The Effects of Biopolymer Encapsulation on Total Lipids and Cholesterol in Egg Yolk during in Vitro Human Digestion
Abstract
:1. Introduction
2. Results
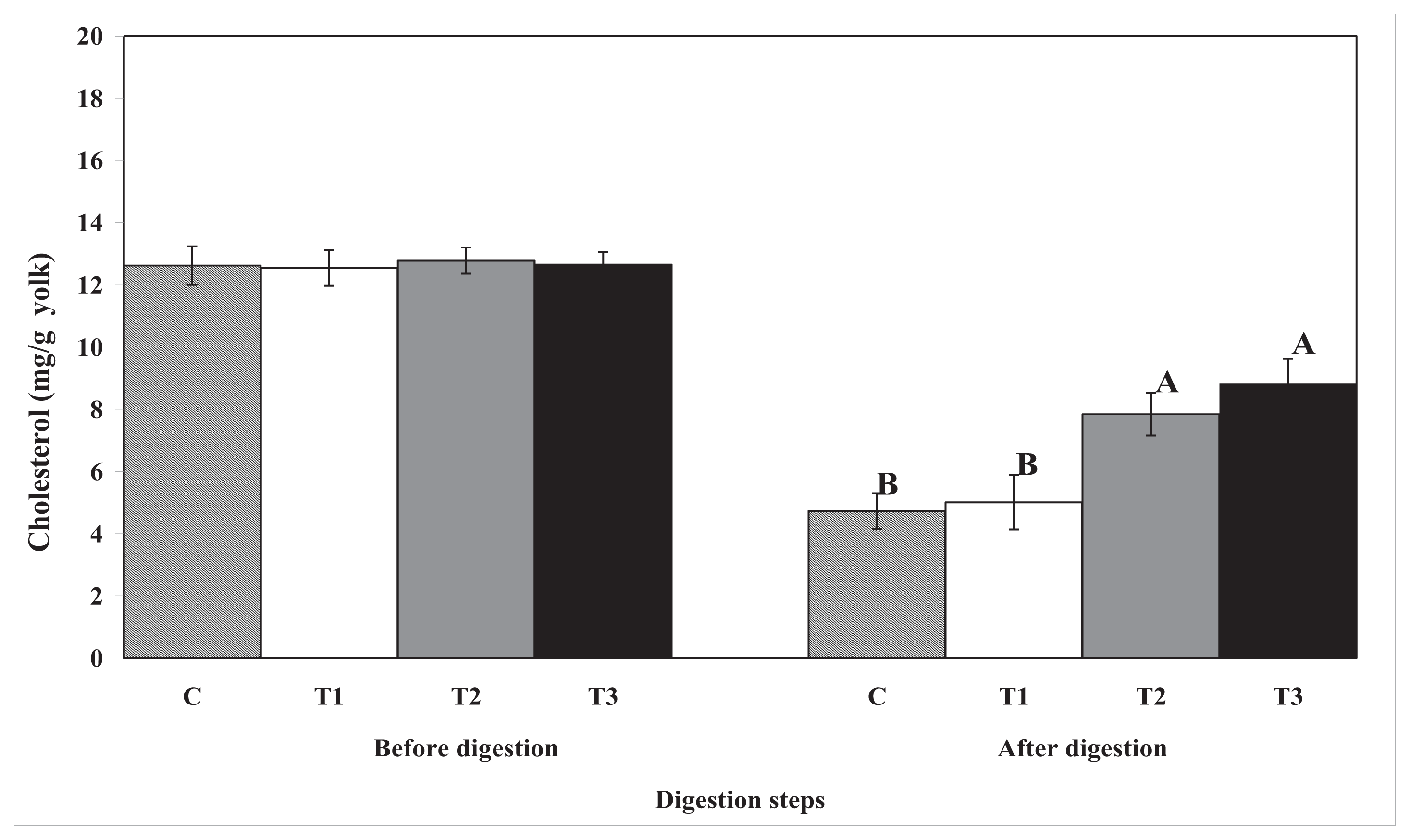
