Molecular and Functional Imaging for Detection of Lymph Node Metastases in Prostate Cancer
Abstract
:1. Introduction
1.1. Conventional Anatomic Imaging
1.2. Pelvic Lymph Node Dissection (PLND)
1.3. Rationale for Molecular and Functional Imaging
2. Molecular and Functional Imaging
2.1. Planar Scintigraphy, Single-Photon Emission Computed Tomography (SPECT) and Gamma Probe
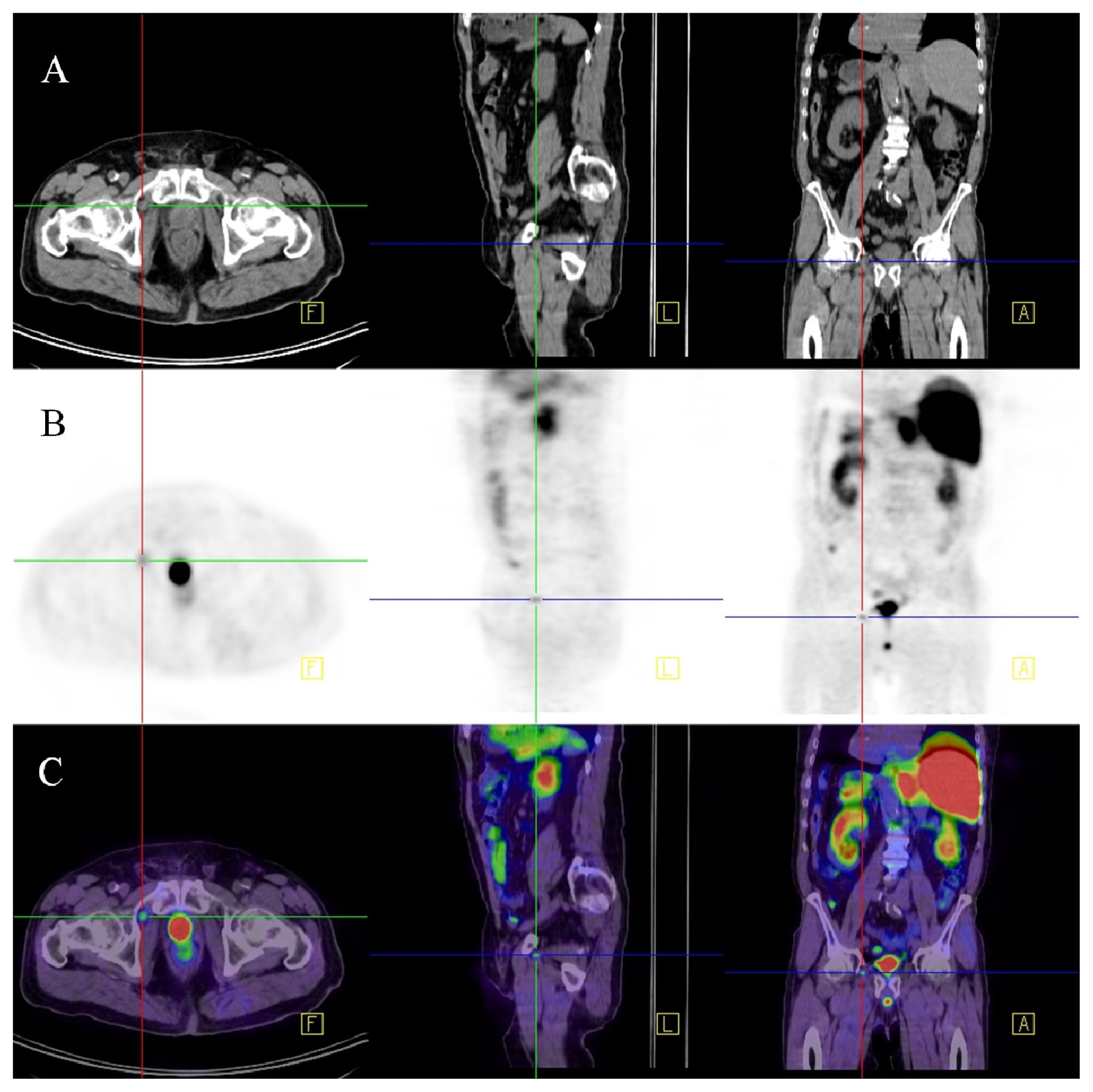
2.1.1. Sentinel Lymph Node Imaging
2.1.2. Radiolabeled Monoclonal Antibodies and Small Molecules
2.2. Positron Emission Tomography (PET)
2.2.1. FDG
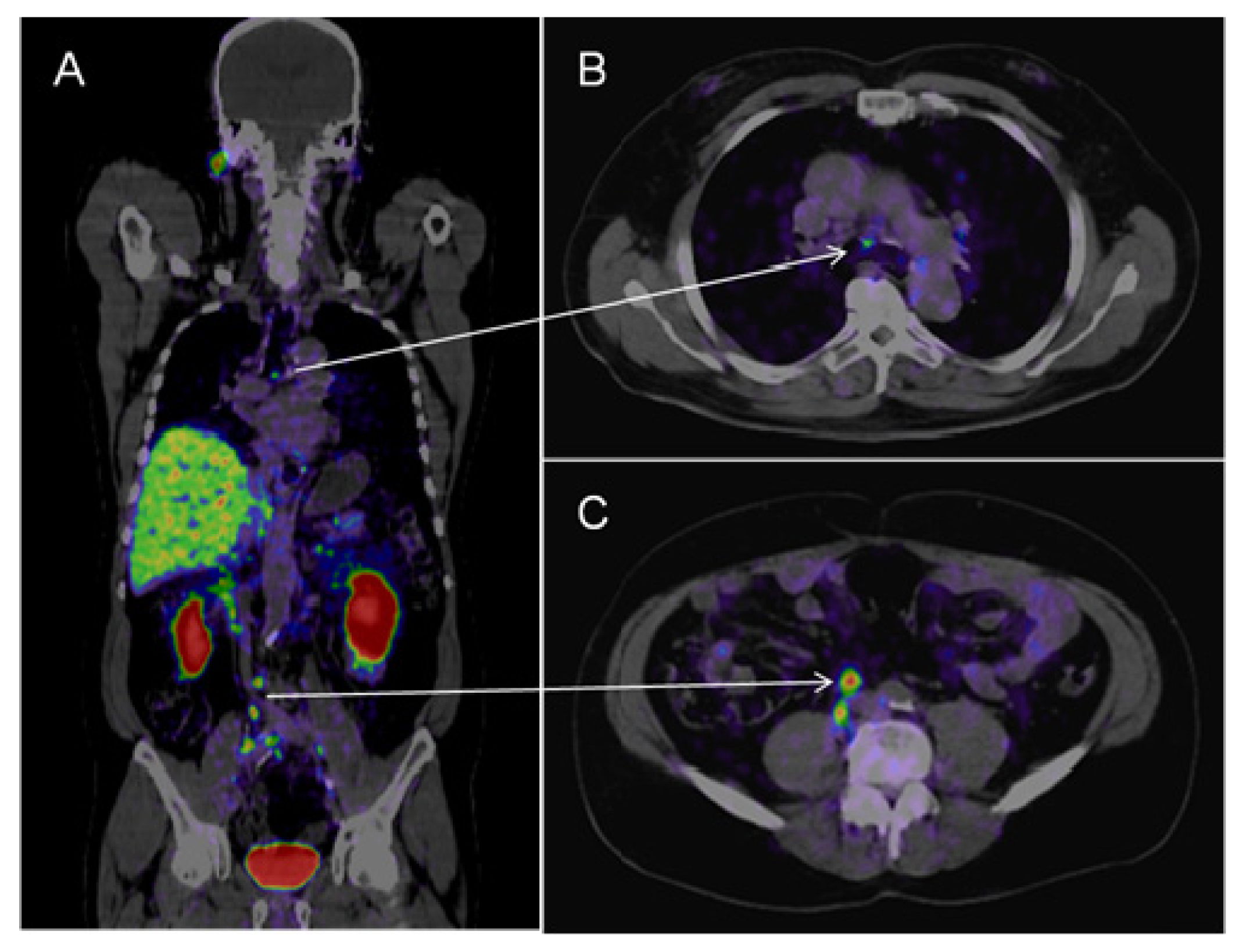
2.2.2. Choline
2.2.3. Acetate
2.2.4. 68Ga-Labelled PSMA Ligand
2.3. Magnetic Resonance Imaging (MRI)
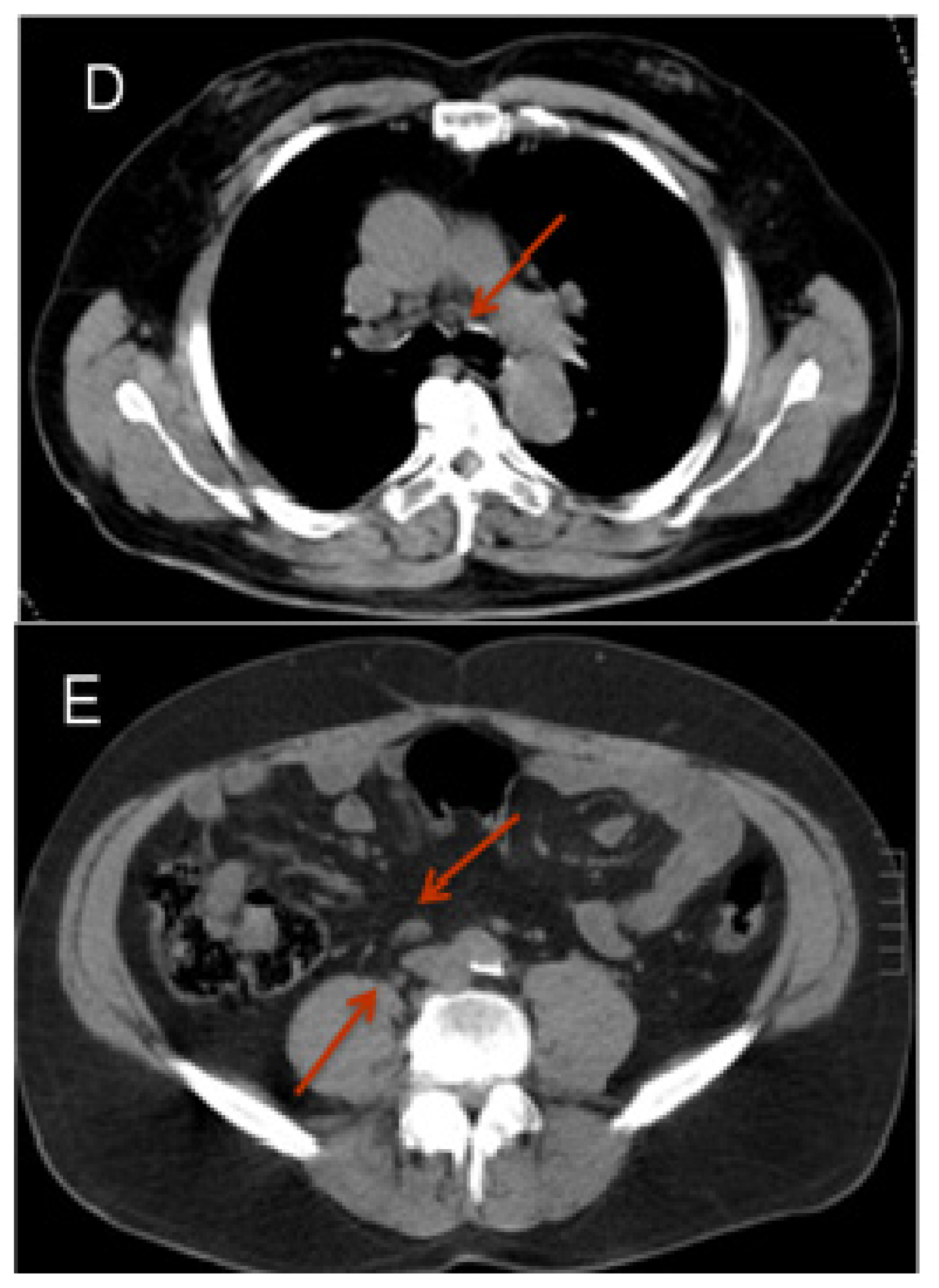
2.3.1. Diffusion Weighted Imaging (DWI)
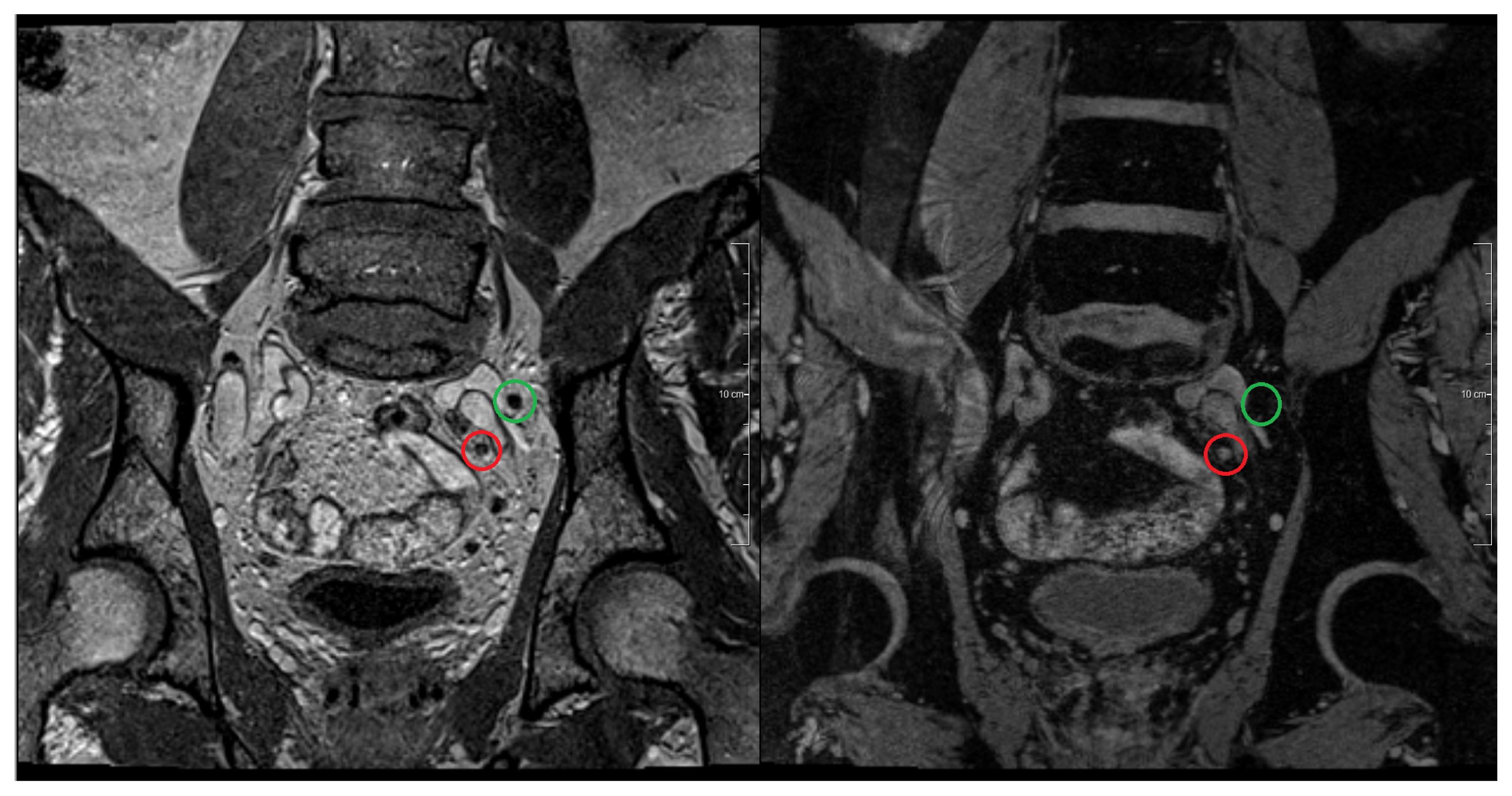
2.3.2. Magnetic Resonance Lymphography (MRL)
3. Focal Treatment Options for Lymph Node Metastases
4. Conclusions
Supplementary Information
ijms-14-13842-s001.pdfConflict of Interest
References
- Daneshmand, S.; Quek, M.L.; Stein, J.P.; Lieskovsky, G.; Cai, J.; Pinski, J.; Skinner, E.C.; Skinner, D.G. Prognosis of patients with lymph node positive prostate cancer following radical prostatectomy: Long-term results. J. Urol. 2004, 172, 2252–2255. [Google Scholar]
- Cheng, L.; Zincke, H.; Blute, M.L.; Bergstralh, E.J.; Scherer, B.; Bostwick, D.G. Risk of prostate carcinoma death in patients with lymph node metastasis. Cancer 2001, 91, 66–73. [Google Scholar]
- Hövels, A.M.; Heesakkers, R.A.; Adang, E.M.; Jager, G.J.; Strum, S.; Hoogeveen, Y.L.; Severens, J.L.; Barentsz, J.O. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: A meta-analysis. Clin. Rad 2008, 63, 387–395. [Google Scholar]
- Hilton, S.; Herr, H.W.; Teitcher, J.B.; Begg, C.B.; Castellino, R.A. CT Detection of retroperitoneal lymph node metastases in patients with clinical stage I testicular nonseminomatous germ cell cancer: Assessment of size and distribution criteria. Am. J. Roentgenol 1997, 169, 521–525. [Google Scholar]
- Brighanti, A.; Larcher, A.; Abdollah, F.; Capitanio, U.; Gallina, A.; Suardi, N.; Bianchi, M.; Freschi, M.; Salonia, A.; Karakiewicz, P.I.; et al. Updated nomogram predicting lymph node invasion in patients with prostate cancer undergoing extended pelvic lymph node dissection: The essential importance of percentage of positive cores. Eur. Urol 2012, 61, 480–487. [Google Scholar]
