Skp2 Regulates Subcellular Localization of PPARγ by MEK Signaling Pathways in Human Breast Cancer
Abstract
:1. Introduction
2. Results
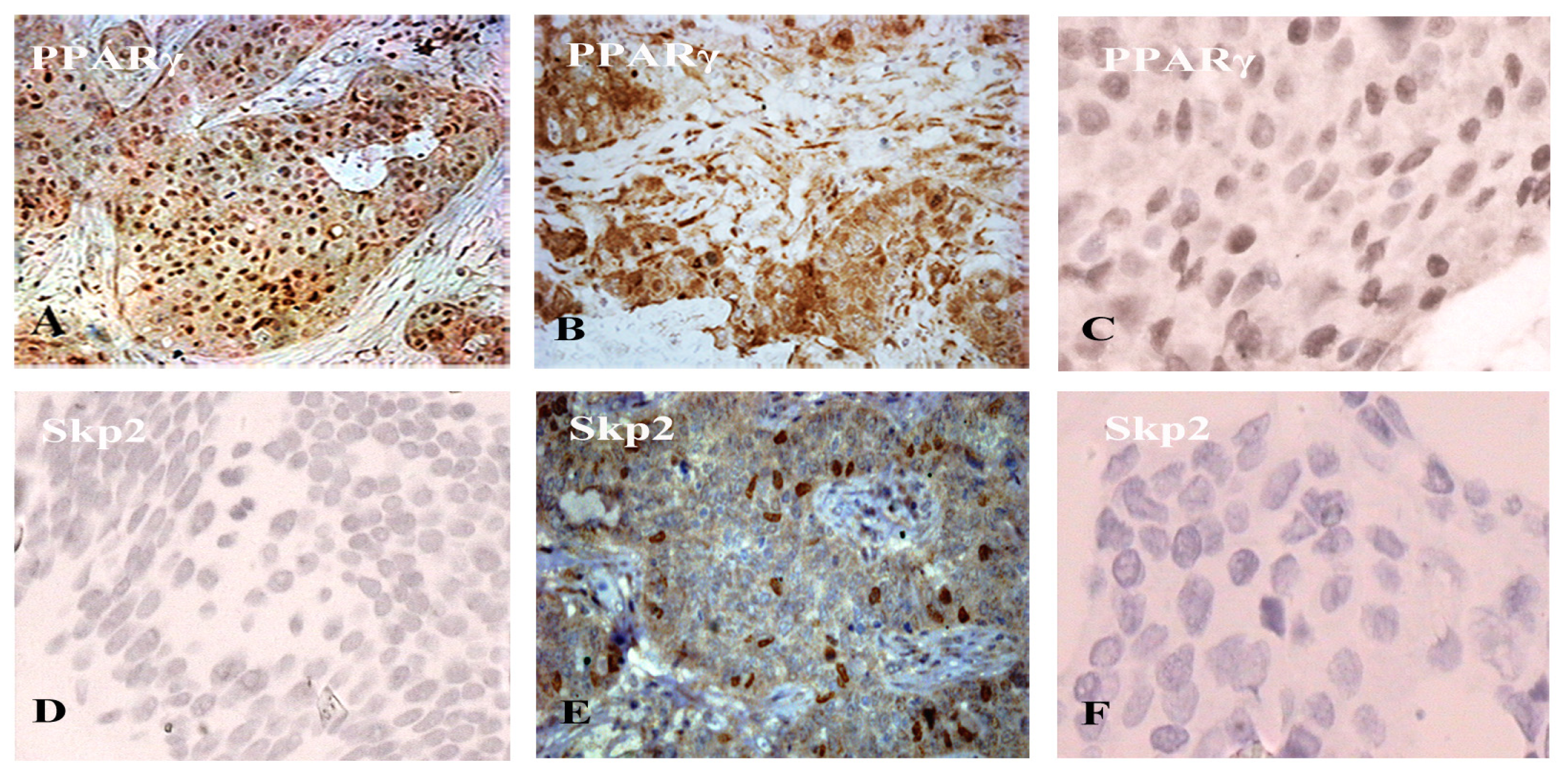
2.1. The Expression of Cytoplasmic PPARγ Is Positively Related with Skp2 Expression
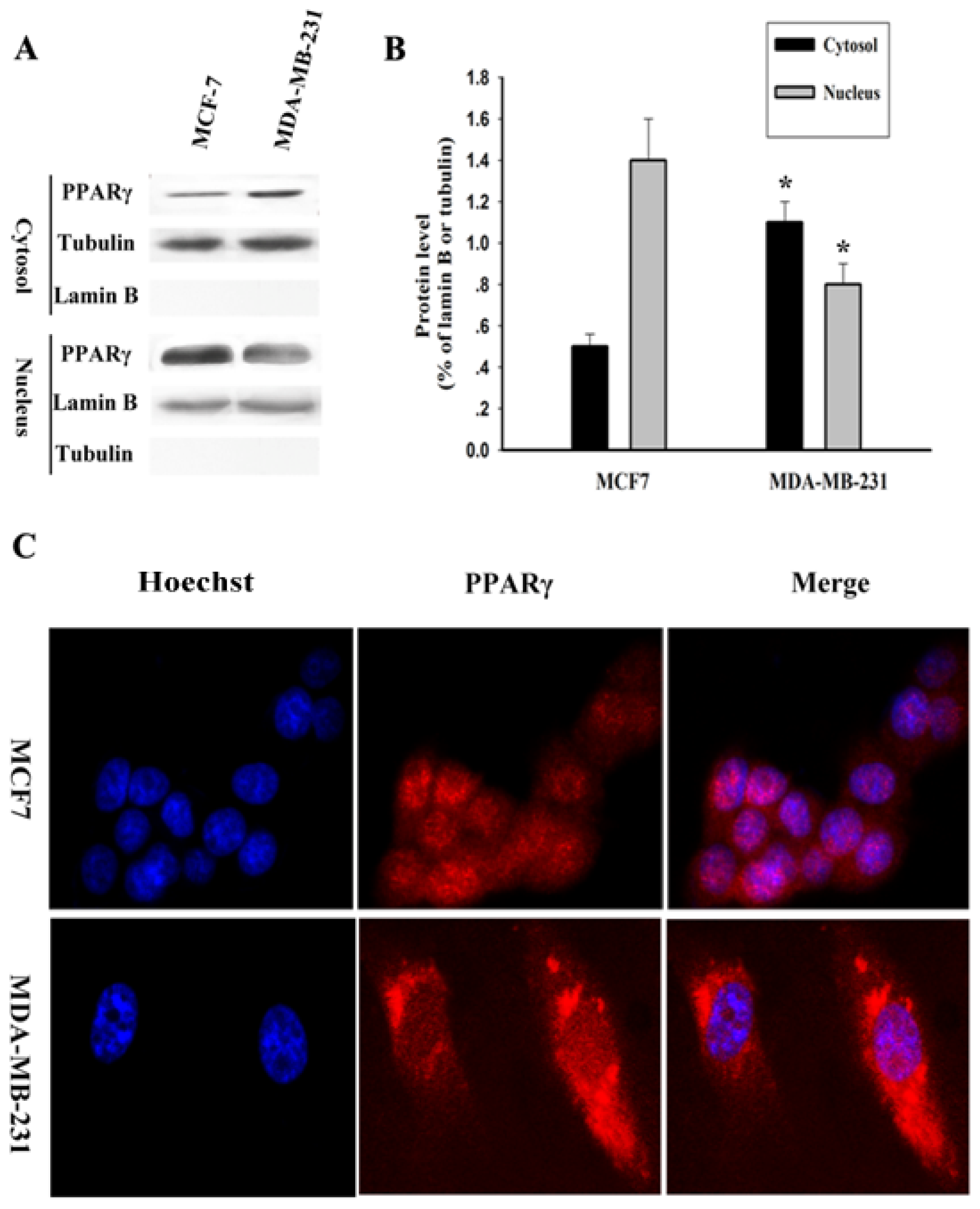
2.2. Subcellular Localization of PPARγ in Human Breast Cancer Cells
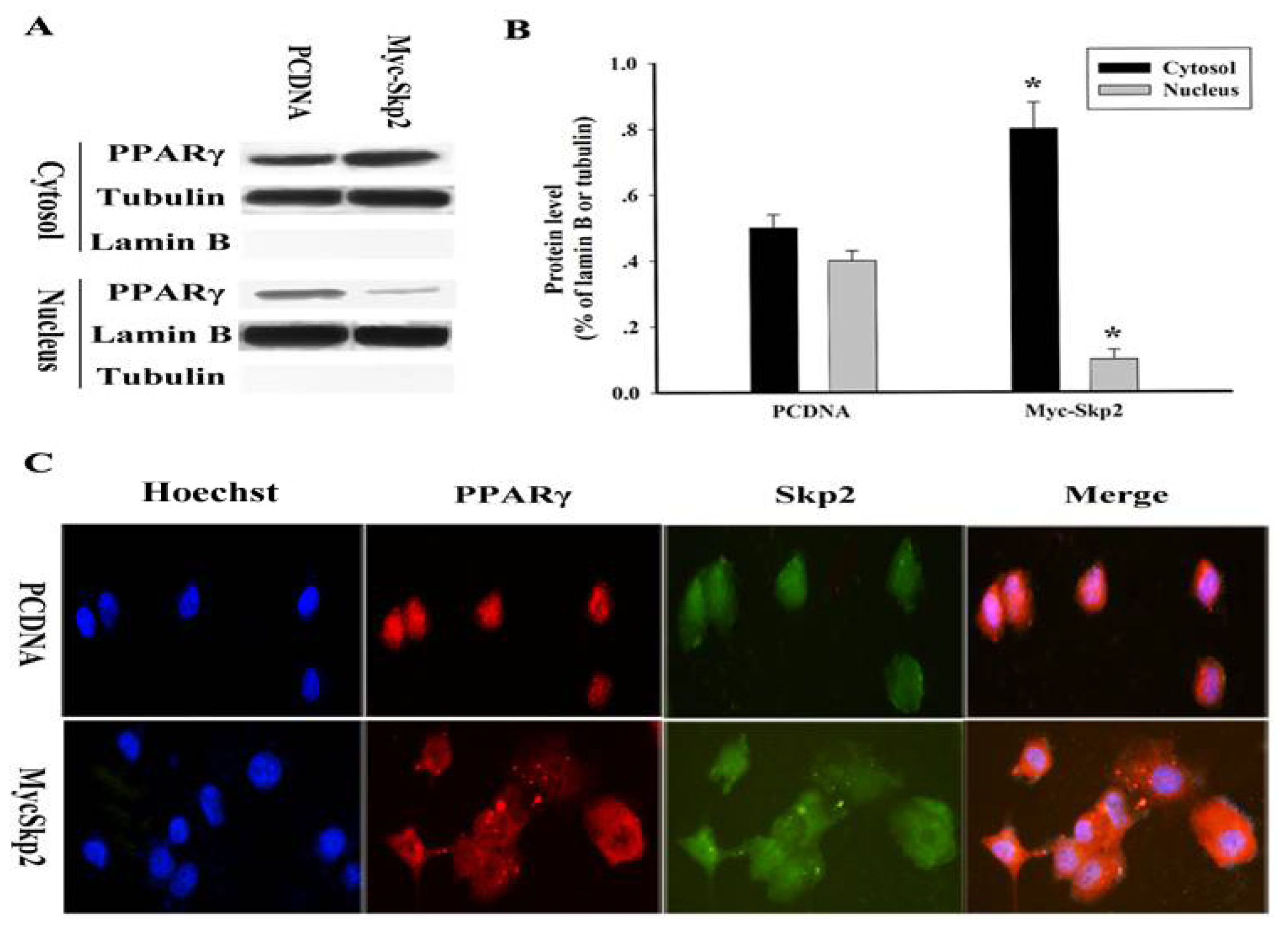
2.3. Cytosolic Retention of PPARγ upon Overexpression of Myc-Skp2
2.4. PPARγ Was Retained in the Cytosol upon MEK1-Dependent Mechanisms in MDA-MB-231 Cells
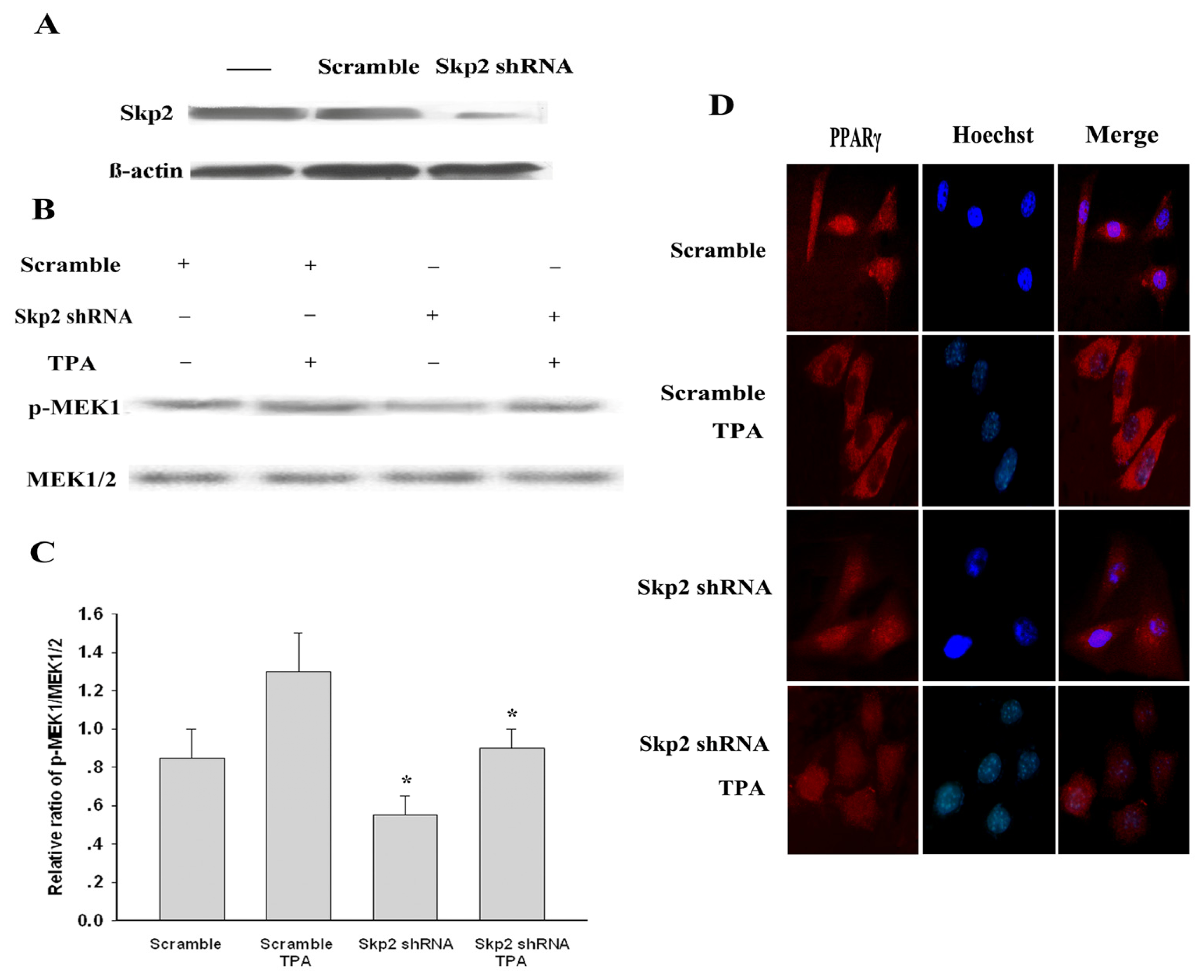
2.5. Down-Regulated Skp2 Reduced Phosphorylation Level of MEK1 and Significantly Reversed TPA-Induced Nuclear Export of PPARγ in MDA-MB-231 Cells
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Cell Culture
4.3. Tissue Samples
4.4. Immunohistochemistry Methods
4.5. Immunohistochemical Evaluation
4.6. Expression Plasmid and Transient Transfection
4.7. Cellular Fractionation
4.8. shRNAs and Transfection
4.9. Western Blot Analysis
4.10. Immunofluorescence Microscopy
4.11. Statistical Analysis
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Houssami, N.; Cuzick, J.; Dixon, J.M. The prevention, detection, and management of breast cancer. Med. J. Aust 2006, 184, 230–234. [Google Scholar]
- Cuzick, J.; Warwick, J.; Pinney, E.; Warren, R.M.; Duffy, S.W. Tamoxifen and breast density in women at increased risk of breast cancer. J. Natl. Cancer Inst 2004, 96, 621–628. [Google Scholar]
