Resveratrol Sensitizes Tamoxifen in Antiestrogen-Resistant Breast Cancer Cells with Epithelial-Mesenchymal Transition Features
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
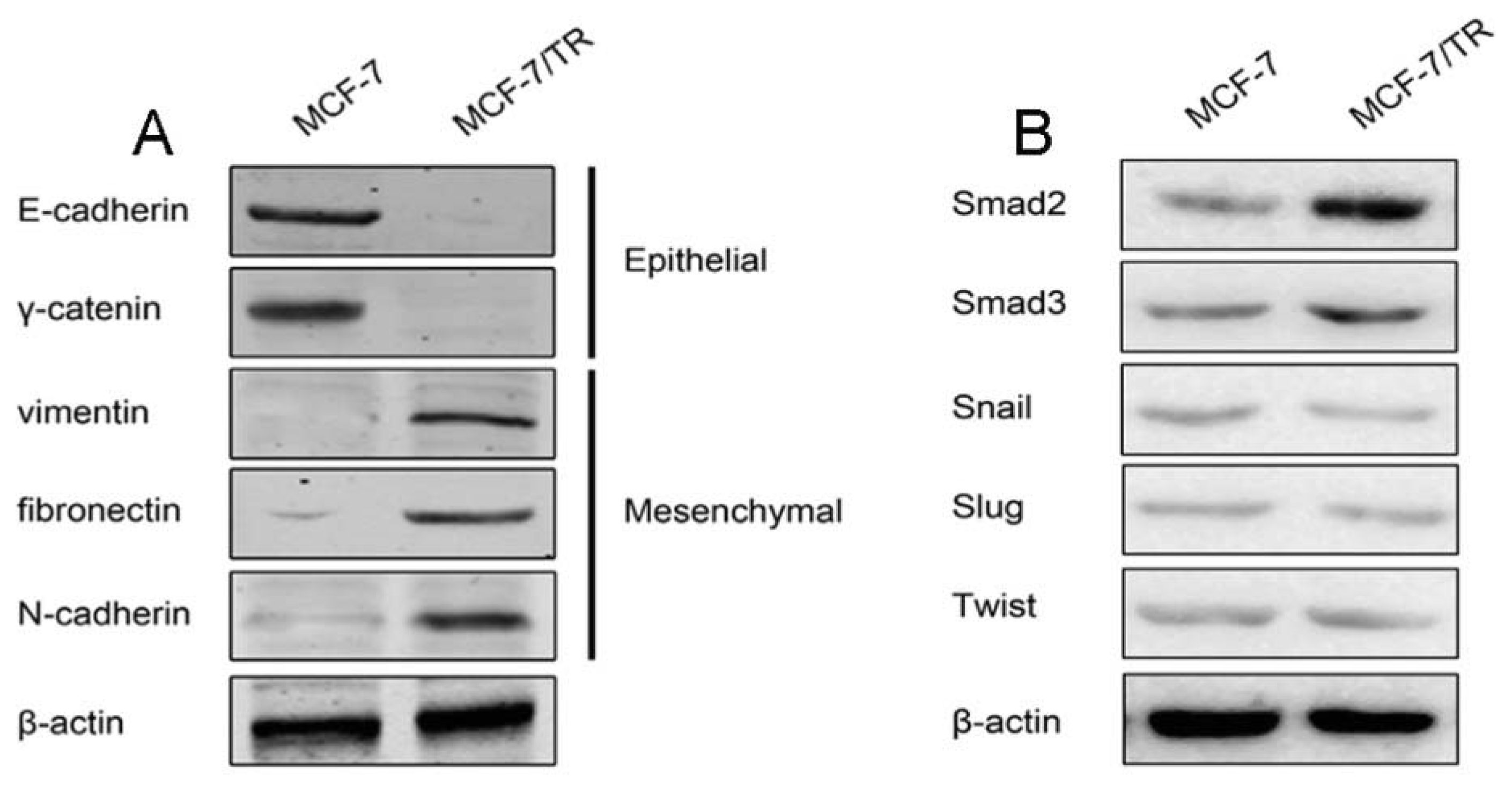
2.1.1. Acquired Tamoxifen Resistant Breast Cancer Cells Undergo EMT
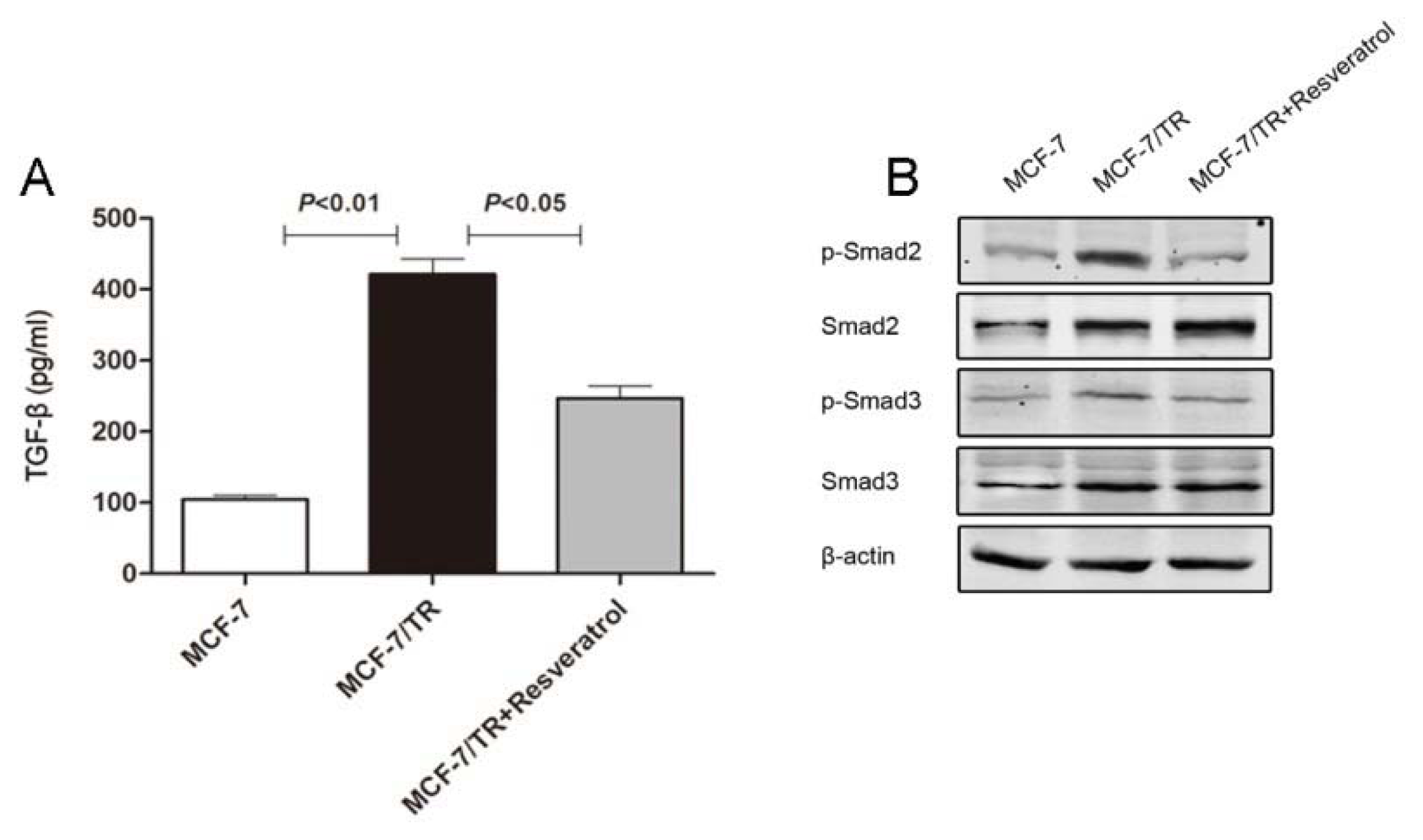
2.1.2. TGF-β Triggers EMT and Confers Tamoxifen Resistance
2.1.3. Effects of Resveratrol on MCF-7 and MCF-7/TR Cell Proliferation
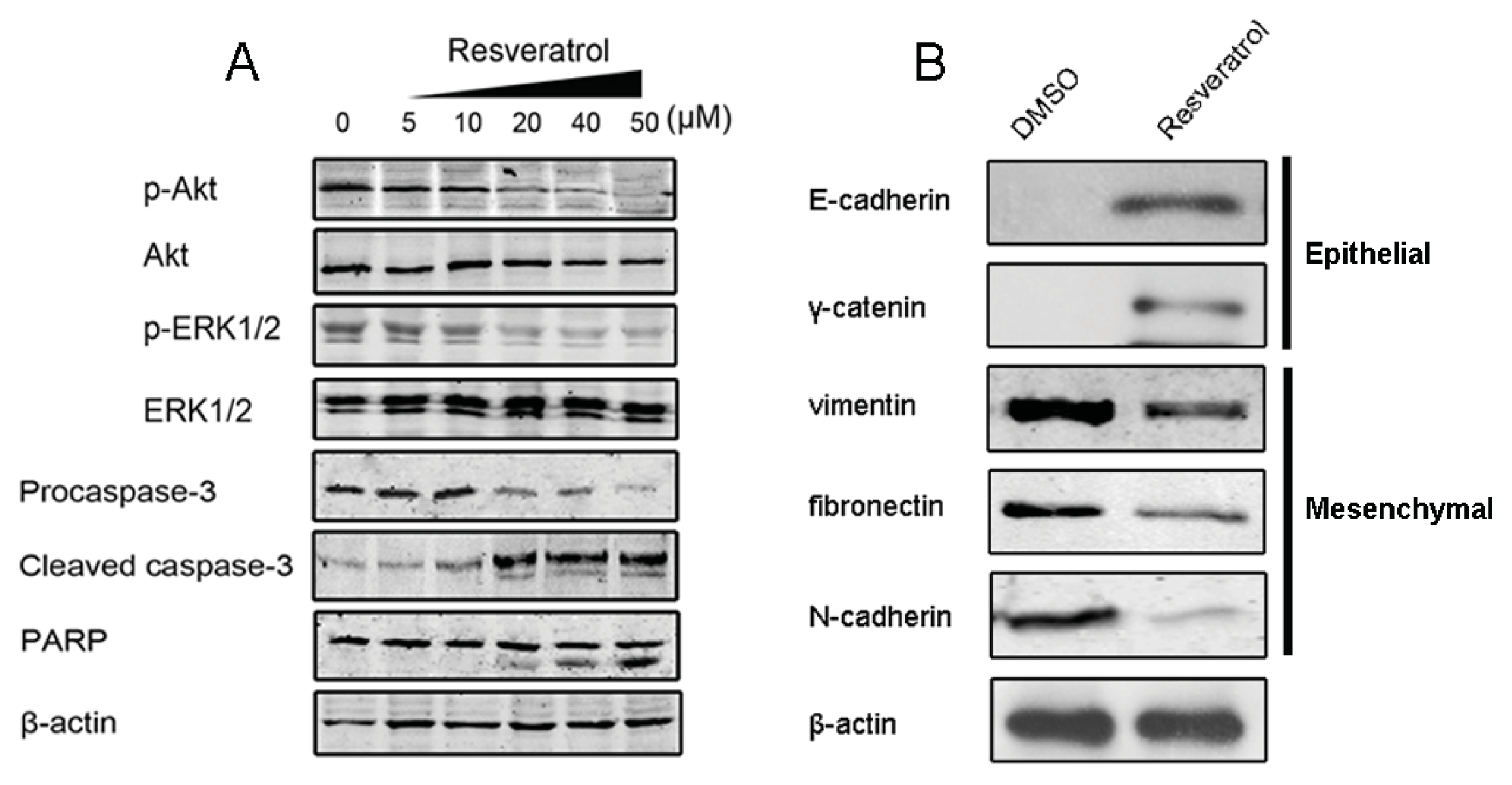
2.1.4. Resveratrol Sensitizes MCF-7/TR Cells to Tamoxifen and Promotes Apoptosis
2.1.5. Resveratrol Inhibits TGF-β Production, Reverses EMT and Overcomes Resistance
2.2. Discussion
3. Experimental Section
3.1. Cell Culture
3.2. Reagents
3.3. Antibodies and Western Blot
3.4. Cell Viability Assay
3.5. Immunocytochemistry
3.6. TGF-β ELISA Analysis
3.7. Apoptosis and Flow Cytometric Studies
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Thun, M.J. Cancer statistics, 2009. CA Cancer J. Clin 2009, 59, 225–249. [Google Scholar]
- Miller, W.R.; Bartlett, J.M.; Canney, P.; Verrill, M. Hormonal therapy for postmenopausal breast cancer: The science of sequencing. Breast Cancer Res. Treat 2007, 103, 149–160. [Google Scholar]
- Perey, L.; Paridaens, R.; Hawle, H.; Zaman, K.; Nolé, F.; Wildiers, H.; Fiche, M.; Dietrich, D.; Clément, P.; Köberle, D.; et al. Clinical benefit of fulvestrant in postmenopausal women with advanced breast cancer and primary or acquired resistance to aromatase inhibitors: Final results of phase II Swiss Group for Clinical Cancer Research Trial (SAKK 21/00). Ann. Oncol 2007, 18, 64–69. [Google Scholar]
