Effects of Calorie Restriction and IGF-1 Receptor Blockade on the Progression of 22Rv1 Prostate Cancer Xenografts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
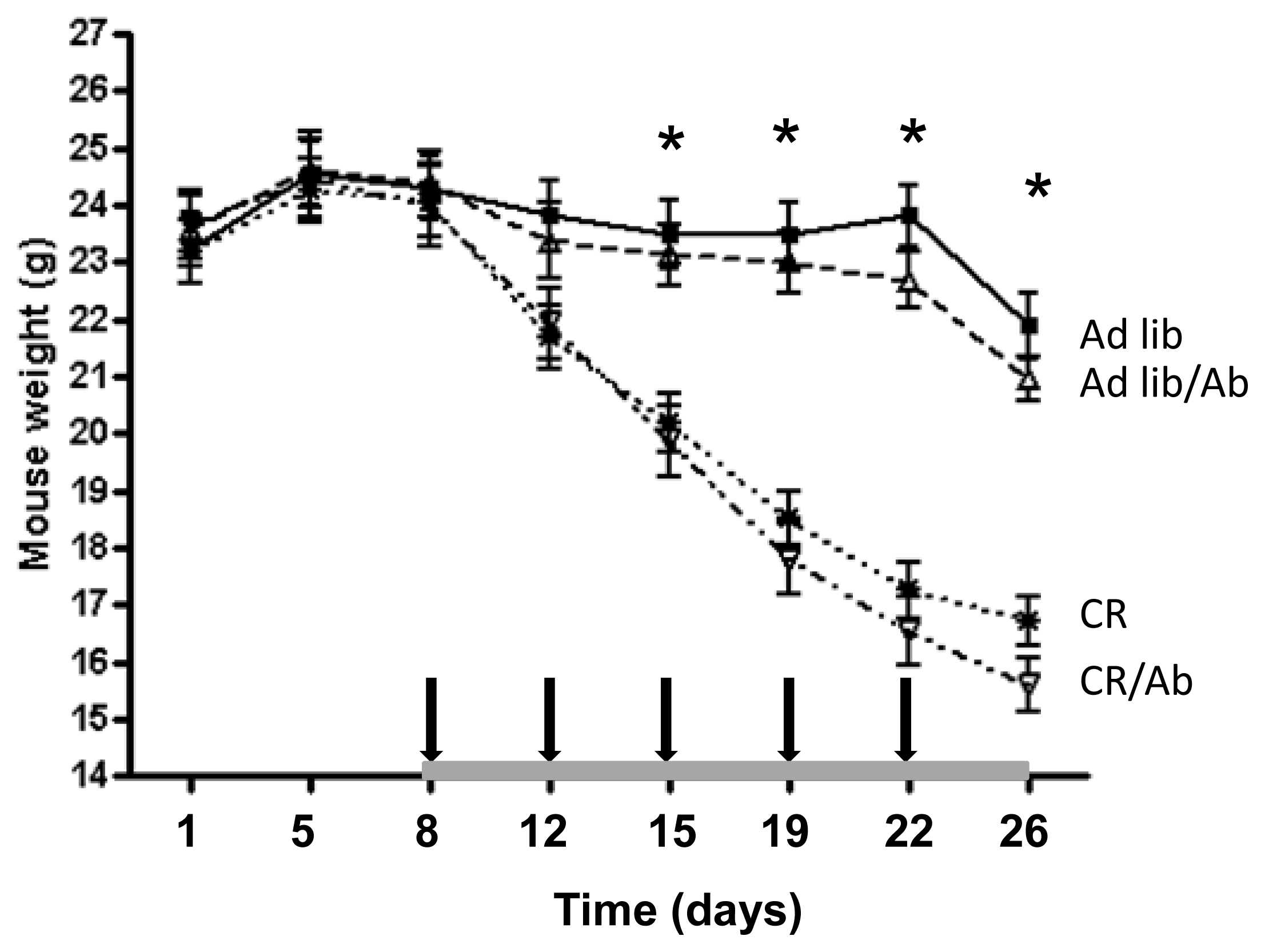
2.1.1. Reduced 22Rv1 Xenograft Growth in the Calorie Restriction and the Combined Therapy Groups
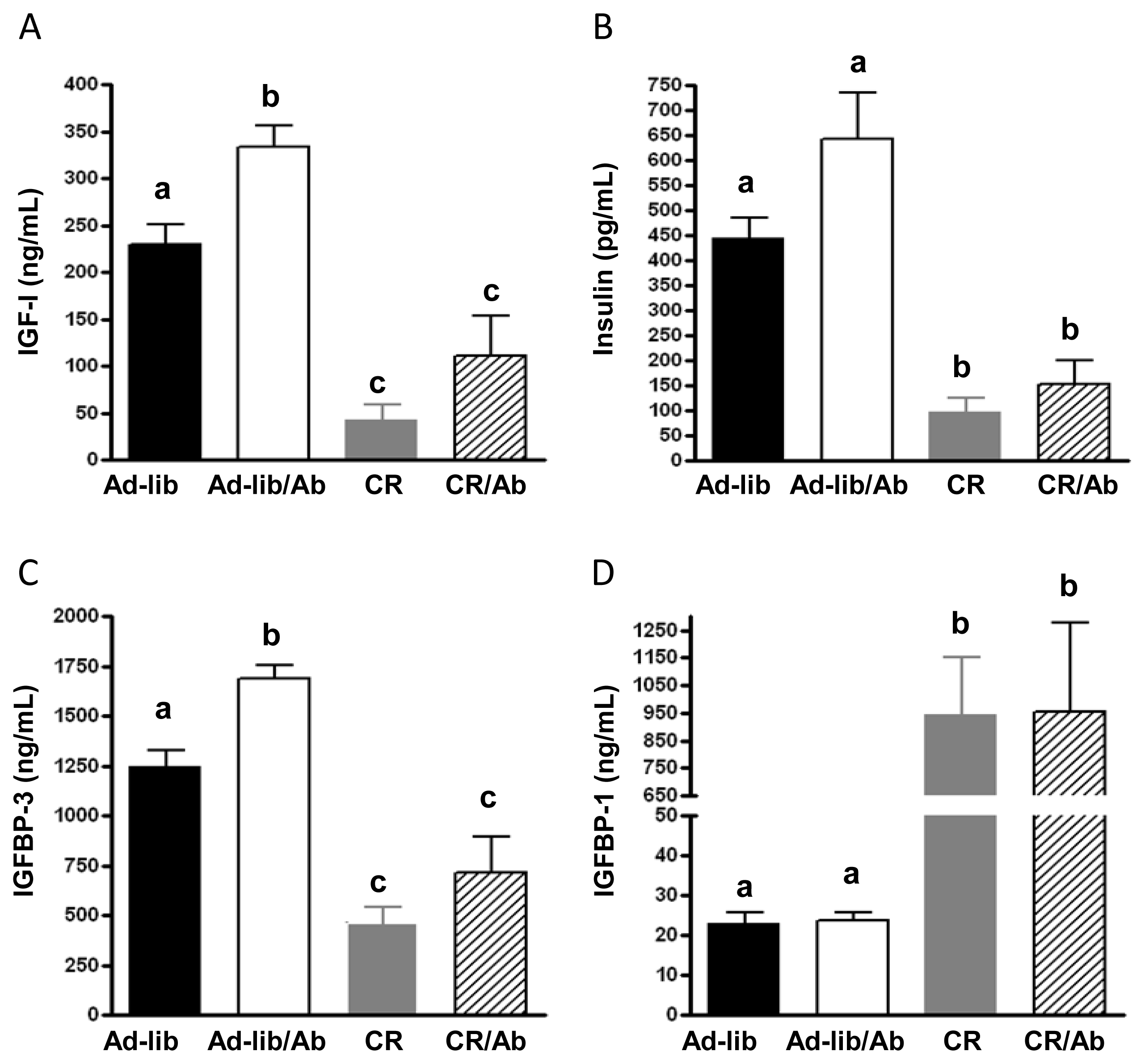
2.1.2. Changes in the IGF Axis in Response to the IGF-1R Blocking Therapy and Calorie Restriction
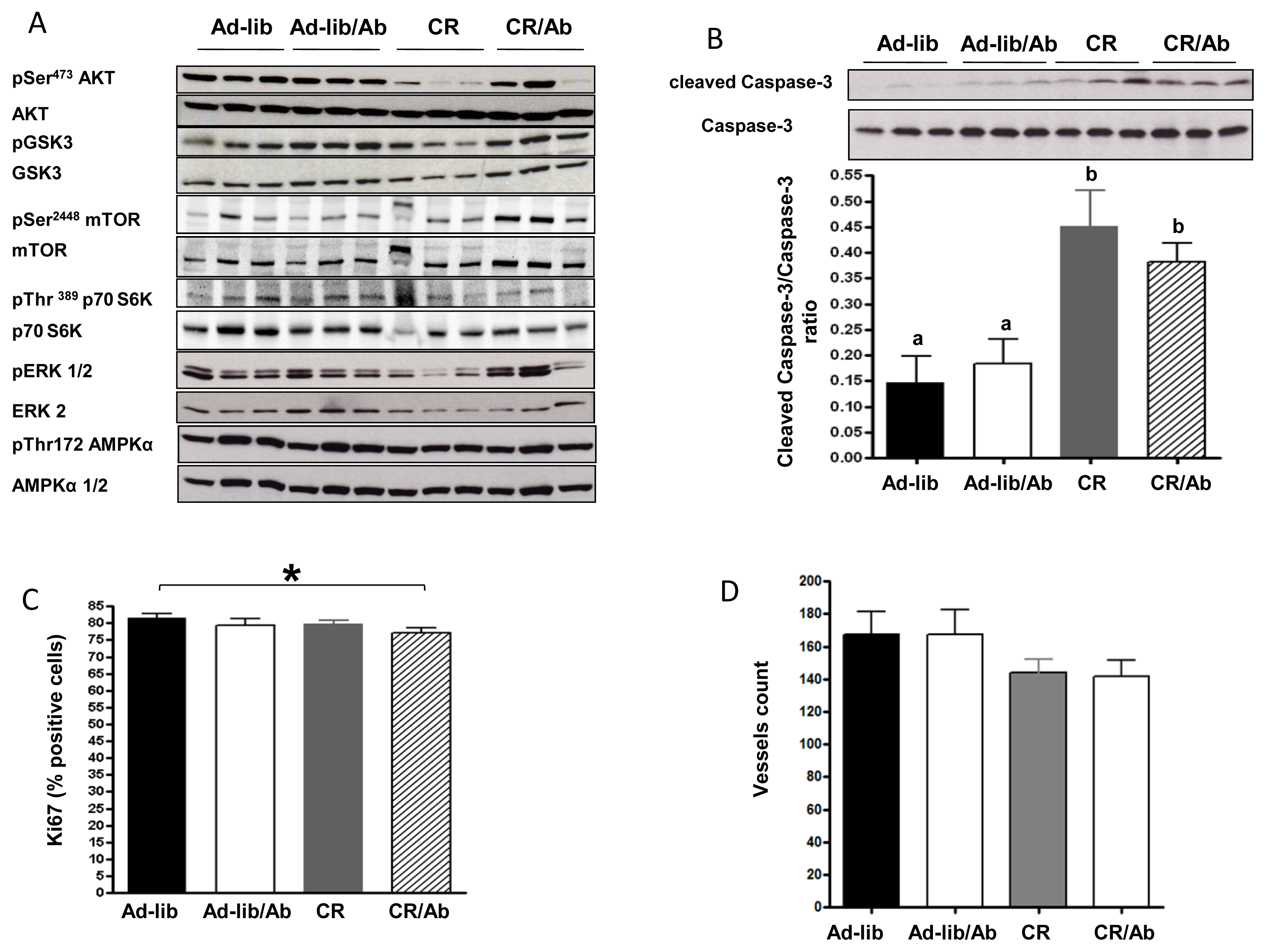
2.1.3. Effect of Calorie Restriction and IGF-1R Blocking Therapy on 22Rv1 Xenograft AKT Activation, Apoptosis and Proliferation
2.2. Discussion
3. Experimental Section
3.1. Animal Husbandry and Feeding Protocol
3.2. 22Rv1 Cell Line
3.3. Experimental Design
3.4. Plasma and Tumor Collection
3.5. Plasma Studies
3.6. Immunohistochemistry
3.7. Western Blot Analysis
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Kohler, B.A.; Ward, E.; McCarthy, B.J.; Schymura, M.J.; Ries, L.A.; Eheman, C.; Jemal, A.; Anderson, R.N.; Ajani, U.A.; Edwards, B.K. Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J. Natl. Cancer Inst 2011, 103, 714–736. [Google Scholar]
- Cohen, P.; Peehl, D.M.; Rosenfeld, R.G. The IGF axis in the prostate. Horm. Metab. Res 1994, 26, 81–84. [Google Scholar]
- Kojima, S.; Inahara, M.; Suzuki, H.; Ichikawa, T.; Furuya, Y. Implications of insulin-like growth factor-I for prostate cancer therapies. Int. J. Urol 2009, 16, 161–167. [Google Scholar]
- Chan, J.M.; Stampfer, M.J.; Giovannucci, E.; Gann, P.H.; Ma, J.; Wilkinson, P.; Hennekens, C.H.; Pollak, M. Plasma insulin-like growth factor-I and prostate cancer risk: A prospective study. Science 1998, 279, 563–566. [Google Scholar]