- Mangelsdorf, D.J.; Thummel, C.; Beato, M.; Herrlich, P.; Schütz, G.; Umesono, K.; Blumberg, B.; Kastner, P.; Mark, M.; Chambon, P.; et al. The nuclear receptor superfamily: The second decade. Cell 1995, 83, 835–839. [Google Scholar]
- Kliewer, S.A.; Forman, B.M.; Blumberg, B.; Ong, E.S.; Borgmeyer, U.; Mangelsdorf, D.J.; Umesono, K.; Evans, R.M. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc. Natl. Acad. Sci. USA 1994, 91, 7355–7359. [Google Scholar]
- Panigrahy, D.; Huang, S.; Kieran, M.W.; Kaipainen, A. PPARγ as a therapeutic target for tumor angiogenesis and metastasis. Cancer Biol. Ther 2005, 4, 687–693. [Google Scholar]
- Ikezoe, T.; Miller, C.W.; Kawano, S.; Heaney, A.; Williamson, E.A.; Hisatake, J.; Green, E.; Hofmann, W.; Taquchi, H.; Koeffler, H.P. Mutational analysis of the peroxisome proliferator-activated receptor γ gene in human malignancies. Cancer Res 2001, 61, 5307–5310. [Google Scholar]
- Burgermeister, E.; Chuderland, D.; Hanoch, T.; Meyer, M.; Liscovitch, M.; Seger, R. Interaction with MEK causes nuclear export and downregulation of peroxisome proliferator-activated receptor γ. Mol. Cell. Biol 2007, 27, 803–817. [Google Scholar]
- Knethen, A.V.; Tzieply, N.; Jennewein, C.; Brüne, B. Casein-kinase-II-dependent phosphorylation of PPARγ provokes CRM1-mediated shuttling of PPARγ from the nucleus to the cytosol. J. Cell. Sci 2010, 123, 192–201. [Google Scholar]
- Khateeb, J.; Kiyan, Y.; Aviram, M.; Tkachuk, S.; Dumler, I.; Fuhrman, B. Urokinase-type plasminogen activator downregulates paraoxonase 1 expression in hepatocytes by stimulating peroxisome proliferator-activated receptor-γ nuclear export. Arterioscler. Thromb. Vasc. Biol 2012, 32, 449–458. [Google Scholar]
