, Italy, 1984; pp. 105–109. [Google Scholar]
- Appel, J.M.; Zerahn, B.; Moller, S.; Christensen, H.M.; Sogaard, P.; Ejlertsen, B.; Fogh-Andersen, N.; Jensen, B.V.; Nielsen, D.L. Long-term heart function after adjuvant epirubicin chemotherapy for breast cancer. Acta Oncol 2012, 51, 1054–1061. [Google Scholar]
- Lo, Y.L.; Hsu, C.Y.; Lin, H.R. pH- and thermo-sensitive pluronic/poly(acrylic acid) in situ hydrogels for sustained release of an anticancer drug. J. Drug Target 2013, 21, 54–66. [Google Scholar]
- Lin, H.R.; Hsu, C.Y.; Lo, Y.L. Preparation and characterization of dual phase transition oral hydrogel for sustained release of epirubicin. Int. J. Polym. Mater. Polym. Biomater 2013, 62, 763–769. [Google Scholar]
- Yong, C.S.; Oh, Y.K.; Jung, S.H.; Rhee, J.D.; Kim, H.D.; Kim, C.K.; Choi, H.G. Preparation of ibuprofen-loaded liquid suppository using eutectic mixture system with menthol. Eur. J. Pharm. Sci 2004, 23, 347–353. [Google Scholar]
- Yun, M.; Choi, H.; Jung, J.; Kim, C. Development of a thermo-reversible insulin liquid suppository with bioavailability enhancement. Int. J. Pharm 1999, 189, 137–145. [Google Scholar]
- Huang, Y.; Yu, H.; Xiao, C. pH-Sensitive cationic guargum/poly(acrylic acid) polyelectrolyte hydrogels: Swelling and in vitro drug release. Carbohydr. Polym 2007, 69, 774–783. [Google Scholar]
- Ranjha, N.M.; Ayub, G.; Naseem, S.; Ansari, M.T. Preparation and characterization of hybrid pH-sensitive hydrogels of chitosan-co-acrylic acid for controlled release of verapamil. J. Mater. Sci. Mater. Med 2010, 21, 2805–2816. [Google Scholar]
- Kim, K.S.; Park, S.J. Effect of porous silica on sustained release behaviors of pH sensitive pluronic F127/poly(acrylic acid) hydrogels containing tulobuterol. Colloids Surf. B Biointerfaces 2010, 80, 240–246. [Google Scholar]
- Park, T.H.; Kim, S.T.; Park, J.S.; Choi, H.G.; Kim, H.T.; Kim, C.K. Effect of zinc oxide on the rheological and mucoadhesive properties of poloxamer 407-based mucoadhesive thermosensitive gel. Drug Dev. Ind. Pharm 2010, 36, 1436–1443. [Google Scholar]
- Zhang, W.; Shi, Y.; Chen, Y.; Hao, J.; Sha, X.; Fang, X. The potential of Pluronic polymeric micelles encapsulated with paclitaxel for the treatment of melanoma using subcutaneous and pulmonary metastatic mice models. Biomaterials 2011, 32, 5934–5944. [Google Scholar]
- Haley, B.; Frenkel, E. Nanoparticles for drug delivery in cancer treatment. Urol. Oncol 2008, 26, 57–64. [Google Scholar]
- Zhang, W.; Shi, Y.; Chen, Y.; Ye, J.; Sha, X.; Fang, X. Multifunctional Pluronic P123/F127 mixed polymeric micelles loaded with paclitaxel for the treatment of multidrug resistant tumors. Biomaterials 2011, 32, 2894–2906. [Google Scholar]


| Hydrogel formulations | Composition (wt %) | |
|---|---|---|
| Formulation 1 | Plu 14%/PAA 0.75%/Epi 50 μg/mL | Liquid suppository |
| Formulation 2 | Plu 14%/PAA 0.375%/Epi 50 μg/mL | |
| Formulation 3 | Plu 14%/PAA 0.1875%/Epi 50 μg/mL | |
| Formulation 4 | Cocoa butter 0.5 g/Plu 14%/PAA 0.75%/Epi 50 μg/mL | Solid suppository |
| Formulation 5 | Cocoa butter 0.5 g/Plu 14%/PAA 0.375%/Epi 50 μg/mL | |
| Formulation 6 | Cocoa butter 0.5 g/Plu 14%/PAA 0.1875%/Epi 50 μg/mL | |
| Suppository formulations | Fmax (g) |
|---|---|
| Formulation 1 | 6.70 ± 0.23 |
| Formulation 2 | 5.39 ± 0.27 |
| Formulation 3 | 3.78 ± 0.13 |
| Formulation 4 | 6.99 ± 0.25 |
| Formulation 5 | 5.64 ± 0.21 |
| Formulation 6 | 4.05 ± 0.29 |
| Cocoa butter | 5.94 ± 0.57 |
| Formulations | Cmax (μg/mL) | Tmax (h) | AUC0→∞ (h × μg/mL) | %Fr |
|---|---|---|---|---|
| Intravenous Epi | 100.00 ± 0.00 †,‡,§,& | 0 | 34.38 ± 0.85 †,‡,§,& | 100.00 ± 2.47 †,‡,§,& |
| Rectal Epi | 2.81 ± 1.38 * | 2 | 17.26 ± 1.95 *,‡,§,& | 50.20 ± 5.67 *,‡,§,& |
| Oral Epi in Plu/PAA | 3.01 ± 1.08 * | 3 | 22.85 ± 2.04 *,† | 66.46 ± 5.93 *,† |
| Epi in liquid suppository | 3.36 ± 2.17 * | 3 | 23.04 ± 2.37 *,† | 67.02 ± 6.89 *,† |
| Epi in solid suppository | 3.16 ± 1.18 * | 3 | 22.14 ± 2.07 *,† | 64.40 ± 6.02 *,† |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lo, Y.-L.; Lin, Y.; Lin, H.-R. Evaluation of Epirubicin in Thermogelling and Bioadhesive Liquid and Solid Suppository Formulations for Rectal Administration. Int. J. Mol. Sci. 2014, 15, 342-360. https://doi.org/10.3390/ijms15010342
Lo Y-L, Lin Y, Lin H-R. Evaluation of Epirubicin in Thermogelling and Bioadhesive Liquid and Solid Suppository Formulations for Rectal Administration. International Journal of Molecular Sciences. 2014; 15(1):342-360. https://doi.org/10.3390/ijms15010342
Chicago/Turabian StyleLo, Yu-Li, Yijun Lin, and Hong-Ru Lin. 2014. "Evaluation of Epirubicin in Thermogelling and Bioadhesive Liquid and Solid Suppository Formulations for Rectal Administration" International Journal of Molecular Sciences 15, no. 1: 342-360. https://doi.org/10.3390/ijms15010342
APA StyleLo, Y.-L., Lin, Y., & Lin, H.-R. (2014). Evaluation of Epirubicin in Thermogelling and Bioadhesive Liquid and Solid Suppository Formulations for Rectal Administration. International Journal of Molecular Sciences, 15(1), 342-360. https://doi.org/10.3390/ijms15010342