- Heesakkers, R.A.; Jager, G.J.; Hövels, A.M.; de Hoop, B.; van den Bosch, H.C.; Raat, F.; Witjes, J.A.; Mulders, P.F.; van der Kaa, C.H.; Barentsz, J.O. Prostate cancer: Detection of lymph node metastases outside the routine surgical area with ferumoxtran-10-enhanced MR Imaging. Radiology 2009, 251, 408–414. [Google Scholar]
- Heidenreich, A.; Varga, Z.; Von Knobloch, R. Extended pelvic lymphadenectomy in patients undergoing radical prostatectomy: High incidence of lymph node metastasis. J. Urol 2002, 167, 1681–1686. [Google Scholar]
- Joniau, W.; van den Bergh, L.; Lerut, E.; Deroose, C.M.; Haustermans, K.; Oyen, R.; Budiharto, T.; Ameye, F.; Bogaerts, K.; van Poppel, H. Mapping of pelvic lymph node metastases in prostate cancer. Eur. Urol 2013, 63, 450–458. [Google Scholar]
- Czerniecki, B.; Bedrosian, I.; Faries, M.; Alavi, A. Revolutionary impact of lymphscintigraphy and intraoperative sentinel node mapping in the clinical practice of oncology. Semin. Nucl. Med 2001, 31, 158–164. [Google Scholar]
- Uren, R.F. Lymphatic drainage of the skin. Ann. Surg. Oncol. 2004. [Google Scholar] [CrossRef]
- Mattei, A.; Fuechsel, F.G.; Bhatta Dhar, N.; Warncke, S.H.; Thalmann, G.N.; Krause, T.; Studer, U.E. The template of the primary lymphatic landing sites of the prostate should be revisited: Results of a multimodality mapping study. Eur. Urol 2008, 53, 118–125. [Google Scholar]
- Ganswindt, U.; Schilling, D.; Muller, A.C.; Bares, R.; Bartenstein, P.; Belka, C. Distribution of prostate sentinel nodes: A SPECT-derived anatomic atlas. Int. J. Radiat. Oncol. Biol. Phys 2011, 79, 1354–1372. [Google Scholar]
- Morikawa, L.K.; Roach, M., III. Pelvic nodal radiotherapy in patients with unfavourable intermediate and high-risk prostate cancer: Evidence, rationale, and future directions. Int. J. Rad. Oncol. Biol. Phys. 2011, 80, 6–16. [Google Scholar]
- Sadeghi, R.; Tabasi, K.T.; Bazaz, S.M.; Kakhki, V.R.; Massoom, A.F.; Ghoami, H.; Zakavi, S.R. Sentinel node mapping in prostate cancer. Nuklearmedizin 2011, 50, 107–115. [Google Scholar]
- Holl, G.; Dorn, R.; Wengenmair, H.; Weckermann, D.; Sciuk, J. Validation of sentinel lymph node dissection in prostate cancer: Experience in more than 2000 patients. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1377–1382. [Google Scholar]