- Krek, W. Proteolysis and the G1-S transition: the SCF connection. Curr. Opin. Genet 1998, 8, 36–42. [Google Scholar]
- Nakayama, K.I.; Nakayama, K. Ubiquitin ligases: Cell-cycle control and cancer. Nat. Rev. Cancer 2006, 6, 369–381. [Google Scholar]
- Signoretti, S.; Di Marcotullio, L.; Richardson, A.; Richardson, A.; Isaac, B.; Rue, M.; Monti, F.; Loda, M.; Pagano, M. Oncogenic role of the ubiquitin ligase subunit Skp2 in human breast cancer. J. Clin. Invest 2002, 110, 633–641. [Google Scholar]
- Gstaiger, M.; Jordan, R.; Lim, M.; Catzavelos, C.; Mestan, J.; Slingerland, J.; Krek, W. Skp2 is oncogenic and overexpressed in human cancers. Proc. Natl. Acad. Sci. USA 2001, 98, 5043–5048. [Google Scholar]
- Seki, R.; Ohshima, K.; Fujisaki, T.; Uike, N.; Kawano, F.; Gondo, H.; Makino, S.; Eto, T.; Moriuchi, Y.; Taguchi, F.; et al. Prognostic significance of S-phase kinase-associated protein 2 and p27kip1 in patients with diffuse large B-cell lymphoma: effects of rituximab. Ann. Oncol 2010, 21, 833–841. [Google Scholar]
- Traub, F.; Mengel, M.; Lück, H.J.; Kreipe, H.H.; von Wasielewski, R. Prognostic impact of Skp2 and p27 in human breast cancer. Breast Cancer Res. Treat 2006, 99, 185–191. [Google Scholar]
- Meng, J.; Ding, Y.; Shen, A.; Yan, M.; He, F.; Ji, H.; Zou, L.; Liu, Y.; Wang, Y.; Lu, X.; et al. Overexpression of PPARγ can down-regulate Skp2 expression in MDA-MB-231 breast tumor cells. Mol. Cell Biochm 2010, 345, 171–180. [Google Scholar]
- Burns, K.A.; Vanden Heuvel, J.P. Modulation of PPAR activity via phosphorylation. Biochim. Biophys. Acta 2007, 1771, 952–960. [Google Scholar]
