Alleviation of Osmotic Stress Effects by Exogenous Application of Salicylic or Abscisic Acid on Wheat Seedlings
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physiological and Morphological Seedling Characteristics under Osmotic Stress
2.1.1. Water Relations
2.1.2. Gas Exchange and Chlorophyll Content
2.1.3. Morphological Traits and MDA
2.2. Osmoprotectants, ABA and Antioxidants in Seedling Leaves under Osmotic Stress
2.2.1. Carbohydrates
2.2.2. Proline
2.2.3. Polyamines
2.2.4. ABA
2.2.5. Antioxidants
2.3. Aftereffects of Osmotic Stress on Yield Components
3. Experimental Section
3.1. Plant Material
3.2. Experimental Design
3.3. Measurements and Analysis
3.4. Osmotic Potential
3.5. Lipid Peroxidation (MDA Content)
3.6. Soluble Carbohydrates
3.7. Proline
3.8. Abscisic Acid
3.9. Total Activity of Low Molecular Antioxidants (TAA)
3.10. Polyamines
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Luo, M.H.; Yuan, S.; Chen, Y.E.; Liu, W.J.; Du, J.B.; Lei, T.; Wang, M.B.; Lin, H.H. Effects of salicylic acid on the photosystem 2 of barley seedlings under osmotic stress. Biol. Plant 2009, 53, 663–669. [Google Scholar]
- Najafian, S.; Khoshkhui, M.; Tavallali, V.; Saharkhiz, M.J. Effect of salicylic acid and salinity in thyme (Thymus Vulgaris L.): Investigation on changes in gas exchange, water relations, and membrane stabilization and biomass accumulation. Aust. J. Basic Appl. Sci 2009, 3, 2620–2626. [Google Scholar]
- Poor, P.; Gemes, K.; Horvath, F.; Szepesi, A.; Simon, M.L.; Tari, I. Salicylic acid treatment via the rooting medium interferes with stomatal response, CO2 fixation rate and carbohydrate metabolism in tomato, and decreases harmful effects of subsequent salt stress. Plant Biol 2011, 13, 105–114. [Google Scholar]
- Yordanova, R.; Popova, L. Effect of exogenous treatment with salicylic acid on photosynthetic activity and antioxidant capacity of chilled wheat plants. Gen. Appl. Plant Physiol 2007, 33, 155–170. [Google Scholar]
- Anjum, S.A.; Farooq, M.; Xie, X.-Y.; Liu, X.-J.; Ijaz, M.F. Antioxidant defense system and proline accumulation enables hot pepper to perform better under drought. Sci. Hort 2012, 140, 66–73. [Google Scholar]
- Capell, T.; Bassie, L.; Christou, P. Modulation of the polyamine biosynthetic pathway in transgenic rice confers tolerance to drought stress. Proc. Natl. Acad. Sci. USA 2004, 101, 9909–9914. [Google Scholar]
- Mohammadkhani, N.; Heidari, R. Drought-induced accumulation of soluble sugars and proline in two maize varieties. World Appl. Sci. J 2008, 13, 448–453. [Google Scholar]
