Bicistronic Gene Transfer Tools for Delivery of miRNAs and Protein Coding Sequences
Abstract
:1. Introduction
2. Results and Discussion
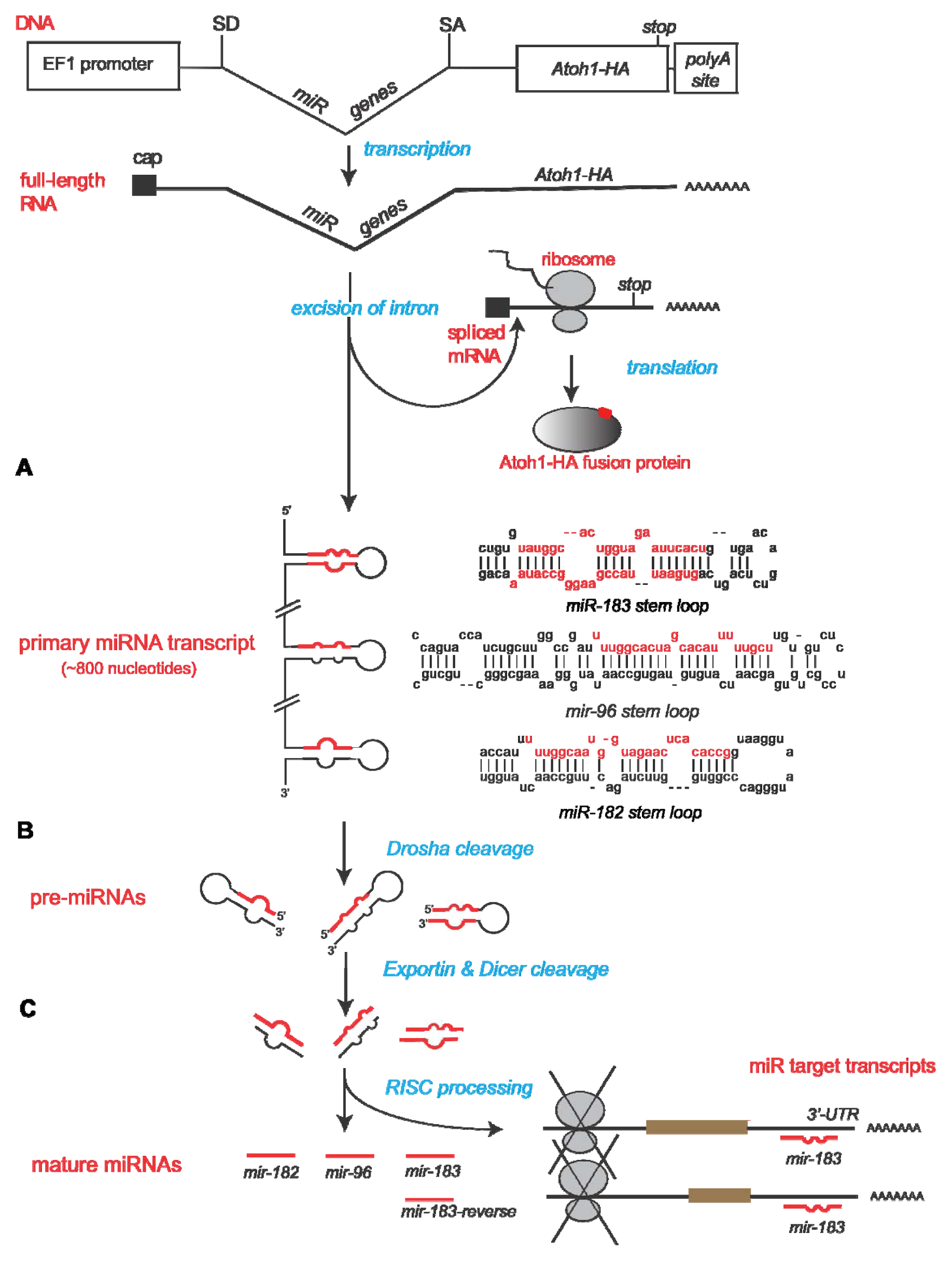
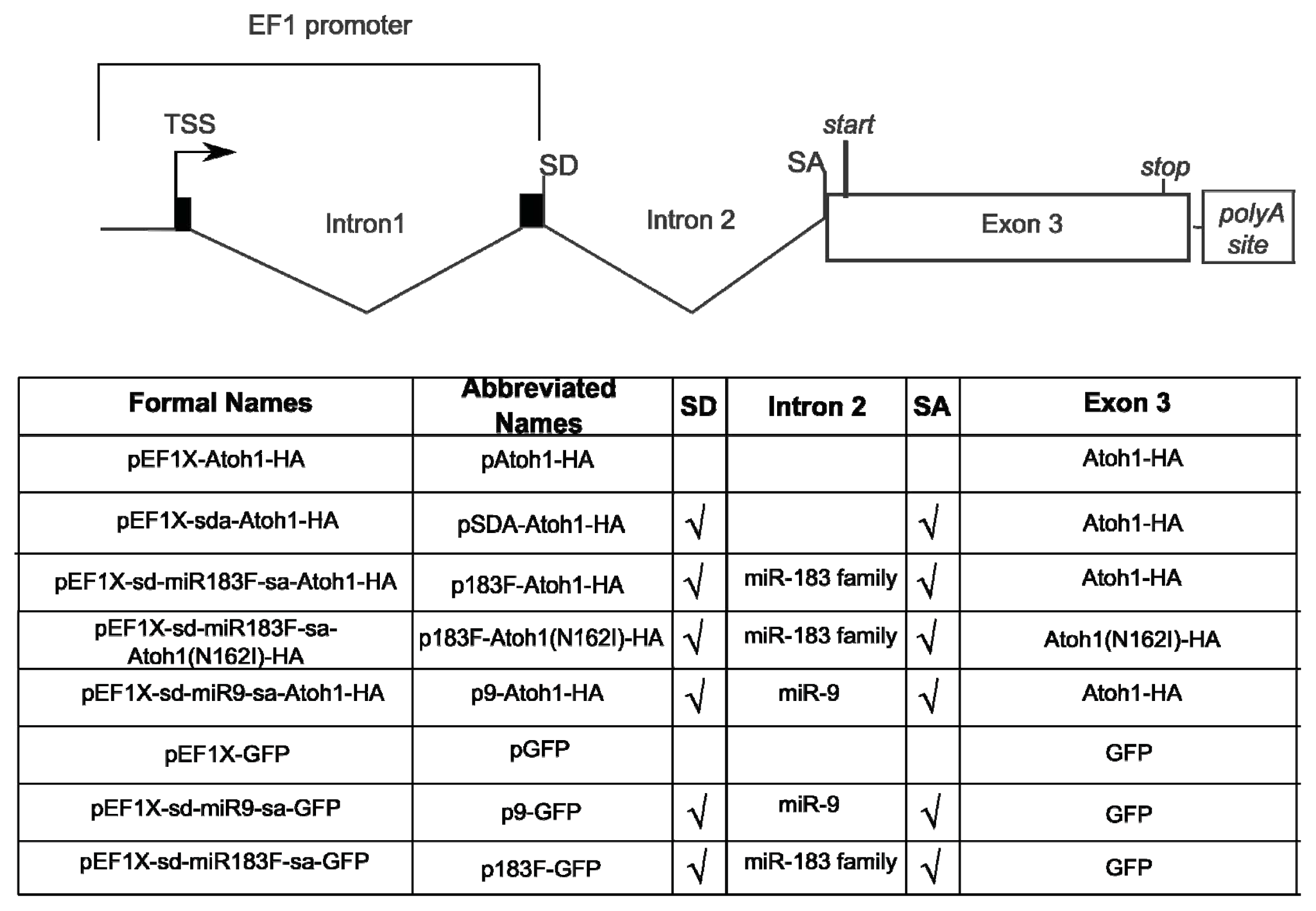
2.1. Construction of Bifunctional Atoh1-HA and miRNA Expression Vector
2.2. Confirmation of Atoh1-HA Production and Function from a Bifunctional Cassette
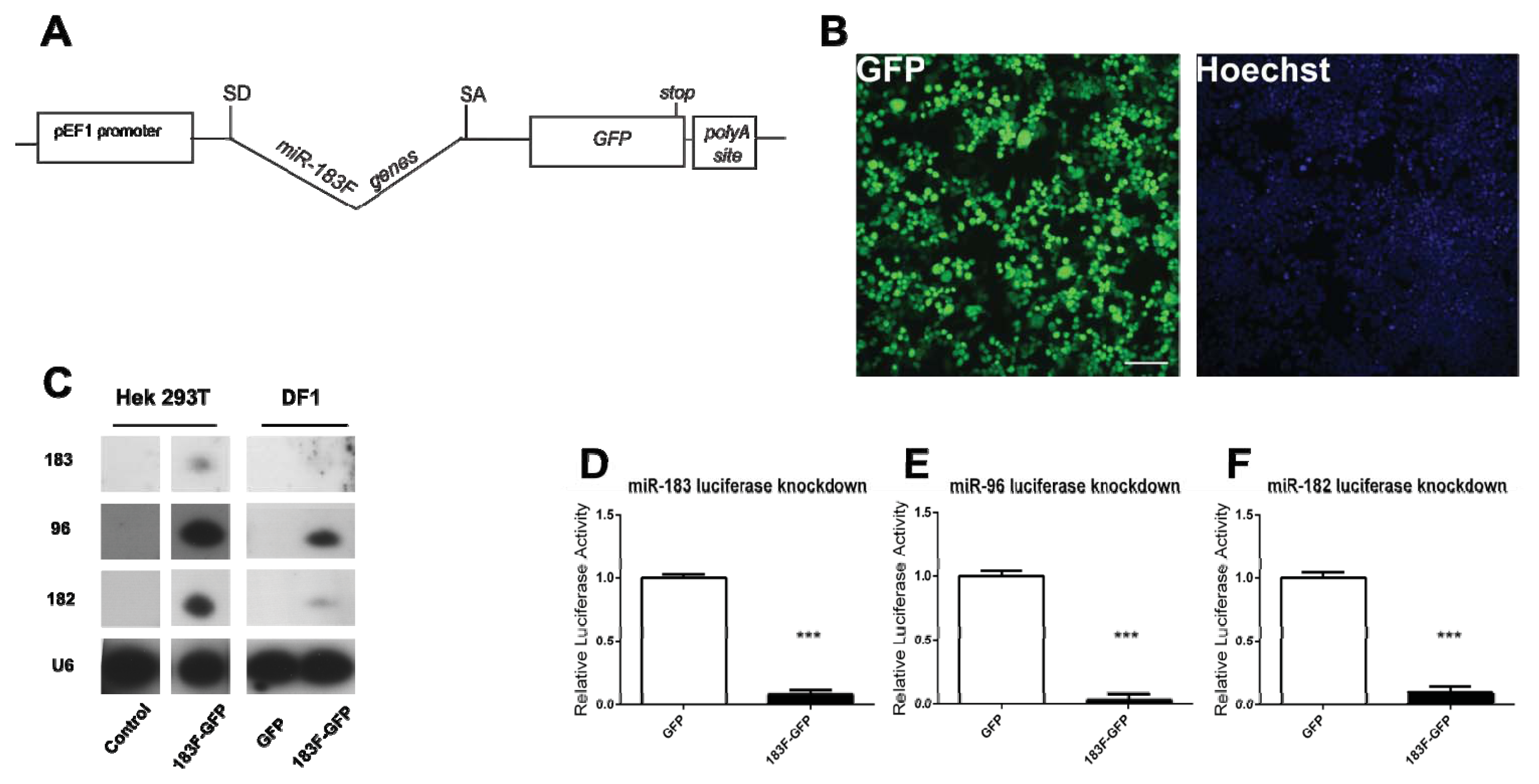
2.3. Confirmation of miRNA Production and Function from a Bifunctional Cassette
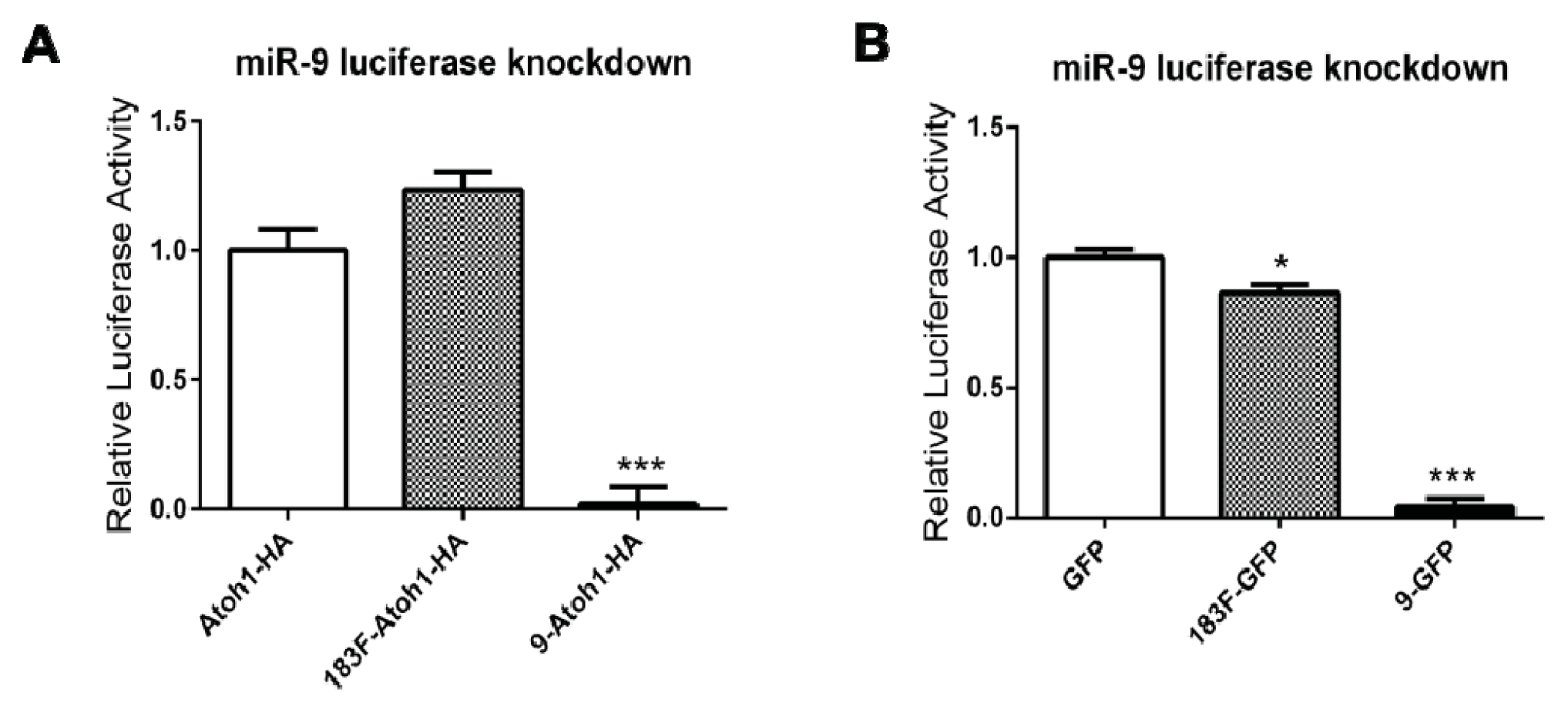
2.4. Overexpression of Functional miRNAs from GFP Expression Vectors
2.5. MiRNAs Produced from Expression Vectors Bind with Specificity
3. Experimental Section
3.1. Bifunctional Plasmid Construction
3.2. Mutation of Atoh1-HA Fusion Protein
3.3. HEK293T Plasmid Transfection
3.4. HEK293T Immunostain and Imaging
3.5. Atoh1 and MiRNA Luciferase Assays
3.6. Northern Blots
3.7. Statistical Analysis
4. Conclusions
Supplementary Information
ijms-14-18239-s001.pdfAcknowledgments
Conflicts of Interest
References
- Ying, S.Y.; Lin, S.L. Intron-mediated RNA interference and microRNA biogenesis. In Methods of Molecular Biology, siRNA and miRNA Gene Silencing; Sioud, M., Ed.; Humana Press: New York, NY, USA, 2009; Volume 487, pp. 387–413. [Google Scholar]
- He, L.; Thomson, J.M.; Hemann, M.T.; Hernando-Monge, E.; Mu, D.; Goodson, S.; Powers, S.; Cordon-Cardo, C.; Lowe, S.W.; Hannon, G.J.; et al. A microRNA polycistron as a potential human oncogene. Nature 2005, 435, 828–833. [Google Scholar]