- Morgan-Parkes, J.H. Metastases: Mechanism, pathways, and cascades. Am. J. Roentgenol 1995, 164, 1075–1082. [Google Scholar]
- David, K.; Milowsky, M.; Kostakoglu, L.; Vallabhajosula, S.; Goldsmith, S.J.; Nanus, D.M.; Bander, N.H. Clinical utility of radiolabeled monoclonal antibodies in prostate cancer. Clin. Genitourin. Cancer 2006, 4, 249–256. [Google Scholar]
- Gosh, A.; Heston, W.D. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J. Cell Biochem 2004, 91, 528–539. [Google Scholar]
- Lamb, H.M.; Faulds, D. Capromab Pendetide. A review of its use as an imaging agent in prostate cancer. Drugs Aging 1998, 12, 293–304. [Google Scholar]
- Rosenthal, S.A.; Haseman, M.K.; Polascik, T.J. Utility of capromab pendetide (ProstaScint) imaging in the management of prostate cancer. Tech. Urol. 2001, 7, 27–37. [Google Scholar]
- Ponsky, L.E.; Cherullo, E.E.; Starkey, R.; Nelson, D.; Neumann, D.; Zippe, C.D. Evaluation of preoperative ProstaScint scans in the prediction of nodal disease. Prostate Cancer Prostatic Dis 2002, 5, 132–135. [Google Scholar]
- Hardie, A.D.; Rieter, W.J.; Bradshaw, M.L.; Gordon, L.L.; Young, M.A.; Keane, T.E. Improved performance of SPECT-CT In-111 capromab pendetide by correlation with diffusion-weighted magnetic resonance imaging for identifying metastatic pelvic lymphadenopathy in prostate cancer. World J. Urol. 2013. [Google Scholar] [CrossRef]
- Troyer, J.K.; Beckett, M.L.; Wright, G.L., Jr. Location of prostate-specific membrane antigen in the LNCaP prostate carcinoma cell line. Prostate 1997, 30, 232–242. [Google Scholar]
- Osborne, J.R.; Akhtar, N.H.; Vallabhajosula, S.; Anand, A.; Deh, K.; Tagawa, S.T. Prostate-specific membrane antigen-based imaging. Urol. Oncol 2013, 31, 144–154. [Google Scholar]
- Holland, J.P.; Divilov, V.; Bander, N.H.; Smith-Jones, P.M.; Larson, S.M.; Lewis, J.S. 89Zr-DFO-J591 for ImmunoPET of Prostate-Specific Membrane Antigen Expression. In Vivo. J. Nucl. Med 2010, 51, 1293–1300. [Google Scholar]
- Barrett, J.A.; Coleman, R.E.; Goldsmith, S.J.; Vallabhajosula, S.; Petry, N.A.; Cho, S.; Armor, T.; Stubbs, J.B.; Maresca, K.P.; Stabin, M.G.; et al. First-in-man evaluation of 2 high-affinity PSMA-avid small molecules for imaging prostate cancer. J. Nucl. Med 2013, 54, 380–387. [Google Scholar]