- Hussain, K.; Nawaz, K.; Majeed, A.; Khan, A.; Lin, F.; Ghani, A.; Raza, G.; Afghan, S.; Zia-ul-Hussnain, S.; Ali, K.; et al. Alleviation of salinity effects by exogenous applications of salicylic acid in pearl millet (Pennisetum glaucum (L.) R. Br.) seedlings. Afr. J. Biotechnol 2010, 9, 8602–8607. [Google Scholar]
- Szepesi, Á.; Csiszár, J.; Bajkán, S.; Gémes, K.; Horváth, F.; Erdei, L.; Deér, A.K.; Simon, M.L.; Tari, I. Role of salicylic acid pre-treatment on the acclimation of tomato plants to salt- and osmotic stress. Acta Biol. Szeged. 2005, 49, 123–125. [Google Scholar]
- Maghsoudi, K.; Arvin, M.J. Response of seed germination and seedling growth of wheat (Triticum aestivum L.) cultivars to interactive effect of salinity and salicylic acid. J. Plant Ecophysiol 2010, 2, 6–2010. [Google Scholar]
- Liu, W.J.; Yuan, S.; Zhang, N.H.; Lei, T.; Duan, H.G.; Liang, H.G.; Lin, H.H. Effect of water stress on photosystem 2 in two wheat cultivars. Biol. Plantarum 2006, 50, 597–602. [Google Scholar]
- Yuan, S.; Liu, W.J.; Zhang, N.H.; Wang, M.B.; Liang, H.G.; Lin, H.H. Effects of water stress on major PSII gene expression and protein metabolism in barley leaves. Physiol. Plant 2005, 125, 464–473. [Google Scholar]
- Noreen, S.; Ashraf, M. Alleviation of adverse effects of salt stress on Sunflower (Helianthus annuus L.) by exogenous application of salicylic acid: Growth and photosynthesis. Pak. J. Bot 2008, 40, 1657–1663. [Google Scholar]
- Sakhabutdinova, A.R.D.; Fatkhutdinova, R.; Bezrukova, M.V.; Shakirova, F.M. Salicylic acid prevents the damaging action of stress factors on wheat plants. Bulg. J. Plant Physiol. 2003, (Special Issue), 314–319. [Google Scholar]
- Sahu, G.K.; Sabat, S.C. Changes in growth, pigment kontent and antioxidants in the root and leaf tissues of wheat plants under the influence of exogenous salicylic acid brazilian society of plant physiology. Braz. J. Plant Physiol 2011, 23, 209–218. [Google Scholar]
- Christmann, A.; Grill, E.; Meinhard, M. Abscisic acid Signalling. In Plant Responses to Abiotic Stress; Hirt, H., Shinozaki, K., Eds.; Topics in Current Genetics; Springer-Verlag: Berlin/Heidelberg, Germany, 2004; pp. 39–71. [Google Scholar]
- Groppa, M.D.; Benavides, M.P. Polyamines and abiotic stress: Recent advances. Amino Acids 2008, 34, 35–45. [Google Scholar]
- Pospisilova, J. Participation of phytohormones in the stomatal regulation of gas exchange during water stress. Biol. Plantarum 2003, 46, 491–506. [Google Scholar]
- Wilkinson, S.; Davies, W.J. ABA-based chemical signalling: The co-ordination of responses to stress in plants. Plant Cell Environ 2002, 25, 195–210. [Google Scholar]
- Sobeih, W.Y.; Dodd, I.C.; Bacon, M.A.; Grierson, D.; Davies, W.J. Long-distance signals regulating stomatal conductance and leaf growth in tomato (Lycopersicon esculentum) plants subjected to partial root-zone drying. J. Exp. Bot 2004, 55, 2353–2363. [Google Scholar]