- Kasinski, A.L.; Slack, F.J. Epigenetics and genetics. MicroRNAs en route to the clinic: Progress in validating and targeting microRNAs for cancer therapy. Nat. Rev. Cancer 2011, 11, 849–864. [Google Scholar]
- Lewis, M.A.; Steel, K.P. MicroRNAs in mouse development and disease. Semin Cell Dev. Biol 2010, 21, 774–780. [Google Scholar]
- Furukawa, N.; Sakurai, F.; Katayama, K.; Seki, N.; Kawabata, K.; Mizuguchi, H. Optimization of a microRNA expression vector for function analysis of microRNA. J. Control Release 2011, 150, 94–101. [Google Scholar]
- Packer, A.N.; Xing, Y.; Harper, S.Q.; Jones, L.; Davidson, B.L. The bifunctional microRNA miR-9/miR-9* regulates REST and CoREST and is downregulated in Huntington’s disease. J. Neurosci 2008, 28, 14341–14346. [Google Scholar]
- Rissland, O.S.; Hong, S.-J.; Bartel, D.P. MicroRNA destabilization enables dynamic regulation of the miR-16 family in response to cell-cycle changes. Mol. Cell 2011, 43, 993–1004. [Google Scholar]
- Otaegi, G.; Pollock, A.; Sun, T. An optimized sponge for microRNA miR-9 affects spinal motor neuron development in vivo. Front. Neurosci 2011, 5, 146. [Google Scholar]
- Shibata, M.; Kurokawa, D.; Nakao, H.; Ohmura, T.; Aizawa, S. MicroRNA-9 modulates Cajal-Retzius cell differentiation by suppressing Foxg1 expression in mouse medial pallium. J. Neurosci 2008, 28, 10415–10421. [Google Scholar]
- Zhao, C.; Sun, G.; Li, S.; Shi, Y. A feedback regulatory loop involving microRNA-9 and nuclear receptor TLX in neural stem cell fate determination. Nat.Struct. Mol. Biol 2009, 16, 365–371. [Google Scholar]