- Gutierrez, M.C.; Detre, S.; Johnston, S.; Mohsin, S.K.; Shou, J.; Allred, D.C.; Schiff, R.; Osborne, C.K.; Dowsett, M. Molecular changes in tamoxifen-resistant breast cancer: Relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinase. J. Clin. Oncol 2005, 23, 2469–2476. [Google Scholar]
- Holm, C.; Rayala, S.; Jirström, K.; Stål, O.; Kumar, R.; Landberg, G. Association between Pak1 expression and subcellular localization and tamoxifen resistance in breast cancer patients. J. Natl. Cancer Inst. 2006, 98, 671–680. [Google Scholar]
- Kirkegaard, T.; Witton, C.J.; McGlynn, L.M.; Tovey, S.M.; Dunne, B.; Lyon, A.; Bartlett, J.M. AKT activation predicts outcome in breast cancer patients treated with tamoxifen. J. Pathol 2005, 207, 139–146. [Google Scholar]
- Riggins, R.B.; Schrecengost, R.S.; Guerrero, M.S.; Bouton, A.H. Pathways to tamoxifen resistance. Cancer Lett 2007, 256, 1–24. [Google Scholar]
- Bachleitner-Hofmann, T.; Pichler-Gebhard, B.; Rudas, M.; Gnant, M.; Taucher, S.; Kandioler, D.; Janschek, E.; Dubsky, P.; Roka, S.; Sporn, E.; Jakesz, R. Pattern of hormone receptor status of secondary contralateral breast cancers in patients receiving adjuvant tamoxifen. Clin. Cancer Res 2002, 8, 3427–3432. [Google Scholar]
- Kuske, B.; Naughton, C.; Moore, K.; Macleod, K.G.; Miller, W.R.; Clarke, R.; Langdon, S.P.; Cameron, D.A. Endocrine therapy resistance can be associated with high estrogen receptor alpha (ERalpha) expression and reduced ERalpha phosphorylation in breast cancer models. Endocr. Relat. Cancer 2006, 13, 1121–1133. [Google Scholar]
- Yang, J.; Weinberg, R.A. Epithelial-mesenchymal transition: At the crossroads of development and tumor metastasis. Dev. Cell 2008, 14, 818–829. [Google Scholar]