- Zhang, J.H.; Jia, W.S.; Yang, J.C.; Ismail, A.M. Role of ABA in integrating plant responses to drought and salt stresses. Field Crops Res 2006, 97, 111–119. [Google Scholar]
- Zhang, S.Q.; Outlaw, W.H. Abscisic acid introduced into the transpiration stream accumulates in the guard-cell apoplast and causes stomatal closure. Plant Cell Environ 2001, 24, 1045–1054. [Google Scholar]
- Okamoto, M.; Tsuboi, Y.; Chikayama, E.; Kikuchi, J.; Hirayama, T. Metabolic movement upon abscisic acid and salicylic acid combined treatments. Plant Biotechnol 2009, 26, 551–560. [Google Scholar]
- Seo, P.J.; Park, C.-M. MYB96-mediated abscisic acid signals induce pathogen resistance response by promoting salicylic acid biosynthesis in Arabidopsis. New Phytol 2010, 186, 471–483. [Google Scholar]
- Chaves, M.M.; Pereira, J.S.; Maroco, J.; Rodrigues, M.L.; Ricardo, C.P.P.; Osorio, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How plants cope with water stress in the field. Photosynthesis and growth. Ann. Bot 2002, 89, 907–916. [Google Scholar]
- Gibson, S.I. Control of plant development and gene expression by sugar signaling. Curr. Opin. Plant Biol 2005, 8, 93–102. [Google Scholar]
- Cha-um, S.; Kirdmanee, C. Effect of osmotic stress on proline accumulation, photosynthetic abilities and growth of sugarcane plantlets (Saccharum officinarum L.). Pak. J. Bot 2008, 40, 2541–2552. [Google Scholar]
- Teixeira, J.; Pereira, S. High salinity and drought act on an organ-dependant manner on potato glutamine synthetase expression and accumulation. Environ. Exp. Bot 2007, 60, 121–126. [Google Scholar]
- Valliyodan, B.; Nguyen, H.T. Understanding regulatory networks and engineering for enhanced drought tolerance in plants. Curr. Opin. Plant Biol 2006, 9, 189–195. [Google Scholar]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar]
- Bandurska, H. Akumulacja wolnej proliny w warunkach deficytu wody w liściach wybranych gatunków roślin uprawnych. Acta Agrobot 2004, 57, 57–67. [Google Scholar]
- Alcazar, R.; Cuevas, J.C.; Patron, M.; Altabella, T.; Tiburcio, A.F. Abscisic acid modulates polyamine metabolism under water stress in Arabidopsis thaliana. Physiol. Plant 2006, 128, 448–455. [Google Scholar]
- Marcińska, I.; Czyczyło-Mysza, I.; Skrzypek, E.; Filek, M.; Grzesiak, S.; Grzesiak, M.T.; Janowiak, F.; Hura, T.; Dziurka, M.; Dziurka, K.; et al. Impact of osmotic stress on physiological and biochemical characteristics in drought susceptible and drought resistant wheat genotypes. Acta Physiol. Plant 2013, 35, 451–461. [Google Scholar]