- Chiang, H.R.; Schoenfeld, L.W.; Ruby, J.G.; Auyeung, V.C.; Spies, N.; Baek, D.; Johnston, W.K.; Russ, C.; Luo, S.; Babiarz, J.E.; et al. Mammalian microRNAs: Experimental evaluation of novel and previously annotated genes. Gene Dev 2010, 24, 992–1009. [Google Scholar]
- Amendola, M.; Passerini, L.; Pucci, F.; Gentner, B.; Bacchetta, R.; Naldini, L. Regulated and multiple miRNA and siRNA delivery into primary cells by a lentiviral platform. Mol. Ther 2009, 17, 1039–1052. [Google Scholar]
- Pierce, M.L.; Weston, M.D.; Fritzsch, B.; Gabel, H.W.; Ruvkun, G.; Soukup, G.A. MicroRNA-183 family conservation and ciliated neurosensory organ expression. Evo. Dev 2008, 10, 106–113. [Google Scholar]
- Mihelich, B.L.; Khramtsova, E.A.; Arva, N.; Vaishnav, A.; Johnson, D.N.; Giangreco, A.A.; Martens-Uzunova, E.; Bagasra, O.; Kajdacsy-Balla, A.; Nonn, L. MiR-183-96-182 cluster is overexpressed in prostate tissue and regulates zinc homeostasis in prostate cells. J. Biol. Chem 2011, 286, 44503–44511. [Google Scholar]
- Hannafon, B.N.; Sebastiani, P.; de las Morenas, A.; Lu, J.; Rosenberg, C.L. Expression of microRNA and their gene targets are dysregulated in preinvasive breast cancer. Breast Cancer Res 2011, 13, R24. [Google Scholar]
- Zhu, W.; Liu, X.; He, J.; Chen, D.; Hunag, Y.; Zhang, Y.K. Overexpression of members of the microRNA-183 family is a risk factor for lung cancer: A case control study. BMC Cancer 2011, 11, 393. [Google Scholar]
- Mencía, A.; Modamio-Høybjør, S.; Redshaw, N.; Morín, M.; Mayo-Merino, F.; Olavarrieta, L.; Aguirre, L.A.; del Castillo, I.; Steel, K.P.; Dalmay, T.; et al. Mutations in the seed region of human miR-96 are responsible for nonsyndromic progressive hearing loss. Nat. Genet 2009, 41, 609–613. [Google Scholar]