- Haberkorn, U.; Markert, A.; Mier, W.; Askoxylakis, V.; Altmann, A. Molecular imaging of tumor metabolism and apoptosis. Oncogene 2011, 30, 4141–4151. [Google Scholar]
- Effert, P.J.; Bares, R.; Handt, S.; Wolff, J.M.; Büll, U.; Jakse, G. Metabolic imaging of untreated prostate cancer by positron emission tomography with 18fluoro-labeled-deoxyglucose. J. Urol 1996, 155, 994–998. [Google Scholar]
- Sanz, G.; Robles, J.E.; Giménez, M.; Arocena, J.; Sánchez, D.; Rodriguez-Rubio, F.; Rosell, D.; Richter, J.A.; Berián, J.M. Positron emission tomography with 18fluorine-labelled deoxyglucose: utility in localized and advanced prostate cancer. BJU Int. 1999, 84, 1028–1031. [Google Scholar]
- Shreve, P.D.; Grossman, H.B.; Gross, M.D.; Wahl, R.L. Metastatic prostate cancer: initial findings of PET with 2-deoxy-2-[F-18]fluoro-D-glucose. Radiology 1996, 199, 751–756. [Google Scholar]
- Ackerstaff, E.; Glunde, K.; Bhujwalla, Z.M. Choline phospholipid metabolism: A target in cancer cells? J. Cell Biochem 2003, 90, 525–523. [Google Scholar]
- Jadvar, H. Prostate cancer: PET with 18F-FDG, 18F- or 11C-acetate, and 18F-or 11C-choline. J. Nucl. Med 2011, 52, 81–89. [Google Scholar]
- Evangelista, L.; Guttilla, A.; Zattoni, F.; Muzzio, P.C.; Zattoni, F. Utility of choline positron tomography/computed tomography for lymph node involvement identification in intermediate-to high-risk prostate cancer: a systematic literature review and meta-analysis. Eur. Urol 2013, 63, 1040–1048. [Google Scholar]
- Fortuin, A.S.; Deserno, W.M.; Meijer, H.J.; Jager, G.J.; Takahashi, S.; Debats, O.A.; Reske, S.N.; Schick, C.; Krause, B.J.; van Oort, I.; et al. Value of PET/CT and MR Lymphography in treatment of prostate cancer patients with lymph node metastases. Int. J. Rad. Oncol. Biol. Phys 2012, 84, 712–718. [Google Scholar]
- DeGrado, T.R.; Coleman, R.E.; Wang, S.; Baldwin, S.W.; Orr, M.D.; Robertson, C.N.; Polascik, T.J.; Price, D.T. Price Synthesis and Evaluation of 18F-labeled Choline as an Oncologic Tracer for Positron Emission Tomography: Initial Findings in Prostate Cancer. Cancer Res 2000, 61, 110–117. [Google Scholar]