- Peinado, H.; Olmeda, D.; Cano, A. Snail, Zeb and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nat. Rev. Cancer 2007, 7, 415–428. [Google Scholar]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar]
- Peña, C.; García, J.M.; Silva, J.; García, V.; Rodríguez, R.; Alonso, I.; Millán, I.; Salas, C.; de Herreros, A.G.; Muñoz, A.; Bonilla, F. E-cadherin and vitamin D receptor regulation by SNAIL and ZEB1 in colon cancer: Clinicopathological correlations. Hum. Mol. Genet 2005, 14, 3361–3370. [Google Scholar]
- Uchikado, Y.; Natsugoe, S.; Okumura, H.; Setoyama, T.; Matsumoto, M.; Ishigami, S.; Aikou, T. Slug Expression in the E-cadherin preserved tumors is related to prognosis in patients with esophageal squamous cell carcinoma. Clin. Cancer Res 2005, 11, 1174–1180. [Google Scholar]
- Arumugam, T.; Ramachandran, V.; Fournier, K.F.; Wang, H.; Marquis, L.; Abbruzzese, J.L.; Gallick, G.E.; Logsdon, C.D.; McConkey, D.J.; Choi, W. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res 2009, 69, 5820–5828. [Google Scholar]
- Shintani, Y.; Okimura, A.; Sato, K.; Nakagiri, T.; Kadota, Y.; Inoue, M.; Sawabata, N.; Minami, M.; Ikeda, N.; Kawahara, K.; et al. Epithelial to mesenchymal transition is a determinant of sensitivity to chemoradiotherapy in non-small cell lung cancer. Ann. Thorac. Surg 2011, 92, 1794–1804. [Google Scholar]
- Creighton, C.J.; Li, X.; Landis, M.; Dixon, J.M.; Neumeister, V.M.; Sjolund, A.; Rimm, D.L.; Wong, H.; Rodriguez, A.; Herschkowitz, J.I.; et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc. Natl. Acad. Sci. USA 2009, 106, 13820–13825. [Google Scholar]