2.1. Digestion Rates of Total Lipids and Cholesterol
2.2. Free Fatty Acid
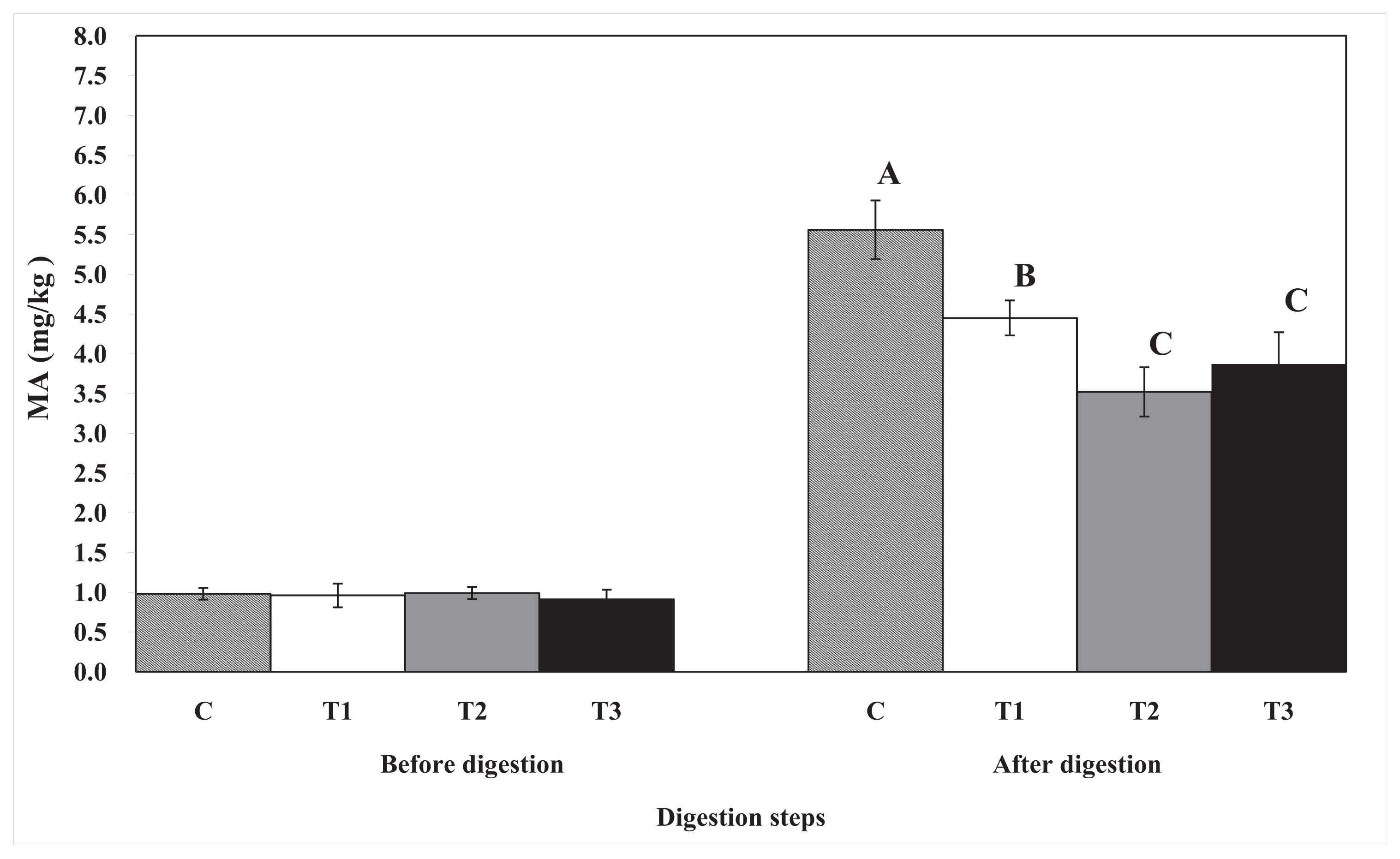
2.3. Lipid Oxidation
2.4. Inhibition of Lipase Activity
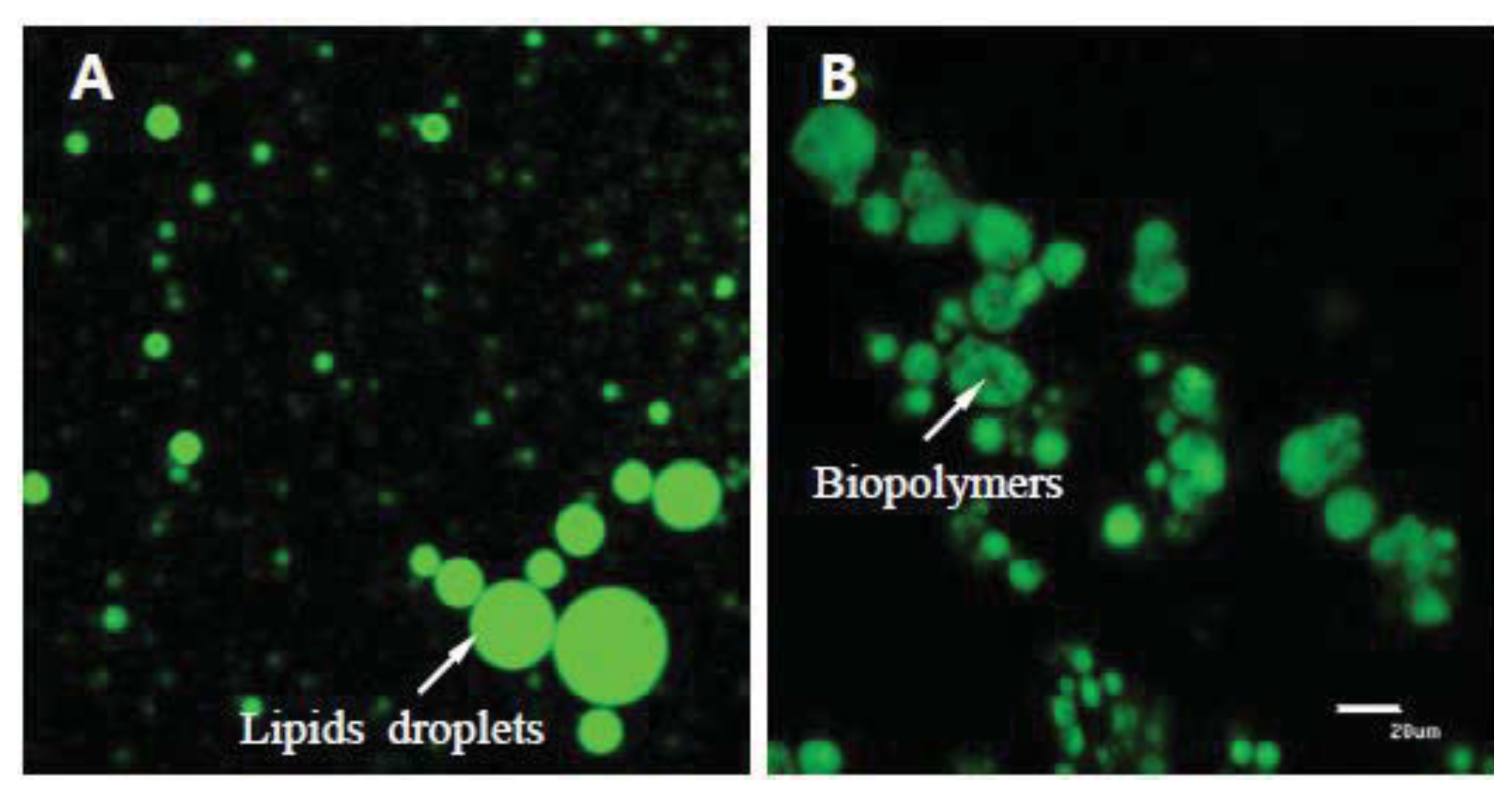
2.5. Particle Size
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Biopolymer Encapsulation Preparation
4.3. In Vitro Human Digestion
- (1)
- Initial system: The initial egg yolk samples encapsulated with 1% biopolymers.