- DeGrado, T.R.; Baldwin, S.W.; Wang, S.; Orr, M.D.; Liao, R.P.; Friedman, H.S.; Reiman, R.; Price, D.T.; Coleman, R.E. Synthesis and evaluation of (18)F-labeled choline analogs as oncologic PET tracers. J. Nucl. Med 2001, 42, 1805–1814. [Google Scholar]
- Tilki, D.; Reich, O.; Graser, A.; Hacker, M.; Silchinger, J.; Becker, A.J.; Khoder, W.; Bartenstein, P.; Stief, C.G.; Loidl, W.; et al. 18F-Fluoroethylcholine PET/CT identifies lymph node metastasis in patients with prostate-specific antigen failure after radical prostatectomy but underestimates its extent. Eur. Urol 2013, 63, 792–796. [Google Scholar]
- Evangelista, L.; Zattoni, F.; Guttilla, A.; Saladini, G.; Zattoni, F.; Colletti, P.M.; Rubello, D. Choline PET or PET/CT and biochemical relapse of prostate Cancer, a systematic review and meta-analysis. Clin. Nucl. Med 2013, 38, 305–314. [Google Scholar]
- Wetter, A.; Lipponer, C.; Nensa, F.; Beiderwellen, K.; Olbricht, T.; Rübben, H.; Bockisch, A.; Schlosser, T.; Heusner, T.A.; Lauenstein, T.C. Simultaneous 18F choline positron emission tomography/magnetic resonance imaging of the prostate. Initial results. Invest. Radiol 2013, 48, 256–262. [Google Scholar]
- Vavere, A.L.; Kridel, S.J.; Wheeler, F.B.; Lewis, J.S. 1-11C-Acetate as a PET radiopharmaceutical for imaging faty acid synthase expression in prostate cancer. J. Nucl. Med 2008, 49, 327–334. [Google Scholar]
- Haseebuddin, M.; Dehdashti, F.; Siegel, B.A.; Liu, J.; Roth, E.B.; Nepple, K.G.; Siegel, C.L.; Fischer, K.C.; Kibel, A.S.; Andriole, G.L.; et al. 11C-Acetate PET/CT before radical prostatectomy: nodal staging and treatment failure prediction. J. Nucl. Med 2013, 54, 699–706. [Google Scholar]
- Fricke, E.; Machtens, S.; Hofmann, M.; van den Hoff, J.; Bergh, S.; Brunkhorst, T.; Meyer, G.J.; Karstens, J.H.; Knapp, W.H.; Boerner, A.R. Positron emission tomography with 11C-acetate and 18F-FDG in prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 607–611. [Google Scholar]
- Afshar-Oromich, A.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Hadaschikc, B.A.; Holland-Letz, T.; Giesel, F.L.; Kratochwil, C.; Haufe, S.; et al. Pet imaging with a [68GA] gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 486–495. [Google Scholar]
- Eder, M.; Eisenhut, M.; Babich, J.; Haberkorn, U. PSMA as a target for radiolabelled small molecules. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 819–823. [Google Scholar]