- Berger, J.; Patel, H.V.; Woods, J.; Hayes, N.S.; Parent, S.A.; Clemas, J.; Leibowitz, M.D.; Elbrecht, A.; Rachubinski, R.A.; Capone, J.P.; et al. A PPARgamma mutant serves as a dominant negative inhibitor of PPAR signaling and is localized in the nucleus. Mol. Cell. Endocrinol 2000, 162, 57–67. [Google Scholar]
- Gurnell, M.; Wentworth, J.M.; Agostini, M.; Adams, M.; Collingwood, T.N.; Provenzano, C.; Browne, P.O.; Rajanayagam, O.; Burris, T.P.; Schwabe, J.W. A dominant-negative peroxisome proliferator-activated receptor gamma (PPARgamma) mutant is a constitutive repressor and inhibits PPARgamma-mediated adipogenesis. J. Biol. Chem 2000, 275, 5754–5759. [Google Scholar]
- Kim, S.; Choi, J.H.; Lim, H.I.; Lee, S.K.; Kim, W.W.; Kim, J.S.; Kim, J.H.; Choe, J.H.; Yang, J.H. The Raf/MEK/ERK pathway in MCF-7 human breast cancer cells. Phytomedicine 2009, 16, 573–580. [Google Scholar]
- Papadaki, I.; Mylona, E.; Giannopoulou, I.; Markaki, S.; Keramopoulos, A.; Nakopoulou, L. PPARgamma expression in breast cancer: clinical value and correlation with ERbeta. Histopathology 2005, 46, 37–42. [Google Scholar]
- Mani, A.; Gelmann, E.P. The ubiquitin-proteasome pathway and its role in cancer. J. Clin. Oncol 2005, 23, 4776–4789. [Google Scholar]
- Kudo, Y.; Kitajima, S.; Sato, S.; Miyauchi, M.; Ogawa, I.; Takata, T. High expression of S-phase kinase-interacting protein 2, human F-box protein, correlate with poor prognosis in oral squamous cell carcinomas. Cancer Res 2001, 61, 7044–7047. [Google Scholar]