- Soldà, G.; Robusto, M.; Primignani, P.; Castorina, P.; Benzoni, E.; Cesarani, A.; Ambrosetti, U.; Asselta, R.; Duga, S. A novel mutation within the MIR96 gene causes non-syndromic inherited hearing loss in an Italian family by altering pre-miRNA processing. Hum. Mol. Genet 2012, 21, 577–585. [Google Scholar]
- Lewis, M.A.; Quint, E.; Glazier, A.M.; Fuchs, H.; de Angelis, M.H.; Langford, C.; van Dongen, S.; Abreu-Goodger, C.; Piipari, M.; Redshaw, N.; et al. An ENU-induced mutation of miR-96 associated with progressive hearing loss in mice. Nat. Genet 2009, 41, 614–618. [Google Scholar]
- Kuhn, S.; Johnson, S.L.; Furness, D.N.; Chen, J.; Ingham, N.; Hilton, J.M.; Steffes, G.; Lewis, M.A.; Zampini, V.; Hackney, C.M.; et al. MiR-96 regulates the progression of differentiation in mammalian cochlear inner and outer hair cells. Proc. Natl. Acad. Sci. USA 2011, 108, 2355–2360. [Google Scholar]
- Xu, S.; Witmer, P.D.; Lumayag, S.; Kovacs, B.; Valle, D. MicroRNA (miRNA) transcriptome of mouse retina and identification of a sensory organ-specific miRNA cluster. J. Biol. Chem 2007, 282, 25053–2366. [Google Scholar]
- Lumayag, S.; Haldin, C.E.; Corbett, N.J.; Wahlin, K.J.; Cowan, C.; Turturro, S.; Larsen, P.E.; Kovacs, B.; Witmer, P.D.; Valle, D.; et al. Inactivation of the microRNA-183/96/182 cluster results in syndromic retinal degeneration. Proc. Natl. Acad. Sci. USA 2013, 110, E507–E516. [Google Scholar]