- Hiscox, S.; Jiang, W.G.; Obermeier, K.; Taylor, K.; Morgan, L.; Burmi, R.; Barrow, D.; Nicholson, R.I. Tamoxifen resistance in MCF7 cells promotes EMT-like behaviour and involves modulation of beta-catenin phosphorylation. Int. J. Cancer 2006, 118, 290–301. [Google Scholar]
- Saiko, P.; Szakmary, A.; Jaeger, W.; Szekeres, T. Resveratrol and its analogs: Defense against cancer, coronary disease and neurodegenerative maladies or just a fad? Mutat. Res 2008, 658, 68–94. [Google Scholar]
- Athar, M.; Back, J.H.; Tang, X.; Kim, K.H.; Kopelovich, L.; Bickers, D.R.; Kim, A.L. Resveratrol: A review of preclinical studies for human cancer prevention. Toxicol. Appl. Pharmacol 2007, 224, 274–283. [Google Scholar]
- Dong, Z. Molecular mechanism of the chemopreventive effect of resveratrol. Mutat. Res 2007, 523–524, 145–150. [Google Scholar]
- Kim, M.Y.; Trudel, L.J.; Wogan, G.N. Apoptosis induced by capsaicin and resveratrol in colon carcinoma cells requires nitric oxide production and caspase activation. Anticancer Res 2009, 29, 3733–3740. [Google Scholar]
- Holme, A.L.; Pervaiz, S. Resveratrol in cell fate decisions. J. Bioenerg. Biomembr 2007, 39, 59–63. [Google Scholar]
- Singh, N.; Nigam, M.; Ranjan, V.; Sharma, R.; Balapure, A.K.; Rath, S.K. Caspase mediated enhanced apoptotic action of cyclophosphamide- and resveratrol-treated MCF-7 cells. J. Pharmacol. Sci 2009, 109, 473–485. [Google Scholar]
- Filomeni, G.; Graziani, I.; Rotilio, G.; Ciriolo, M.R. trans-Resveratrol induces apoptosis in human breast cancer cells MCF-7 by the activation of MAP kinases pathways. Genes Nutr 2007, 2, 295–305. [Google Scholar] [Green Version]
- Garvin, S.; Ollinger, K.; Dabrosin, C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Lett 2006, 231, 113–122. [Google Scholar]