- Eiber, M.; Beer, A.J.; Holzapfel, K.; Tauber, R.; Weirich, G.; Krause, B.J.; Rummeny, E.J.; Gaa, J. Preliminary results for characterization of pelvic lymph nodes in patients with prostate cancer by diffusion-weighted MR-imaging. Invest. Radiol 2010, 45, 15–23. [Google Scholar]
- Beer, A.J.; Eiber, M.; Holzapfel, K.; Tauber, R.; Ganter, C.; Weirich, G.; Krause, B.J.; Rummeny, E.J.; Gaa, J. Restricted water diffusibility as measured by diffusion-weighted MR Imaging and choline uptake in 11C-choline PET/CT are correlated in pelvic lymph nodes in patients with prostate cancer. Mol. Imaging Biol 2011, 13, 352–361. [Google Scholar]
- Budiharto, T.; Joniau, S.; Lerut, E.; van den Bergh, L.; Mottaghy, F.; Deroose, C.M.; Oyen, R.; Ameye, F.; Bogaerts, K.; Haustermans, K.; et al. Prospective Evaluation of 11C-choline positron emission Tomograhpy/computed tomography and diffusion-weighted magnetic resonance imaging for the nodal staging of prostate cancer with a high risk of lymph node metastases. Eur. Urol 2011, 60, 125–130. [Google Scholar]
- Harisinghani, M.G.; Barentsz, J.O.; Hahn, P.F.; Deserno, W.M.; Tabatabaei, S.; van de Kaa, C.H.; de la Rosette, J.; Weissleder, R. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N. Engl. J. Med 2003, 348, 2491–2499. [Google Scholar]
- Harisinghani, M.G.; Barentsz, J.O.; Hahn, P.F.; Deserno, W.M.; de la Rosette, J.; Saini, S.; Weissleder, R. MR lymphangiography for detection of minimal nodal disease in patients with prostate cancer. Acad. Radiol 2002, 9, 312–313. [Google Scholar]
- Heesakkers, R.A.; Hövels, A.M.; Jager, G.J.; van den Bosch, H.C.; Witjes, J.A.; Raat, H.P.; Severens, J.L.; Adang, E.M.; van der Kaa, C.H.; Fütterer, J.J.; et al. MRI with a lymph-node-specific contrast agent as an alternative to CT scan and lymph-node dissection in patients with prostate cancer: A prospective multicohort study. Lancet. Oncol 2008, 9, 850–856. [Google Scholar]
- Triantafyllou, M.; Studer, U.E.; Birkhäuser, F.D.; Fleischmann, A.; Bains, L.J.; Petralia, G.; Christe, A.; Froehlich, J.M.; Thoeny, H.C. Ultrasmall superparamagnetic particles of iron oxide allow for the detection of metastases in normal sized pelvic lymph nodes of patients with bladder and/or prostate cancer. Eur. J. Cancer 2013, 49, 616–624. [Google Scholar]