- Weston, M.D.; Pierce, M.L.; Jensen-Smith, H.C.; Fritzsch, B.; Rocha-Sanchez, S.; Beisel, K.W.; Soukup, G.A. MicroRNA-183 family expression in hair cell development and requirement of microRNAs for hair cell maintenance and survival. Dev. Dynam 2011, 240, 808–819. [Google Scholar]
- Weston, M.D.; Pierce, M.L.; Rocha-Sanchez, S.; Beisel, K.W.; Soukup, G.A. MicroRNA gene expression in the mouse inner ear. Brain Res 2006, 1111, 95–104. [Google Scholar]
- Sacheli, R.; Nguyen, L.; Borgs, L.; Vandenbosch, R.; Bodson, M.; Lefebvre, P.; Malgrange, B. Expression patterns of miR-96, miR-182 and miR-183 in the development inner ear. Gene Expr. Patterns 2009, 9, 364–370. [Google Scholar]
- Li, H.; Kloosterman, W.; Fekete, D.M. MicroRNA-183 family members regulate sensorineural fates in the inner ear. J. Neurosci 2010, 30, 3254–3263. [Google Scholar]
- Izumikawa, M.; Minoda, R.; Kawamoto, K.; Abrashkin, K.A.; Swiderski, D.L.; Dolan, D.F.; Brough, D.E.; Raphael, Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat. Med 2005, 11, 271–276. [Google Scholar]
- Yang, S.-M.; Chen, W.; Guo, W.-W.; Jia, S.; Sun, J.-H.; Liu, H.-Z.; Young, W.-Y.; He, D.Z.Z. Regeneration of stereocilia of hair cells by forced Atoh1 expression in the adult mammalian cochlea. PLoS One 2012, 7, e46355. [Google Scholar]
- Groves, A.K.; Zhang, K.D.; Fekete, D.M. The genetics of hair cell development and regeneration. Annu. Rev. Neurosci 2013, 36, 361–381. [Google Scholar]
- Agarwala, S.; Sanders, T.A.; Ragsdale, C.W. Sonic hedgehog control of size and shape in midbrain pattern formation. Science 2001, 291, 2147–2150. [Google Scholar]