- (2)
- Mouth: 10 g of initial egg yolk samples was mixed with 12 mL of simulated saliva solution (pH 6.8) and then stirred for 5 min at 37 °C.
- (3)
- Stomach: 24 mL of simulated gastric juice (pH 2) was then added, and the mixture was stirred for 2 h at 37 °C.
- (4)
- Small intestine: 24 mL of duodenal juice, 12 mL of bile juice, and 2 mL of HCO3 solution (pH 6.5 to 7) was then added. The total solution was placed in a 250 mL flask, and then the dialysis tubing (molecular weight cutoff of 50,000, flat width 34 mm, thickness 18 μm, Membrane Filtration Products, Inc. Seguin, TX, USA) containing 10 mL of phosphate buffer (pH 7) was placed in a 250 mL flask, and the mix was stirred for 2 h at 37 °C.
4.4. Total Lipid and Cholesterol Contents
4.5. Free Fatty Acid Content
4.6. Thiobarbituric Acid-Reactive Substances
4.7. Lipase Activity
4.8. Confocal Laser Scanning Microscopy
4.9. Particle Size
4.10. Statistics
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Majumder, K.; Wu, J. Angiotensin I converting enzyme inhibitory peptides from simulated in vitro gastrointestinal digestion of cooked eggs. J. Agric. Food Chem 2009, 57, 471–477. [Google Scholar]
- Anonymous Composition of Foods, Dairy and Egg Product, Raw-Processed-Prepared; U.S. Department of Agriculture, U.S. Government Printing Office: Washington, DC, USA, 1989.
- Nourooz-Zadeh, J.; Appelqvist, L.A. Cholesterol oxides in Swedish foods and food ingredients: Milk powder products. J. Food Sci 1988, 53, 74–79. [Google Scholar]
- Cohn, J.; Kamili, A.; Wat, E.; Chung, R.W.; Tandy, S. Dietary phospholipids and intestinal cholesterol absorption. Nutrients 2010, 2, 116–127. [Google Scholar]
- Siri-Tarino, P.; Sun, Q.; Hu, F.; Krauss, R. Saturated fatty acids and risk of coronary heart disease: Modulation by replacement nutrients. Curr. Atheroscler. Rep 2010, 12, 384–390. [Google Scholar]
- Lee, A.; Griffin, B. Dietary cholesterol, eggs and coronary heart disease risk in perspective. Nutr. Bull 2006, 31, 21–27. [Google Scholar]
- Hur, S.J.; Kim, D.H.; Chun, S.C.; Lee, S.K. Effects of dietary conjugated linoleic acid and biopolymer encapsulation on lipid metabolism in mice. Inter. J. Mol. Sci 2013, 14, 6848–6862. [Google Scholar]
- Lairon, D. Soluble fibers and dietary lipids, in dietary fiber in health and disease. Diet. Fiber Health Dis 1997, 427, 99–108. [Google Scholar]
- Beysseriat, M.; Decker, E.A.; McClements, D.J. Preliminary study of the influence of dietary fiber on the properties of oil-in-water emulsions passing through an in vitro human digestion model. Food Hydrocoll 2006, 20, 800–809. [Google Scholar]
- Jenkins, D.J.A.; Kendall, C.W.C.; Ransom, T.P.P. Dietary fiber, the evolution of the human diet and coronary heart disease. Nutr. Res 1998, 18, 633–652. [Google Scholar]
- Lairon, D.; Play, B.; Jourdheuil-Rahmani, D. Digestible and indigestible carbohydrates: Interactions with postprandial lipid metabolism. J. Nutr. Biochem 2007, 18, 217–227. [Google Scholar]