- Nemeth, M.; Janda, T.; Horvath, E.; Paldi, E.; Szalai, G. Exogenous salicylic acid increases polyamine content but may decrease drought tolerance in maize. Plant Sci 2002, 162, 569–574. [Google Scholar]
- Safrankova, I.; Hejnak, V.; Stuchlikova, K.; Ceska, J. The effect of abscisic acid on rate of photosynthesis and transpiration in six barley genotypes under water stress. Cereal Res. Commun 2007, 35, 1013–1016. [Google Scholar]
- Agehara, S.; Leskovar, D.I. Characterizing concentration effects of exogenous abscisic acid on gas exchange, water relations, and growth of Muskmelon seedlings during water stress and rehydration. J. Am. Soc. Hort. Sci 2012, 137, 400–410. [Google Scholar]
- Grzesiak, M.T.; Grzesiak, S.; Skoczowski, A. Changes of leaf water potential and gas exchange during and after drought in triticale and maize genotypes differing in drought tolerance. Photosynthetica 2006, 44, 561–568. [Google Scholar]
- Janda, T.; Szalai, G.; Tari, I.; Paldi, E. Hydroponic treatment with salicylic acid decreases the effects of chilling injury in maize (Zea mays L.) plants. Planta 1999, 208, 175–180. [Google Scholar]
- Duan, B.; Yang, Y.; Lu, Y.; Korpelainen, H.; Berninger, F.; Li, C. Interactions between water deficit, ABA, and provenances in Picea asperata. J. Exp. Bot 2007, 58, 3025–3036. [Google Scholar]
- Khan, S.U.; Bano, A.; Ud-Din, J.U.; Gurmani, A.R. Abscisic acid and salicylic acid seed treatment as potent inducer of drought tolerance in wheat (Triticum aestivum L.). Pak. J. Bot. 2012, 44(Special Issue), 43–49. [Google Scholar]
- Mostajeran, A.; Rahimi-Eichi, V. Effects of drought stress on growth and yield of rice (Oryza sativa) cultivars and accumulation of proline and soluble sugars in sheath and blades of their different ages leaves. Am.-Eurasian J. Agric. Environ. Sci 2009, 5, 264–272. [Google Scholar]
- Izanloo, A.; Condon, A.G.; Langridge, P.; Tester, M.; Schnurbusch, T. Different mechanisms of adaptation to cyclic water stress in two South Australian bread wheat cultivars. J. Exp. Bot 2008, 59, 3327–3346. [Google Scholar]
- Watanabe, S.; Kojima, K.; Ide, Y.; Satohiko, S. Effects of saline and osmotic stress on praline and sugar accumulation in Populus euphratica in vitro. Plant Cell Tiss. Org 2000, 63, 199–206. [Google Scholar]
- Kishor, P.B.K.; Sangam, S.; Amrutha, R.N.; Laxmi, P.S.; Naidu, K.R.; Rao, K.R.S.S.; Rao, S.; Reddy, K.J.; Theriappan, P.; Sreenivasulu, N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci 2005, 88, 424–438. [Google Scholar]
- Delauney, A.; Verma, D.P.S. Proline biosynthesis and osmoregulation in plants. Plant J 1993, 4, 215–223. [Google Scholar]