- Lin, S.L.; Chang, D.; Wu, D.Y.; Ying, S.Y. A novel RNA splicing-mediated gene silencing mechanism potential for genome evolution. Biochem. Biophy. Res. Commun 2003, 310, 754–760. [Google Scholar]
- Lin, S.-L.; Ying, S.-Y. New drug design for gene therapy—Taking advantage of introns. Lett. Drug Des. Discov 2004, 1, 256–262. [Google Scholar]
- Gubbels, S.P.; Woessner, D.W.; Mitchell, J.C.; Ricci, A.J.; Brigande, J.V. Functional auditory hair cells produced in the mammalian cochlea by in utero gene transfer. Nature 2008, 455, 537–541. [Google Scholar]
- Krizhanovsky, V.; Soreq, L.; Kliminski, V.; Ben-arie, N. Math1 target genes are enriched with evolutionarily conserved clustered E-box binding sites. J. Mol. Neurosci 2006, 28, 211–229. [Google Scholar]
- Jarman, A.P.; Grell, E.H.; Ackerman, L.; Jan, L.J.; Jan, Y.N. Atonal is the proneural gene drosophila photoreceptors. Nature 1994, 369, 398–400. [Google Scholar]
- Mulvaney, J.; Dabdoub, A. Atoh1, an essential transcription factor in neurogenesis and intestinal and inner ear development: Function, regulation, and context dependency. JARO 2012, 13, 281–293. [Google Scholar]
- Hughes, S.H. The RCAS vector system. Folia Biol 2004, 50, 107–119. [Google Scholar]
- Kwan, K.M.; Fujimoto, E.; Grabher, C.; Mangum, B.D.; Hardy, M.E.; Campbell, D.S.; Parant, J.M.; Yost, H.J.; Kanki, J.P.; Chien, C.-B. The Tol2kit: A multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dynam 2007, 236, 3088–3099. [Google Scholar]