- Scheel, C.; Eaton, E.N.; Li, S.H.; Chaffer, C.L.; Reinhardt, F.; Kah, K.J.; Bell, G.; Guo, W.; Rubin, J.; Richardson, A.L.; Weinberg, R.A. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell 2011, 145, 926–940. [Google Scholar]
- Engelman, J.A. Targeting PI3K signalling in cancer: Opportunities, challenges and limitations. Nat. Rev. Cancer 2009, 9, 550–562. [Google Scholar]
- Ryan, P.D.; Goss, P.E. Adjuvant hormonal therapy in peri- and postmenopausal breast cancer. Oncologist 2006, 11, 718–731. [Google Scholar]
- Zavadil, J.; Böttinger, E.P. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene 2005, 24, 5764–5774. [Google Scholar]
- Radisky, D.C. miR-200c at the nexus of epithelial-mesenchymal transition, resistance to apoptosis, and the breast cancer stem cell phenotype. Breast Cancer Res 2011, 13, 110, , doi:10.1186/bcr2885.. [Google Scholar]
- Zhang, W.; Feng, M.; Zheng, G.; Chen, Y.; Wang, X.; Pen, B.; Yin, J.; Yu, Y.; He, Z. Chemoresistance to 5-fluorouracil induces epithelial-mesenchymal transition via up-regulation of Snail in MCF7 human breast cancer cells. Biochem. Biophys. Res. Commun 2012, 417, 679–685. [Google Scholar]
- Rho, J.K.; Choi, Y.J.; Lee, J.K.; Ryoo, B.Y.; Na, I.I.; Yang, S.H.; Kim, C.H.; Lee, J.C. Epithelial to mesenchymal transition derived from repeated exposure to gefitinib determines the sensitivity to EGFR inhibitors in A549, a non-small cell lung cancer cell line. Lung Cancer 2009, 63, 219–226. [Google Scholar]
- Patel, B.; Patel, S.; Hoffman, R. Inhibition of cyclo-oxygenase-2 expression in mouse macrophages by 4-(3-methyl-but-1-enyl)-3,5,3′,4′-tetrahydroxystilbene, a resveratrol derivative from peanuts. Phytother. Res 2005, 19, 552–555. [Google Scholar]