- Li, X.; Yang, Y.; Jia, L.; Chen, H.; Wei, X. Zinc-induced oxidative damage, antioxidant enzyme response and proline metabolism in roots and leaves of wheat plants. Ecotoxicol. Environ. Saf. 2012. [Google Scholar] [CrossRef]
- Maiale, S.; Sanchez, D.H.; Guirado, A.; Vidal, A.; Ruiz, O.A. Spermine accumulation under salt stress. J. Plant Physiol 2004, 161, 35–42. [Google Scholar]
- Urano, K.; Yoshiba, Y.; Nanjo, T.; Igarashi, Y.; Seki, M.; Sekiguchi, F.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Characterization of Arabidopsis genes involvedbiosynthesis of polyamines in abiotic stress responses and developmental stages. Plant Cell Environ 2003, 26, 1917–1926. [Google Scholar]
- Legocka, J.; Kluk, A. Effect of salt and osmotic stress on changes in polyamine content and arginine decarboxylase activity in Lupinus luteus seedlings. J. Plant Physiol 2005, 162, 662–668. [Google Scholar]
- Tonon, G.; Kevers, C.; Faivre-Rampant, O.; Grazianil, M.; Gaspar, T. Effect of NaCl and mannitol iso-osmotic stresses on proline and free polyamine levels in embryogenic Fraxinus angustifolia callus. J. Plant Physiol 2004, 161, 701–708. [Google Scholar]
- Do, P.T.; Degenkolbe, T.; Erban, A.; Heyer, A.G.; Kopka, J.; Kohl, K.I.; Hincha, D.K.; Zuther, E. Dissecting rice polyamine metabolism under controlled long-term drought stress. PLoS One 2013, 8, e60325. [Google Scholar]
- Quarrie, S.A. Implications of Genetic Differences in ABA Accumulation for Crop Production. In Abscisic Acid Physiology and Biochemistry; Davies, W.J., Jones, H.G., Eds.; BIOS Scientific Publishers: Oxford, UK, 1991; pp. 227–243. [Google Scholar]
- Ozfidan, C.; Turkan, I.; Sekmen, A.H.; Seckin, B. Abscisic acid-regulated responses of aba2-1 under osmotic stress: The abscisic acid-inducible antioxidant defence system and reactive oxygen species production. Plant Biol 2012, 14, 337–346. [Google Scholar]
- Nan, R.; Carman, J.G.; Salisbury, F.B. Water stress, CO2 and photoperiod influence hormone levels in wheat. J. Plant Physiol 2002, 159, 307–312. [Google Scholar]
- Guoth, A.; Tari, I.; Galle, A.; Csiszar, J.; Pecsvaradi, A.; Cseuz, L.; Erdei, L. Comparison of the drought stress responses of tolerant and sensitive wheat cultivars during grain filling: Changes in flag leaf photosynthetic activity, ABA levels, and grain yield. J. Plant Growth Regul 2009, 28, 167–176. [Google Scholar]
- Doerffling, K.; Doerffling, H.; Luck, E. Improved frost tolerance and winter hardiness in proline overaccumulating winter wheat mutants obtained by in vitro-selection is associated with increased carbohydrate, soluble protein and abscisic acid (ABA) levels. Euphytica 2009, 165, 545–556. [Google Scholar]