- Stattin, P.; Rinaldi, S.; Biessy, C.; Stenman, U.H.; Hallmans, G.; Kaaks, R. High levels of circulating insulin-like growth factor-I increase prostate cancer risk: A prospective study in a population-based nonscreened cohort. J. Clin. Oncol 2004, 22, 3104–3112. [Google Scholar]
- Chitnis, M.M.; Yuen, J.S.; Protheroe, A.S.; Pollak, M.; Macaulay, V.M. The type 1 insulin-like growth factor receptor pathway. Clin. Cancer Res 2008, 14, 6364–6370. [Google Scholar]
- Rodon, J.; DeSantos, V.; Ferry, R.J., Jr; Kurzrock, R. Early drug development of inhibitors of the insulin-like growth factor-I receptor pathway: Lessons from the first clinical trials. Mol. Cancer Ther. 2008, 7, 2575–2588. [Google Scholar]
- Wu, J.D.; Odman, A.; Higgins, L.M.; Haugk, K.; Vessella, R.; Ludwig, D.L.; Plymate, S.R. In vivo effects of the human type I insulin-like growth factor receptor antibody A12 on androgen-dependent and androgen-independent xenograft human prostate tumors. Clin. Cancer Res 2005, 11, 3065–3074. [Google Scholar]
- Beltran, P.J.; Mitchell, P.; Chung, Y.A.; Cajulis, E.; Lu, J.; Belmontes, B.; Ho, J.; Tsai, M.M.; Zhu, M.; Vonderfecht, S.; et al. AMG 479, a fully human anti-insulin-like growth factor receptor type I monoclonal antibody, inhibits the growth and survival of pancreatic carcinoma cells. Mol. Cancer Ther 2009, 8, 1095–1105. [Google Scholar]
- Rowinsky, E.K.; Schwartz, J.D.; Zojwalla, N.; Youssoufian, H.; Fox, F.; Pultar, P.; Ludwig, D.L. Blockade of insulin-like growth factor type-1 receptor with cixutumumab (IMC-A12): A novel approach to treatment for multiple cancers. Curr. Drug Targets 2011, 12, 2016–2033. [Google Scholar]