- Karali, M.; Manfredi, A.; Puppo, A.; Marrocco, E.; Gargiulo, A.; Allocca, M.; Corte, M.D.; Rossi, S.; Giunti, M.; Bacci, M.L.; et al. MicroRNA-restricted transgene expression in the retina. PLoS One 2011, 6, e22166. [Google Scholar]
- Chung, K.-H. Polycistronic RNA polymerase II expression vectors for RNA interference based on BIC/miR-155. Nucleic Acids Res 2006, 34, e53. [Google Scholar]
- Shin, K.-J.; Wall, E.A.; Zavzavadjian, J.R.; Santat, L.A.; Liu, J.; Hwang, J.-I.; Rebres, R.; Roach, T.; Seaman, W.; Simon, M.I.; et al. A single lentiviral vector platform for microRNA-based conditional RNA interference and coordinated transgene expression. Proc. Natl. Acad. Sci. USA 2006, 103, 13759–13764. [Google Scholar]
- Zhu, X.; Santat, L.A.; Chang, M.S.; Liu, J.; Zavzavadjian, J.R.; Wall, E.A.; Kivork, C.; Simon, M.I.; Fraser, I.D. A versatile approach to multiple gene RNA interference using microRNA-based short hairpin RNAs. BMC Mol. Biol 2007, 8, 98. [Google Scholar]
- Stegmeier, F.; Hu, G.; Rickles, R.J.; Hannon, G.J.; Elledge, S.J. A lentiviral microRNA-based system for single-copy polymerase II-regulated RNA interference in mammalian cells. Proc. Natl. Acad. Sci. USA 2005, 102, 13212–13217. [Google Scholar]
- Wang, X.; Liu, P.; Liu, H.; Yang, W.; Liu, Z.; Zhuo, Z.; Gao, Y. Delivery of interferons and siRNA targeting STAT3 using lentiviral vectors suppresses the growth of murine melanoma. Cancer Gene Ther 2012, 19, 822–827. [Google Scholar]
- Du, G.; Yonekubo, J.; Zeng, Y.; Osisami, M.; Frohman, M.A. Design of expression vectors for RNA interference based on miRNAs and RNA splicing. FEBS J 2006, 273, 5421–5427. [Google Scholar]