- Janowiak, F.; Maas, B.; Dörffling, K. Importance of abscisic acid for chilling tolerance of maize seedlings. J. Plant Physiol 2002, 159, 635–643. [Google Scholar]
- Fujita, Y.; Fujita, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. ABA-mediated transcriptional regulation in response to osmotic stress in plants. J. Plant Res 2011, 124, 509–525. [Google Scholar]
- Sanchez-Moreno, C. Review: Methods used to evaluate the free radical scavenging activity in foods and biological systems. Food Sci. Technol. Int 2002, 8, 121–137. [Google Scholar]
- Hong-Bo, S.; Li-Ye, C.; Ming-An, S.; Jaleel, C.A.; Hong-Mei, M. Higher plant antioxidants and redox signaling under environmental stresses. C. R. Biol 2008, 331, 433–441. [Google Scholar]
- Kolarovic, L.; Valentovic, P.; Luxova, M.; Gasparikova, O. Changes in antioxidants and cell damage in heterotrophic maize seedlings differing in drought sensitivity after exposure to short-term osmotic stress. Plant Growth Regul 2009, 59, 21–26. [Google Scholar]
- Kellos, T.; Timar, I.; Szilagyi, V.; Szalai, G.; Galiba, G.; Kocsy, G. Stress hormones and abiotic stresses have different effects on antioxidants in maize lines with different sensitivity. Plant Biol 2008, 10, 563–572. [Google Scholar]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol 2004, 161, 1189–1202. [Google Scholar]
- Guo, Z.; Ou, W.; Lu, S.; Zhong, Q. Differential responses of antioxidative system to chilling and drought in four rice cultivars differing in sensitivity. Plant Phys. Bioch 2006, 44, 828–836. [Google Scholar]
- Loutfy, N.; El-Tayeb, M.A.; Hassanen, A.M.; Moustafa, M.F.M.; Sakuma, Y.; Inouhe, M. Changes in the water status and osmotic solute contents in response to drought and salicylic acid treatments in four different cultivars of wheat (Triticum aestivum). J. Plant Res 2012, 125, 173–184. [Google Scholar]
- Cairns, J.E.; Sanchez, C.; Vargas, M.; Ordonez, R.; Araus, J.L. Dissecting maize productivity: Ideotypes associated with grain yield under drought stress and well-watered conditions. J. Integr. Plant Biol 2012, 54, 1007–1020. [Google Scholar]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlation with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot 1981, 32, 96–101. [Google Scholar]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A colorimetric method forth determination of sugars. Nature 1951, 168, 167–168. [Google Scholar]
- Ting, S.V.; Rouseff, R.L. Proline content in Florida frozen concentrated orange juice and canned grapefruit juice. Proc. Fla. State Hortic. Soc 1979, 92, 143–145. [Google Scholar]
- Walker-Simmons, M.K.; Abrams, S.R. Use of ABA Immunoassays. In Abscisic Acid Physiology and Biochemistry; Davies, W.J., Jones, H.G., Eds.; BIOS Scientific Publishers: Oxford, UK, 1991; pp. 53–61. [Google Scholar]