- Gee, J.M.; Blackburn, N.A.; Johnson, I.T. The influence of guar gum on intestinal cholesterol transport in the rat. Br. J. Nutr 1983, 50, 215–224. [Google Scholar]
- Kamp, F.; Zakim, D.; Zhang, F.; Noy, N.; Hamilton, J.A. Fatty acid flip-flop in phospholipid bilayers is extremely fast. Biochemistry 1995, 34, 11928–11937. [Google Scholar]
- Klinkesorn, U.; McClements, D.J. Impact of lipase, bile salts, and polysaccharides on properties and digestibility of tuna oil multilayer emulsions stabilized by lecithin-chitosan. Food Biophys 2010, 5, 73–81. [Google Scholar]
- Fernandez, M.L. Distinct mechanisms of plasma LDL lowering by dietary fiber in the guinea pig: Specific effects of pectin, guar gum, and psyllium. J. Lipid Res 1995, 36, 2394–2404. [Google Scholar]
- Moundras, C.; Behr, S.R.; Demigné, C.; Mazur, A.; Rémésy, C. Fermentable polysaccharides that enhance fecal bile acid excretion lower plasma cholesterol and apolipoprotein E-rich HDL in rats. J. Nutr 1994, 124, 2179–2188. [Google Scholar]
- Mun, S.; Decker, E.; Park, Y.; Weiss, J.; McClements, D. Influence of interfacial composition on in vitro digestibility of emulsified lipids: Potential mechanism for chitosan’s ability to inhibit fat digestion. Food Biophys 2006, 1, 21–29. [Google Scholar]
- Meyer, J.H.; Doty, J.E. GI transit and absorption of solid food: Multiple effects of guar. Am. J. Clin. Nutr 1988, 48, 267–273. [Google Scholar]
- Khan, M.; Nakkeeran, E.; Umesh-Kumar, S. Potential application of pectinase in developing functional foods. Annu. Rev. Food Sci. Technol 2013, 4, 21–34. [Google Scholar]
- Cui, S.W. Food Carbohydrates: Chemistry, Physical Properties and Application; Taylor and Francis: Boca Raton, FL, USA, 2005. [Google Scholar]
- Yamaguchi, F.; Uchida, S.; Watabe, S.; Kojima, H.; Shimizu, N.; Hatanaka, C. Relationship between molecular weights of pectin and hypocholesterolemic effects in rats. BioSci. Biotechnol. Biochem 1995, 59, 2130–2131. [Google Scholar]
- Dongowski, G. Effect of pH on the in vitro interactions between bile acids and pectin. Z. Lebensm. Unters. Forsch 1997, 205, 185–192. [Google Scholar]
- Versantvoort, C.H.M.; Oomen, A.G.; van de Kamp, E.; Rompelberg, C.J.M.; Sips, A.J.A.M. Applicability of an in vitro digestion model in assessing the bioaccessibility of mycotoxins from food. Food Chem. Toxicol 2005, 43, 31–40. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem 1957, 226, 497–509. [Google Scholar]
- Russo, M.V.; De Leonardis, A.; Macciola, V. Solid phase extraction—Gas-chromatographic method to determine free cholesterol in animal fats. J. Food Compos. Anal 2005, 18, 617–624. [Google Scholar]
- Association Office Analytical Chemists (AOAC), Official Methods of Analysis of AOAC International, 16th ed.; Patricia, C. (Ed.) AOAC: Arlington, VA, USA, 1995.