- Malik, S.N.; Brattain, M.; Ghosh, P.M.; Troyer, D.A.; Prihoda, T.; Bedolla, R.; Kreisberg, J.I. Immunohistochemical demonstration of phospho-Akt in high Gleason grade prostate cancer. Clin. Cancer Res 2002, 8, 1168–1171. [Google Scholar]
- Latres, E.; Chiarle, R.; Schulman, B.A.; Pavletich, N.P.; Pellicer, A.; Inghirami, G.; Pagano, M. Role of the F-box protein Skp2 in lymphomagenesis. Proc. Natl. Acad. Sci. USA 2001, 98, 2515–2520. [Google Scholar]
- Hershko, D.; Bornstein, G.; Ben-Izhak, O.; Carrano, A.; Pagano, M.; Krausz, M.M.; Hershko, A. Inverse relation between levels of p27 (Kip1) and of its ubiquitin ligase subunit Skp2 in colorectal carcinomas. Cancer 2001, 91, 1745–1751. [Google Scholar]
- Shapira, M.; Ben-Izhak, O.; Linn, S.; Futerman, B.; Minkov, I.; Hershko, D.D. The prognostic impact of the ubiquitin ligase subunit Skp2 and Cks1 in colorectal carcinoma. Cancer 2005, 103, 1336–1346. [Google Scholar]
- Masuda, T.; Inoue, H.; Sonoda, H.; Mine, S.; Yoshikawa, Y.; Nakayama, K.; Nakayama, K.; Mori, M. Clinical and biological significance of S-phase kinase-associated protein 2 (Skp2) gene expression in gastric carcinoma: Modulation of malignant phenotype by Skp2 overexpression, possibly via p27 proteolysis. Cancer Res 2002, 62, 3819–3825. [Google Scholar]
- Oliveira, A.M.; Okuno, S.H.; Nascimento, A.G. Lloyd RVSkp2 protein expression in soft tissue sarcomas. J. Clin. Oncol 2003, 21, 722–727. [Google Scholar]
- Min, Y.H.; Cheong, J.W.; Lee, M.H.; Kim, J.Y.; Lee, S.T.; Hahn, J.S.; Ko, Y.W. Elevated S-phase kinase-associated protein 2 protein expression in acute myelogenous leukemia: Its association with constitutive phosphorylation of phosphatase and tensin homologue protein and poor prognosis. Clin. Cancer Res 2004, 10, 5123–5130. [Google Scholar]