- Smith, M.A.; Davies, P.J. Separation and quantitation of polyamines in plant-tissue by high-performance liquid-chromatography of their dansyl derivatives. Plant Physiol 1985, 78, 89–91. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free-radical method to evaluate antioxidant activity. Food Sci. Technol.-Lebensm.-Wiss. Technol 1995, 28, 25–30. [Google Scholar]
- Plazek, A.; Dubert, F.; Janowiak, F.; Krepski, T.; Tatrzanska, M. Plant age and in vitro or in vivo propagation considerably affect cold tolerance of Miscanthus x giganteus. Eur. J. Agron 2011, 34, 163–171. [Google Scholar]
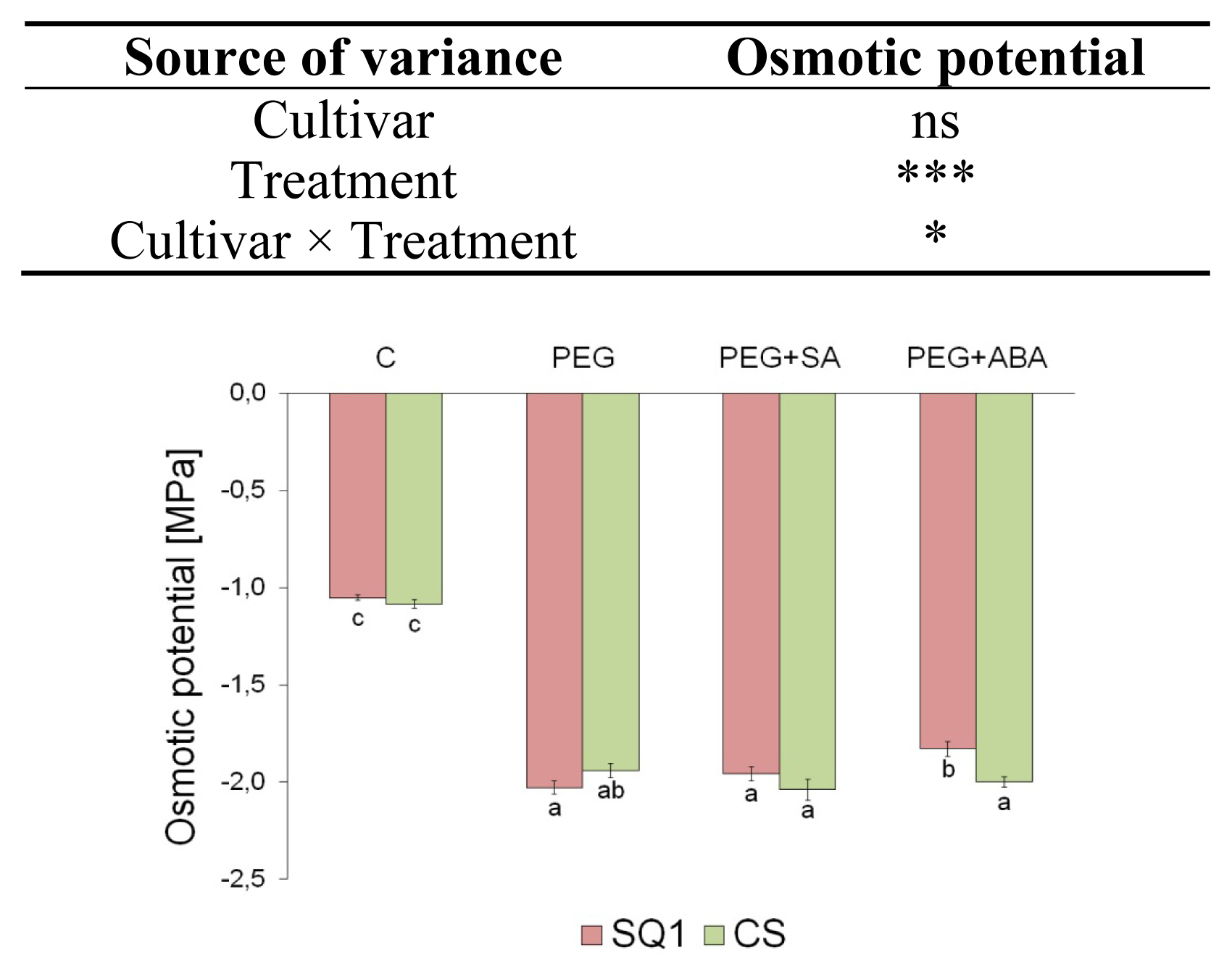
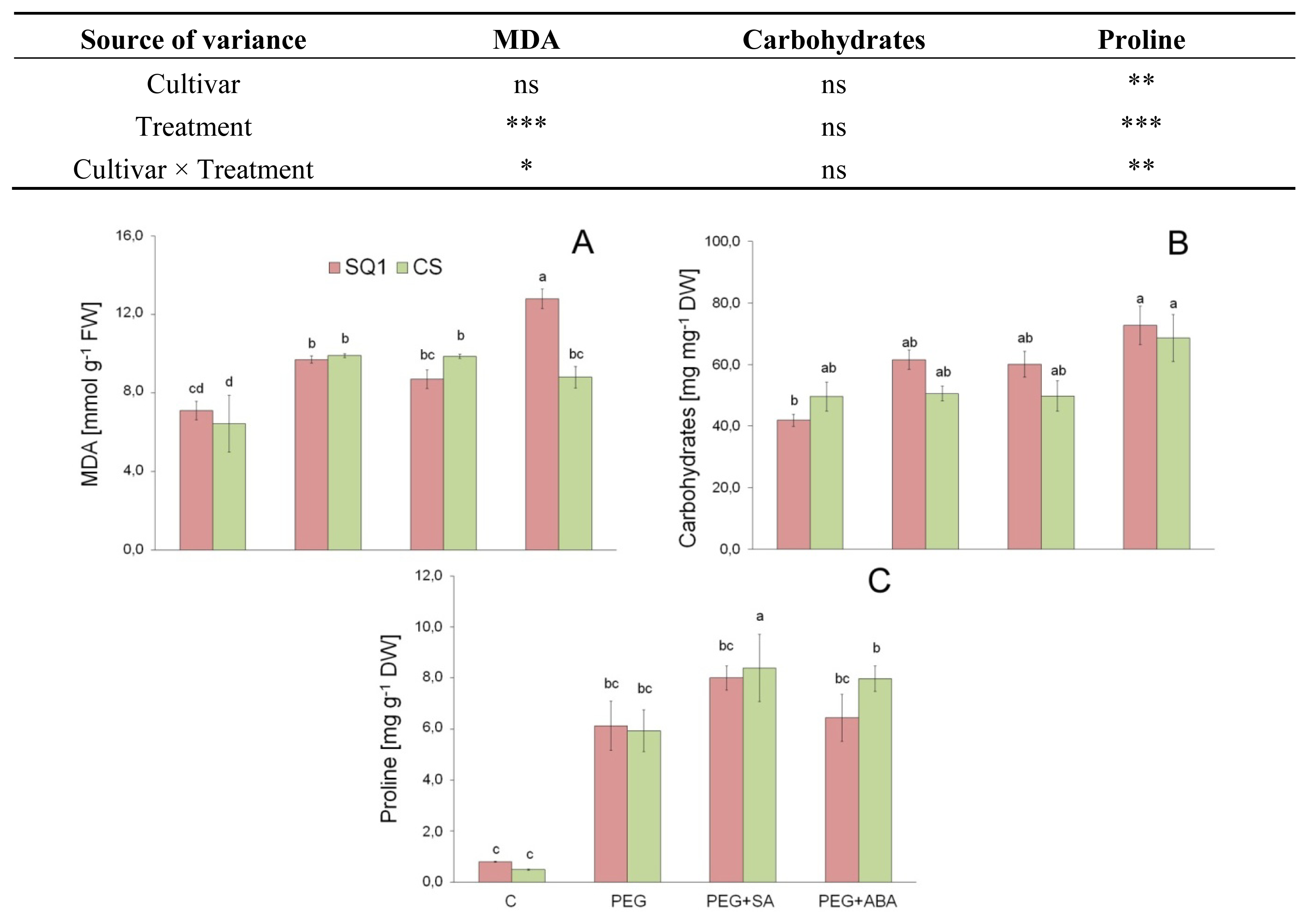
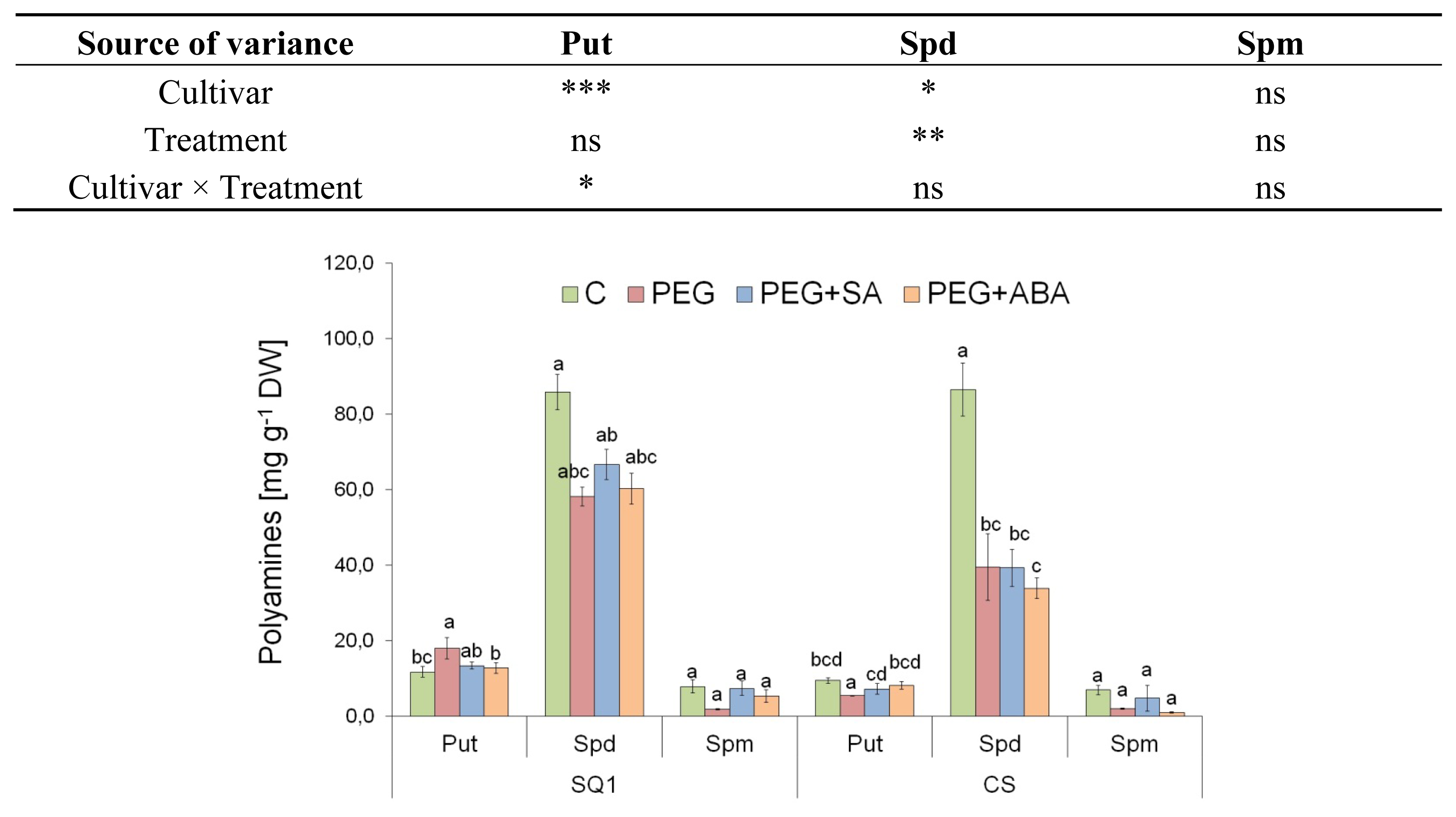
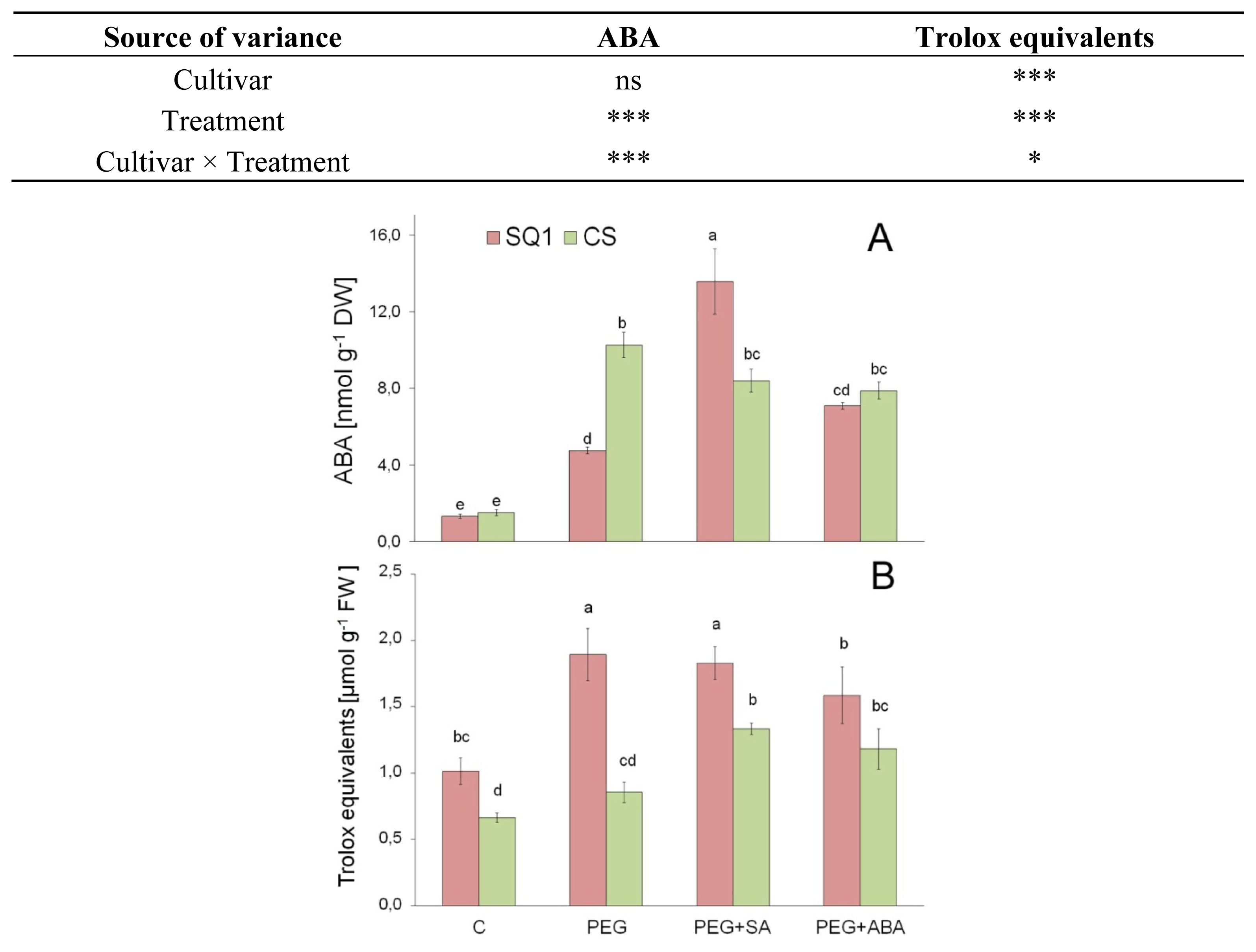
| Parameter | Cultivar | Treatment | |||
|---|---|---|---|---|---|
| C | PEG | PEG + SA | PEG + ABA | ||
| Leaves length (mm) | SQ1 | 341 a,b (100%) | 258 c (76%) | 276 c (81%) | 252 c (74%) |
| CS | 410 a (100%) | 374 a (91%) | 303 b,c (74%) | 341 a,b (83%) | |
| Roots length (mm) | SQ1 | 244 a,b (100%) | 152 c (62%) | 202 b,c (83%) | 184 b,c (75%) |
| CS | 283 a,b (100%) | 288 a (102%) | 188 b,c (67%) | 221 a,b,c (78%) | |
| Water content in leaves (%) (WC) | SQ1 | 85.6 a,b (100%) | 81.0 a,b (95%) | 80.9 a,b (94%) | 78.5 b (92%) |
| CS | 87.8 a (100%) | 83.8 a,b (95%) | 82.0 c (93%) | 83.1 a,b (95%) | |
| Water content in roots [%] (WC) | SQ1 | 91.9 a,b (100%) | 89.4 b,c (97%) | 85.9 c,d (93%) | 88.1 c (96%) |
| CS | 93.0 a (100%) | 89.1 a (96%) | 83.2 b,c (89%) | 87.8 c (94%) | |
| Source of variance | Leaves length | Roots length | WC in leaves | WC in roots | |
| Cultivar | *** | ** | ns | ns | |
| Treatment | *** | * | *** | *** | |
| Cultivar × Treatment | ns | * | ** | ** | |
| Parameter | Cultivar | Treatment | |||
|---|---|---|---|---|---|
| C | PEG | PEG + SA | PEG + ABA | ||
| Net photosynthesis (Pn) (μmol CO2 cm−2 s−1) | SQ1 | 16.20 a (100%) | 11.90 d (73%) | 12.84 c,d (79%) | 11.55 d (71%) |
| CS | 17.62 a (100%) | 8.60 b,c (49%) | 9.62 b (55%) | 8.80 b,c (50%) | |
| Transpiration (E) (mmol H2O cm−2 s−1) | SQ1 | 5.93 a (100%) | 5.27 b (89%) | 5.50 b (93%) | 4.75 b (80%) |
| CS | 7.58 b (100%) | 5.48 b (72%) | 4.84 b (64%) | 4.67 b (61%) | |
| Water use efficiency (WUE) (μmol CO2mmol−1 H2O) | SQ1 | 2.78 a,b,c (100%) | 2.45 d (88%) | 2.33 c,d (84%) | 2.54 d (91%) |
| CS | 2.35 a (100%) | 1.66 a,b (71%) | 1.99 b,c (85%) | 1.89 a,b (80%) | |
| Stomatal conductance (gs) (mmol H2O cm−2 s−1) | SQ1 | 117.33a (100%) | 65.17 c (56%) | 79.80 c (68%) | 76.83 c (65%) |
| CS | 137.83 b (100%) | 64.00 c (46%) | 74.20 c (54%) | 69.67 c (51%) | |