- Chi, K.N.; Gleave, M.E.; Fazli, L.; Goldenberg, S.L.; So, A.; Kollmannsberger, C.; Murray, N.; Tinker, A.V.; Pollak, M.N. A phase II pharmacodynamic study of pre-operative figitumumab in patients with localized prostate cancer. Clin. Cancer Res 2012, 18, 3407–3413. [Google Scholar]
- Scagliotti, G.V.; Novello, S. The role of the insulin-like growth factor signaling pathway in non-small cell lung cancer and other solid tumors. Cancer Treat. Rev 2012, 38, 292–302. [Google Scholar]
- Konijeti, R.; Koyama, S.; Gray, A.; Barnard, R.J.; Said, J.W.; Castor, B.; Elashoff, D.; Wan, J.; Beltran, P.J.; Calzone, F.J.; et al. Effect of a low-fat diet combined with IGF-1 receptor blockade on 22Rv1 prostate cancer xenografts. Mol. Cancer Ther 2012, 11, 1539–1546. [Google Scholar]
- Longo, V.D.; Fontana, L. Calorie restriction and cancer prevention: Metabolic and molecular mechanisms. Trends Pharmacol. Sci 2010, 31, 89–98. [Google Scholar]
- Hursting, S.D.; Smith, S.M.; Lashinger, L.M.; Harvey, A.E.; Perkins, S.N. Calories and carcinogenesis: Lessons learned from 30 years of calorie restriction research. Carcinogenesis 2010, 31, 83–89. [Google Scholar]
- Giovannucci, E.; Liu, Y.; Platz, E.A.; Stampfer, M.J.; Willett, W.C. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int. J. Cancer 2007, 121, 1571–1578. [Google Scholar]
- Ngo, T.H.; Barnard, R.J.; Tymchuk, C.N.; Cohen, P.; Aronson, W.J. Effect of diet and exercise on serum insulin, IGF-1, and IGFBP-1 levels and growth of LNCaP cells in vitro (United States). Cancer Causes Control 2002, 13, 929–935. [Google Scholar]
- Blando, J.; Moore, T.; Hursting, S.D.; Jiang, G.; Saha, A.; Beltran, L.; Shen, J.; Repass, J.; Strom, S.S.; Digiovanni, J. Dietary energy balance modulates prostate cancer progression in Hi-Myc mice. Cancer Prev. Res 2011, 4, 2002–2014. [Google Scholar]