- Kundu, J.K.; Shin, Y.K.; Kim, S.H.; Surh, Y.J. Resveratrol inhibits phorbol ester-induced expression of COX-2 and activation of NF-kappaB in mouse skin by blocking IkappaB kinase activity. Carcinogenesis 2006, 27, 1465–1474. [Google Scholar]



© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shi, X.-P.; Miao, S.; Wu, Y.; Zhang, W.; Zhang, X.-F.; Ma, H.-Z.; Xin, H.-L.; Feng, J.; Wen, A.-D.; Li, Y. Resveratrol Sensitizes Tamoxifen in Antiestrogen-Resistant Breast Cancer Cells with Epithelial-Mesenchymal Transition Features. Int. J. Mol. Sci. 2013, 14, 15655-15668. https://doi.org/10.3390/ijms140815655
Shi X-P, Miao S, Wu Y, Zhang W, Zhang X-F, Ma H-Z, Xin H-L, Feng J, Wen A-D, Li Y. Resveratrol Sensitizes Tamoxifen in Antiestrogen-Resistant Breast Cancer Cells with Epithelial-Mesenchymal Transition Features. International Journal of Molecular Sciences. 2013; 14(8):15655-15668. https://doi.org/10.3390/ijms140815655
Chicago/Turabian StyleShi, Xiao-Peng, Shan Miao, Yin Wu, Wei Zhang, Xiao-Fang Zhang, Hua-Zhao Ma, Hai-Li Xin, Juan Feng, Ai-Dong Wen, and Yan Li. 2013. "Resveratrol Sensitizes Tamoxifen in Antiestrogen-Resistant Breast Cancer Cells with Epithelial-Mesenchymal Transition Features" International Journal of Molecular Sciences 14, no. 8: 15655-15668. https://doi.org/10.3390/ijms140815655
APA StyleShi, X.-P., Miao, S., Wu, Y., Zhang, W., Zhang, X.-F., Ma, H.-Z., Xin, H.-L., Feng, J., Wen, A.-D., & Li, Y. (2013). Resveratrol Sensitizes Tamoxifen in Antiestrogen-Resistant Breast Cancer Cells with Epithelial-Mesenchymal Transition Features. International Journal of Molecular Sciences, 14(8), 15655-15668. https://doi.org/10.3390/ijms140815655