| Chlorophyll content SPAD | SQ1 | 6.68 a (100%) | 4.86 b,c (73%) | 5.10 b (76%) | 4.55 c (68%) |
| CS | 7.06 a (100%) | 4.84 b,c (69%) | 5,23 b,c (74%) | 4.51 b,c (64%) | |
| Source of variance | Pn | E | WUE | gs | SPAD |
| Cultivar | *** | ** | ** | *** | *** |
| Treatment | ** | ns | *** | ns | ns |
| Cultivar × Treatment | * | ns | ns | ns | ns |
| Yield component | Cultivar | Treatment | |||
|---|---|---|---|---|---|
| C | PEG | PEG + SA | PEG + ABA | ||
| Grain number | SQ1 | 83.3 c (100%) | 49.3 c,d (59%) | 58 c,d (70%) | 42 d (50%) |
| CS | 189.3 a (100%) | 135.0 b (71%) | 137.7 b (73%) | 172.7 a,b (91%) | |
| Grain mass (g) | SQ1 | 4.0 b,c (100%) | 2.2 c,d (55%) | 2.6 c,d (65%) | 1.9 d (48%) |
| CS | 6.6 a (100%) | 4.6 a,b (70%) | 5.0 a,b (76%) | 6.0 a (91%) | |
| Biomass (g) | SQ1 | 5.7 c,d (100%) | 3.1 d,e (54%) | 3.7 d,e (65%) | 2.6 e (46%) |
| CS | 11.2 a (100%) | 7.6 b,c (68%) | 8.8 a,b (79%) | 10.0 a,b (89%) | |
| Source of variance | Grain number | Grain mass | Biomass | ||
| Cultivar | *** | *** | *** | ||
| Treatment | ns | * | * | ||
| Cultivar × Treatment | ns | ns | ns | ||
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Marcińska, I.; Czyczyło-Mysza, I.; Skrzypek, E.; Grzesiak, M.T.; Janowiak, F.; Filek, M.; Dziurka, M.; Dziurka, K.; Waligórski, P.; Juzoń, K.; et al. Alleviation of Osmotic Stress Effects by Exogenous Application of Salicylic or Abscisic Acid on Wheat Seedlings. Int. J. Mol. Sci. 2013, 14, 13171-13193. https://doi.org/10.3390/ijms140713171
Marcińska I, Czyczyło-Mysza I, Skrzypek E, Grzesiak MT, Janowiak F, Filek M, Dziurka M, Dziurka K, Waligórski P, Juzoń K, et al. Alleviation of Osmotic Stress Effects by Exogenous Application of Salicylic or Abscisic Acid on Wheat Seedlings. International Journal of Molecular Sciences. 2013; 14(7):13171-13193. https://doi.org/10.3390/ijms140713171
Chicago/Turabian StyleMarcińska, Izabela, Ilona Czyczyło-Mysza, Edyta Skrzypek, Maciej T. Grzesiak, Franciszek Janowiak, Maria Filek, Michał Dziurka, Kinga Dziurka, Piotr Waligórski, Katarzyna Juzoń, and et al. 2013. "Alleviation of Osmotic Stress Effects by Exogenous Application of Salicylic or Abscisic Acid on Wheat Seedlings" International Journal of Molecular Sciences 14, no. 7: 13171-13193. https://doi.org/10.3390/ijms140713171
APA StyleMarcińska, I., Czyczyło-Mysza, I., Skrzypek, E., Grzesiak, M. T., Janowiak, F., Filek, M., Dziurka, M., Dziurka, K., Waligórski, P., Juzoń, K., Cyganek, K., & Grzesiak, S. (2013). Alleviation of Osmotic Stress Effects by Exogenous Application of Salicylic or Abscisic Acid on Wheat Seedlings. International Journal of Molecular Sciences, 14(7), 13171-13193. https://doi.org/10.3390/ijms140713171