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol 1978, 52, 302–310. [Google Scholar]
- Gooda Sahib, N.; Abdul Hamid, A.; Saari, N.; Abas, F.; Pak Dek, M.S.; Rahim, M. Anti-pancreatic lipase and antioxidant activity of selected tropical herbs. Inter. J. Food Prop 2011, 15, 569–578. [Google Scholar]





| Biopolymer type | Electrical charge | Molecular weight | Hydrophobicity |
|---|---|---|---|
| Cellulose | Non-ionic | 100 kDa | Low |
| Pectin | Anionic (–COO−) Degree of methylaton: 50 | 100 kDa | Variable (–CH3) |
| Chitosan | Cationic (NH3+) Degree of acetylation: 50 | 100 kDa | Variable (–COCH3) |
| Saliva | Gastric juice | Duodenal juice | Bile juice | |
|---|---|---|---|---|
| Inorganic components | 10 mL KCl 89.6 g/L | 15.7 mL NaCl 175.3 g/L | 40 mL NaCl 175.3 g/L | 30 mL NaCl 175.3 g/L |
| 10 mL KSCN 20 g/L | 3.0 mL NaH2PO4 88.8 g/L | 40 mL NaHCO3 84.7 g/L | 68.3 mL NaHCO3 84.7 g/L | |
| 10 mL NaH2PO4 88.8 g/L | 9.2 mL KCl 89.6 g/L | 10 mL KH2PO4 8 g/L | 4.2 mL KCl 89.6 g/L | |
| 10 mL NaSO4 57 g/L | 18 mL CaCl2·2H2O 22.2 g/L | 6.3 mL KCl 89.6 g/L | 150 μL HCl 37% g/g | |
| 1.7 mL NaCl 175.3 g/L | 10 mL NH4Cl 30.6 g/L | 10 mL MgCl2 5 g/L | - | |
| 20 mL NaHCO3 84.7 g/L | 6.5 mL HCl 37% g/g | 180 μL HCl 37% g/g | 20 mL NaHCO3 84.7 g/L | |
| Organic components | 8 mL urea 25 g/L | 10 mL glucose 65 g/L | 4 mL urea 25 g/L | 10 mL urea 25 g/L |
| 10 mL glucuronic acid 2 g/L | ||||
| 3.4 mL urea 25 g/L | ||||
| 10 mL glucosamine hydrochloride 33 g/L | ||||
| Add to mixture of organic + inorganic components | 290 mg α-amylase | 1 g BSA | 9 mL CaCl2·2H2O 22.2 g/L | 10 mL CaCl2·2H2O 22.2 g/L |
| 15 mg uric acid | 2.5 g pepsin | 1 g BSA | 1.8 g BSA | |
| 25 mg mucin | 3 g mucin | 9 g pancreatin | 30 g bile | |
| 1.5 g lipase | ||||
| pH | 6.8 ± 0.2 | 1.30 ± 0.02 | 8.1 ± 0.2 | 8.2 ± 0.2 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hur, S.-J.; Kim, Y.-C.; Choi, I.; Lee, S.-K. The Effects of Biopolymer Encapsulation on Total Lipids and Cholesterol in Egg Yolk during in Vitro Human Digestion. Int. J. Mol. Sci. 2013, 14, 16333-16347. https://doi.org/10.3390/ijms140816333
Hur S-J, Kim Y-C, Choi I, Lee S-K. The Effects of Biopolymer Encapsulation on Total Lipids and Cholesterol in Egg Yolk during in Vitro Human Digestion. International Journal of Molecular Sciences. 2013; 14(8):16333-16347. https://doi.org/10.3390/ijms140816333
Chicago/Turabian StyleHur, Sun-Jin, Young-Chan Kim, Inwook Choi, and Si-Kyung Lee. 2013. "The Effects of Biopolymer Encapsulation on Total Lipids and Cholesterol in Egg Yolk during in Vitro Human Digestion" International Journal of Molecular Sciences 14, no. 8: 16333-16347. https://doi.org/10.3390/ijms140816333
APA StyleHur, S.-J., Kim, Y.-C., Choi, I., & Lee, S.-K. (2013). The Effects of Biopolymer Encapsulation on Total Lipids and Cholesterol in Egg Yolk during in Vitro Human Digestion. International Journal of Molecular Sciences, 14(8), 16333-16347. https://doi.org/10.3390/ijms140816333