- Fortuin, A.S.; Barentsz, J.O. Comments on Ultrasmall superparamagnetic particles of iron oxide allow for the detection of metastases in normal sized pelvic lymph nodes of patients with bladder and/or prostate cancer. Eur. J. Cancer 2013, 49, 1789–1790. [Google Scholar]
- Thoeny, H.; Triantafyllou, M.; Birkhaeuser, F.D.; Froehlich, J.M.; Tshering, D.W.; Binser, T.; Fleischmann, A.; Vermathen, P.; Studer, U.E. Combined ultrasmall superparamagnetic particles of iron oxide-enhanced and diffusion-weighted magnetic resonance imaging reliably detect pelvic lymph node metastases in normal-sized nodes of bladder and prostate cancer patients. Eur. Urol 2009, 55, 761–769. [Google Scholar]
- Meijer, H.J.; Fortuin, A.S.; van Lin, E.N.; Debats, O.A.; Witjes, J.A.; Kaanders, J.H.; Barentsz, J.O. Geographical distribution of lymph node metastases on MR lymphography in prostate cancer patients. Radiother. Oncol 2013, 106, 59–63. [Google Scholar]
- Meijer, H.J.; van Lin, E.N.; Debats, O.A.; Witjes, J.A.; Span, P.N.; Kaanders, J.H.; Barentsz, J.O. High occurrence of aberrant lymph node spread on magnetic resonance lymphography in prostate cancer patients with a biochemical recurrence after radical prostatectomy. Int. J. Rad. Oncol. Biol. Phys 2012, 82, 1405–1410. [Google Scholar]
- Joslyn, S.A.; Konety, B.R. Impact of extent of lymphadenectomy on survival after radical prostatectomy for prostate cancer. Urology 2006, 68, 121–125. [Google Scholar]
- Burkhard, F.C.; Studer, U.E. The role of lymphadenectomy in high-risk prostate cancer. World J. Urol 2008, 26, 231–236. [Google Scholar]
- Thurairaja, R.; Studer, U.E.; Burkhard, F.C. Indications, extend, and benefits of pelvic lymph node dissection for patients with bladder and prostate cancer. Oncologist 2009, 14, 40–51. [Google Scholar]
- Allaf, M.E.; Palapattu, G.S.; Trock, B.J.; Carter, H.B.; Walsh, P.C. Anatomical extent of lymph node dissection: impact on men with clinically localized prostate cancer. J. Urol 2004, 172, 1840–1844. [Google Scholar]
- Winter, A.; Uphoff, J.; Henke, R.P.; Wawroschek, F. First results of 11C-choline PET/CT-guided secondary lymph node surgery single lymph node recurrence after radical retropubic prostatectomy. Urol. Int 2010, 84, 418–423. [Google Scholar]