- Mukherjee, P.; Sotnikov, A.V.; Mangian, H.J.; Zhou, J.R.; Visek, W.J.; Clinton, S.K. Energy intake and prostate tumor growth, angiogenesis, and vascular endothelial growth factor expression. J. Natl. Cancer Inst. 1999, 91, 512–523. [Google Scholar]
- Buschemeyer, W.C., III; Klink, J.C.; Mavropoulos, J.C.; Poulton, S.H.; Demark-Wahnefried, W.; Hursting, S.D.; Cohen, P.; Hwang, D.; Johnson, T.L.; Freedland, S.J. Effect of intermittent fasting with or without caloric restriction on prostate cancer growth and survival in SCID mice. Prostate 2010, 70, 1037–1043. [Google Scholar]
- Desbois-Mouthon, C.; Baron, A.; Blivet-Van Eggelpoel, M.J.; Fartoux, L.; Venot, C.; Bladt, F.; Housset, C.; Rosmorduc, O. Insulin-like growth factor-1 receptor inhibition induces a resistance mechanism via the epidermal growth factor receptor/HER3/AKT signaling pathway: Rational basis for cotargeting insulin-like growth factor-1 receptor and epidermal growth factor receptor in hepatocellular carcinoma. Clin. Cancer Res 2009, 15, 5445–5456. [Google Scholar]
- Buck, E.; Gokhale, P.C.; Koujak, S.; Brown, E.; Eyzaguirre, A.; Tao, N.; Rosenfeld-Franklin, M.; Lerner, L.; Chiu, M.I.; Wild, R.; et al. Compensatory insulin receptor (IR) activation on inhibition of insulin-like growth factor-1 receptor (IGF-1R): Rationale for cotargeting IGF-1R and IR in cancer. Mol. Cancer Ther. 2010, 9, 2652–2664. [Google Scholar]
- Bosma, G.C.; Custer, R.P.; Bosma, M.J. A severe combined immunodeficiency mutation in the mouse. Nature 1983, 301, 527–530. [Google Scholar]