- Karwacz, K.; Bricogne, C.; MacDonald, D.; Arce, F.; Bennett, C.L.; Collins, M.; Escors, D. PD-L1 co-stimulation contributes to ligand-induced T cell receptor down-modulation on CD8+ T cells. EMBO Mol. Med 2011, 3, 581–592. [Google Scholar]
- Arce, F.; Breckpot, K.; Stephenson, H.; Karwacz, K.; Ehrenstein, M.R.; Collins, M.; Escors, D. Selective ERK activation differentiates mouse and human tolerogenic dendritic cells, expands antigen-specific regulatory T cells, and suppresses experimental inflammatory arthritis. Arthritis Rheum 2011, 63, 84–95. [Google Scholar]


© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Stoller, M.L.; Chang, H.C.; Fekete, D.M. Bicistronic Gene Transfer Tools for Delivery of miRNAs and Protein Coding Sequences. Int. J. Mol. Sci. 2013, 14, 18239-18255. https://doi.org/10.3390/ijms140918239
Stoller ML, Chang HC, Fekete DM. Bicistronic Gene Transfer Tools for Delivery of miRNAs and Protein Coding Sequences. International Journal of Molecular Sciences. 2013; 14(9):18239-18255. https://doi.org/10.3390/ijms140918239
Chicago/Turabian StyleStoller, Michelle L., Henry C. Chang, and Donna M. Fekete. 2013. "Bicistronic Gene Transfer Tools for Delivery of miRNAs and Protein Coding Sequences" International Journal of Molecular Sciences 14, no. 9: 18239-18255. https://doi.org/10.3390/ijms140918239
APA StyleStoller, M. L., Chang, H. C., & Fekete, D. M. (2013). Bicistronic Gene Transfer Tools for Delivery of miRNAs and Protein Coding Sequences. International Journal of Molecular Sciences, 14(9), 18239-18255. https://doi.org/10.3390/ijms140918239