- Winter, A.; Uphoff, J.; Henke, R.P.; Wawroschek, F. Complete PSA remission without adjuvant therapy after secondary lymph node surgry in selected patients with biochemical relaps after radical prostatectomy and pelvic lymph node dissection. Adv. Urol. 2012. [Google Scholar] [CrossRef]
- Jereczek-Fossa, B.A.; Beltramo, G.; Fariselli, L.; Fodor, C.; Santoro, L.; Vavassori, A.; Zerini, D.; Gherardi, F.; Ascione, C.; Bossi-Zanetti, I.; et al. Robotic image-guided stereotactic radiotherapy, for isolated recurrent primary, lymph node or metastatic prostate cancer. Int. J. Radiat. Oncol. Boil. Phys 2012, 82, 889–897. [Google Scholar]
- Meijer, H.J.; Debats, O.A.; Kunze-Busch, M.; van Kollenburg, P.; Leer, J.W.; Witjes, J.A.; Kaanders, J.H.; Barentsz, J.O.; van Lin, E.N. Magnetic resonance lymphography-guided selective high-dose lymph node irradiation in prostate cancer. Int. J. Radiat. Oncol. Biol. Phys 2012, 82, 175–183. [Google Scholar]
- Weidner, A.M.; van Lin, E.N.; Dinter, D.J.; Rozema, T.; Schoenberg, S.O.; Wenz, F.; Barentsz, J.O.; Lohr, F. Ferumoxstran-10 MR lymphography for target definition and follow-up in a patient undergoing image-guided, dose-escalated radiotherapy of lymph nodes upon PSA relaps. Strahlenther. Onkol 2011, 187, 206–212. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fortuin, A.; De Rooij, M.; Zamecnik, P.; Haberkorn, U.; Barentsz, J. Molecular and Functional Imaging for Detection of Lymph Node Metastases in Prostate Cancer. Int. J. Mol. Sci. 2013, 14, 13842-13857. https://doi.org/10.3390/ijms140713842
Fortuin A, De Rooij M, Zamecnik P, Haberkorn U, Barentsz J. Molecular and Functional Imaging for Detection of Lymph Node Metastases in Prostate Cancer. International Journal of Molecular Sciences. 2013; 14(7):13842-13857. https://doi.org/10.3390/ijms140713842
Chicago/Turabian StyleFortuin, Ansje, Maarten De Rooij, Patrik Zamecnik, Uwe Haberkorn, and Jelle Barentsz. 2013. "Molecular and Functional Imaging for Detection of Lymph Node Metastases in Prostate Cancer" International Journal of Molecular Sciences 14, no. 7: 13842-13857. https://doi.org/10.3390/ijms140713842
APA StyleFortuin, A., De Rooij, M., Zamecnik, P., Haberkorn, U., & Barentsz, J. (2013). Molecular and Functional Imaging for Detection of Lymph Node Metastases in Prostate Cancer. International Journal of Molecular Sciences, 14(7), 13842-13857. https://doi.org/10.3390/ijms140713842