- Lee, C.; Safdie, F.M.; Raffaghello, L.; Wei, M.; Madia, F.; Parrella, E.; Hwang, D.; Cohen, P.; Bianchi, G.; Longo, V.D. Reduced levels of IGF-1 mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res 2010, 70, 1564–1572. [Google Scholar]
- Safdie, F.M.; Dorff, T.; Quinn, D.; Fontana, L.; Wei, M.; Lee, C.; Cohen, P.; Longo, V.D. Fasting and cancer treatment in humans: A case series report. Aging 2009, 1, 988–1007. [Google Scholar]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending healthy life span—From yeast to humans. Science 2010, 328, 321–326. [Google Scholar]
- Cangemi, R.; Friedmann, A.J.; Holloszy, J.O.; Fontana, L. Long-term effects of calorie restriction on serum sex-hormone concentrations in men. Aging Cell 2010, 9, 236–242. [Google Scholar]
- Fontana, L.; Klein, S.; Holloszy, J.O. Effects of long-term calorie restriction and endurance exercise on glucose tolerance, insulin action, and adipokine production. Age 2010, 32, 97–108. [Google Scholar]
- Fontana, L. Calorie restriction and cardiometabolic health. Eur. J. Prev. Cardiol 2008, 15, 3–9. [Google Scholar]
- Redman, L.M.; Heilbronn, L.K.; Martin, C.K.; de Jonge, L.; Williamson, D.A.; Delany, J.P.; Ravussin, E. Metabolic and behavioral compensations in response to caloric restriction: Implications for the maintenance of weight loss. PLoS One 2009, 4, e4377. [Google Scholar]
- Tolcher, A.W.; Sarantopoulos, J.; Patnaik, A.; Papadopoulos, K.; Lin, C.C.; Rodon, J.; Murphy, B.; Roth, B.; McCaffery, I.; Gorski, K.S.; et al. Phase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1. J. Clin. Oncol 2009, 27, 5800–5807. [Google Scholar]
- The R Project for Statistical Computing. Available online: http://www.r-project.org/ (on accessed 1 July 2013).