- Radke, S.; Pirkmaier, A.; Germain, D. Differential expression of the F-box proteins Skp2 and Skp2B in breast cancer. Oncogene 2005, 24, 3448–3458. [Google Scholar]
- Kohno, M.; Pouyssegur, J. Targeting the ERK signaling pathway in cancer therapy. Ann. Med 2006, 38, 200–211. [Google Scholar]
- Adeyinka, A.; Nui, Y.; Cherlet, T.; Snell, L.; Watson, P.H.; Murphy, L.C. Activated mitogen-activated protein kinase expression during human breast tumorigenesis and breast cancer progression. Clin. Cancer Res 2002, 8, 1747–1753. [Google Scholar]
- Davidovich, S.; Ben-Izhak, O.; Shapira, M.; Futerman, B.; Hershko, D.D. Over-expression of Skp2 is associated with resistance to preoperative doxorubicin-based chemotherapy in primary breast cancer. Breast Cancer Res 2008, 10, R63. [Google Scholar]
- Ravaioli, A.; Monti, F.; Regan, M.M.; Maffini, F.; Mastropasqua, M.G.; Spataro, V.; Castiglione-Gertsch, M.; Panzini, I.; Gianni, L.; Goldhirsch, A. p27 and Skp2 immunoreactivity and its clinical significance with endocrine and chemo-endocrine treatments in node-negative early breast cancer. Ann. Oncol 2008, 19, 660–668. [Google Scholar]
- Dai, S.; Wan, T.; Wang, B.; Zhou, X.; Xiu, F.; Chen, T.; Wu, Y.; Cao, X. More efficient induction of HLA-A*0201-restricted and carcinoembryonic antigen (CEA)-specific CTL response by immunization with Exosomes prepared from heat-stressed CEA-positive tumor cells. Clin. Cancer Res 2005, 11, 7554–7563. [Google Scholar]

| Criteria | Levels of cytoplasmic PPARγ | ||
|---|---|---|---|
| Low | High | p value a | |
| ER | |||
| 0 | 5 | 8 | 0.026 * |
| 1 | 27 | 10 | |
| PR | |||
| 0 | 9 | 10 | 0.055 |
| 1 | 23 | 8 | |
| HER-2 | |||
| Negative | 11 | 6 | 0.941 |
| Positive | 21 | 12 | |
| p53 | |||
| Negative | 13 | 11 | 0.164 |
| Positive | 19 | 7 | |
| TNM | |||
| I | 9 | 4 | 0.580 |
| II | 18 | 9 | |
| III | 5 | 5 | |
| Grade | |||
| 1 | 10 | 1 | 0.029 * |
| 2 | 18 | 10 | |
| 3 | 4 | 7 | |
| Ki-67 | |||
| Negative (<19) | 8 | 7 | 0.304 |
| Positive (≥19) | 24 | 11 | |
| Skp2 | |||
| Low (<10) | 25 | 0 | 0.000 * |
| High (≥10) | 7 | 18 | |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cheng, H.; Meng, J.; Wang, G.; Meng, Y.; Li, Y.; Wei, D.; Fu, C.; Deng, K.; Shen, A.; Wang, H.; et al. Skp2 Regulates Subcellular Localization of PPARγ by MEK Signaling Pathways in Human Breast Cancer. Int. J. Mol. Sci. 2013, 14, 16554-16569. https://doi.org/10.3390/ijms140816554
Cheng H, Meng J, Wang G, Meng Y, Li Y, Wei D, Fu C, Deng K, Shen A, Wang H, et al. Skp2 Regulates Subcellular Localization of PPARγ by MEK Signaling Pathways in Human Breast Cancer. International Journal of Molecular Sciences. 2013; 14(8):16554-16569. https://doi.org/10.3390/ijms140816554
Chicago/Turabian StyleCheng, Hongge, Jie Meng, Guisheng Wang, Yuming Meng, Yu Li, Dong Wei, Chunyun Fu, Kaifeng Deng, Aiguo Shen, Huimin Wang, and et al. 2013. "Skp2 Regulates Subcellular Localization of PPARγ by MEK Signaling Pathways in Human Breast Cancer" International Journal of Molecular Sciences 14, no. 8: 16554-16569. https://doi.org/10.3390/ijms140816554