| Ingredient | kcal/g | Ad libitum diet | Calorie restriction diet | ||
|---|---|---|---|---|---|
| g/kg | kcal/kg | g/kg | kcal/kg | ||
| Casein | 3.6 | 200 | 716 | 200 | 716 |
| L-Cystine | 4 | 3 | 12 | 3 | 12 |
| Sucrose | 4 | 84 | 336 | 84 | 336 |
| Cornstarch | 3.6 | 397.5 | 1431 | 378.5 | 1363 |
| Dyetrose | 3.8 | 132 | 501.6 | 132 | 501.6 |
| Corn Oil | 9 | 86 | 774 | 86 | 774 |
| Cellulose | 0 | 50 | 0 | 50 | 0 |
| Mineral Mix #210025 | 0.9 | 35 | 30.8 | 49 | 43.1 |
| Vitamin Mix #310025 | 3.9 | 10 | 38.7 | 14 | 54.2 |
| Choline Bitartrate | 0 | 2.5 | 0 | 3.5 | 0 |
| Total | 1000 | 3840.1 | 1000 | 3799.9 | |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Galet, C.; Gray, A.; Said, J.W.; Castor, B.; Wan, J.; Beltran, P.J.; Calzone, F.J.; Elashoff, D.; Cohen, P.; Aronson, W.J. Effects of Calorie Restriction and IGF-1 Receptor Blockade on the Progression of 22Rv1 Prostate Cancer Xenografts. Int. J. Mol. Sci. 2013, 14, 13782-13795. https://doi.org/10.3390/ijms140713782
Galet C, Gray A, Said JW, Castor B, Wan J, Beltran PJ, Calzone FJ, Elashoff D, Cohen P, Aronson WJ. Effects of Calorie Restriction and IGF-1 Receptor Blockade on the Progression of 22Rv1 Prostate Cancer Xenografts. International Journal of Molecular Sciences. 2013; 14(7):13782-13795. https://doi.org/10.3390/ijms140713782
Chicago/Turabian StyleGalet, Colette, Ashley Gray, Jonathan W. Said, Brandon Castor, Junxiang Wan, Pedro J. Beltran, Franck J. Calzone, David Elashoff, Pinchas Cohen, and William J. Aronson. 2013. "Effects of Calorie Restriction and IGF-1 Receptor Blockade on the Progression of 22Rv1 Prostate Cancer Xenografts" International Journal of Molecular Sciences 14, no. 7: 13782-13795. https://doi.org/10.3390/ijms140713782




