Reinvestigation of the Oxidative Folding Pathways of Hen Egg White Lysozyme: Switching of the Major Pathways by Temperature Control
Abstract
:1. Introduction
2. Results
2.1. Stoichiometric SS Formation of R Using DHSox
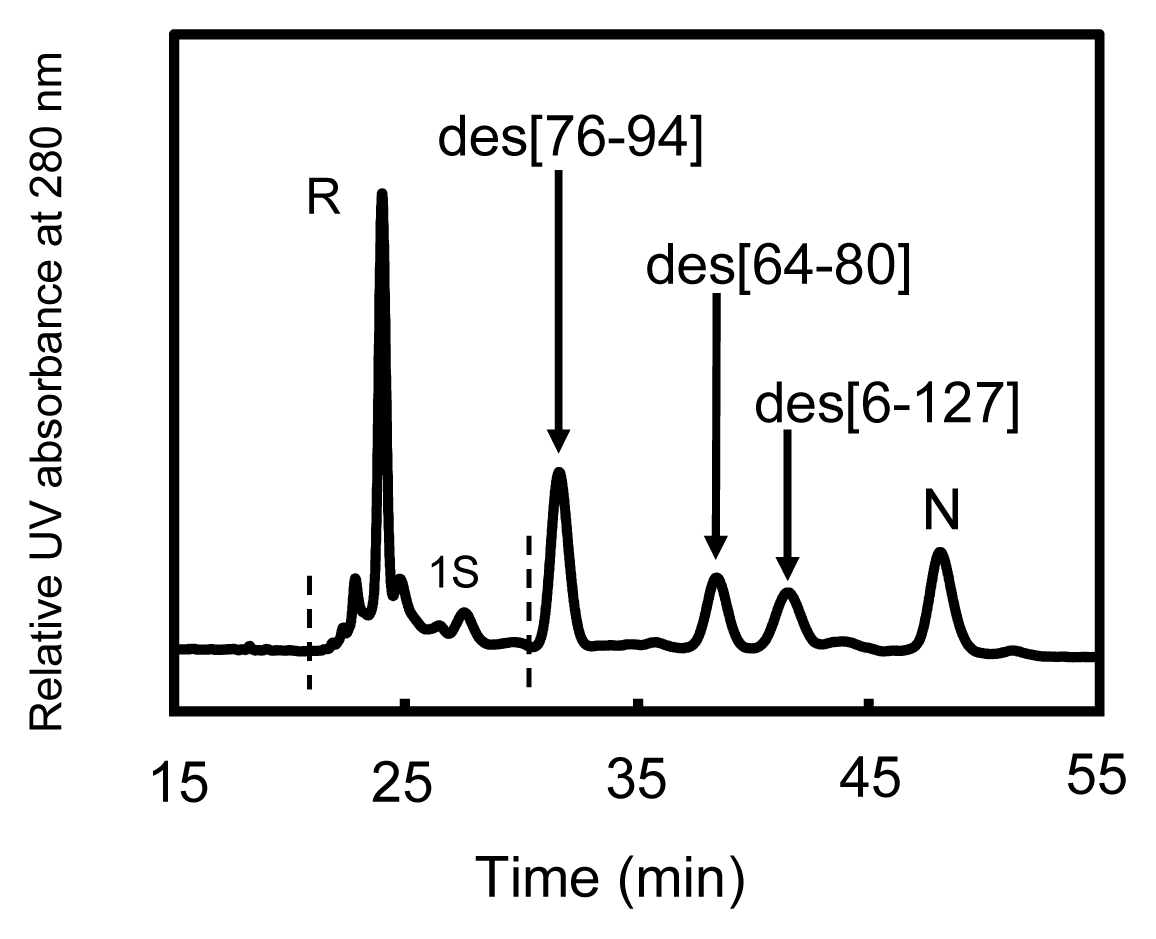
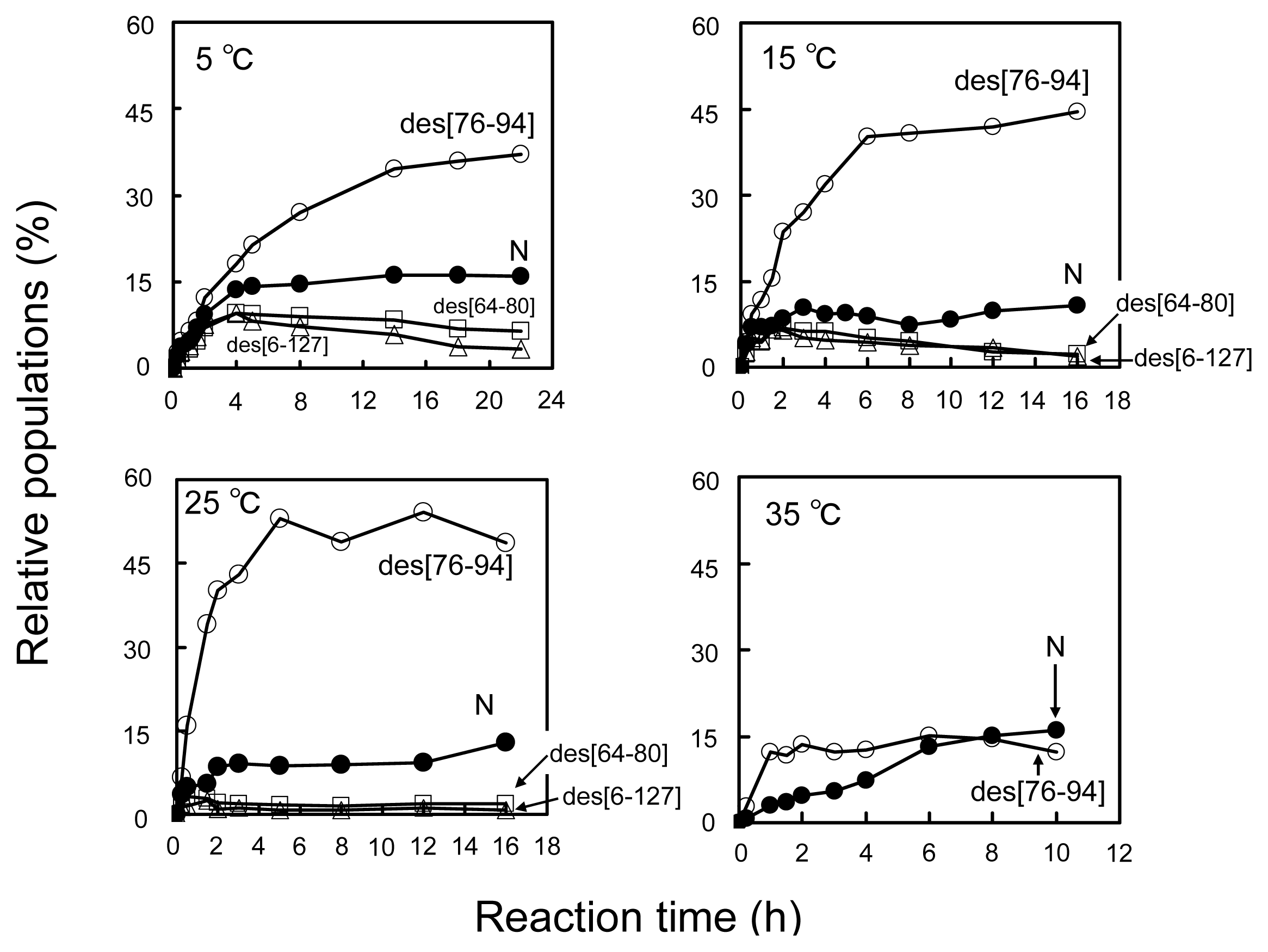
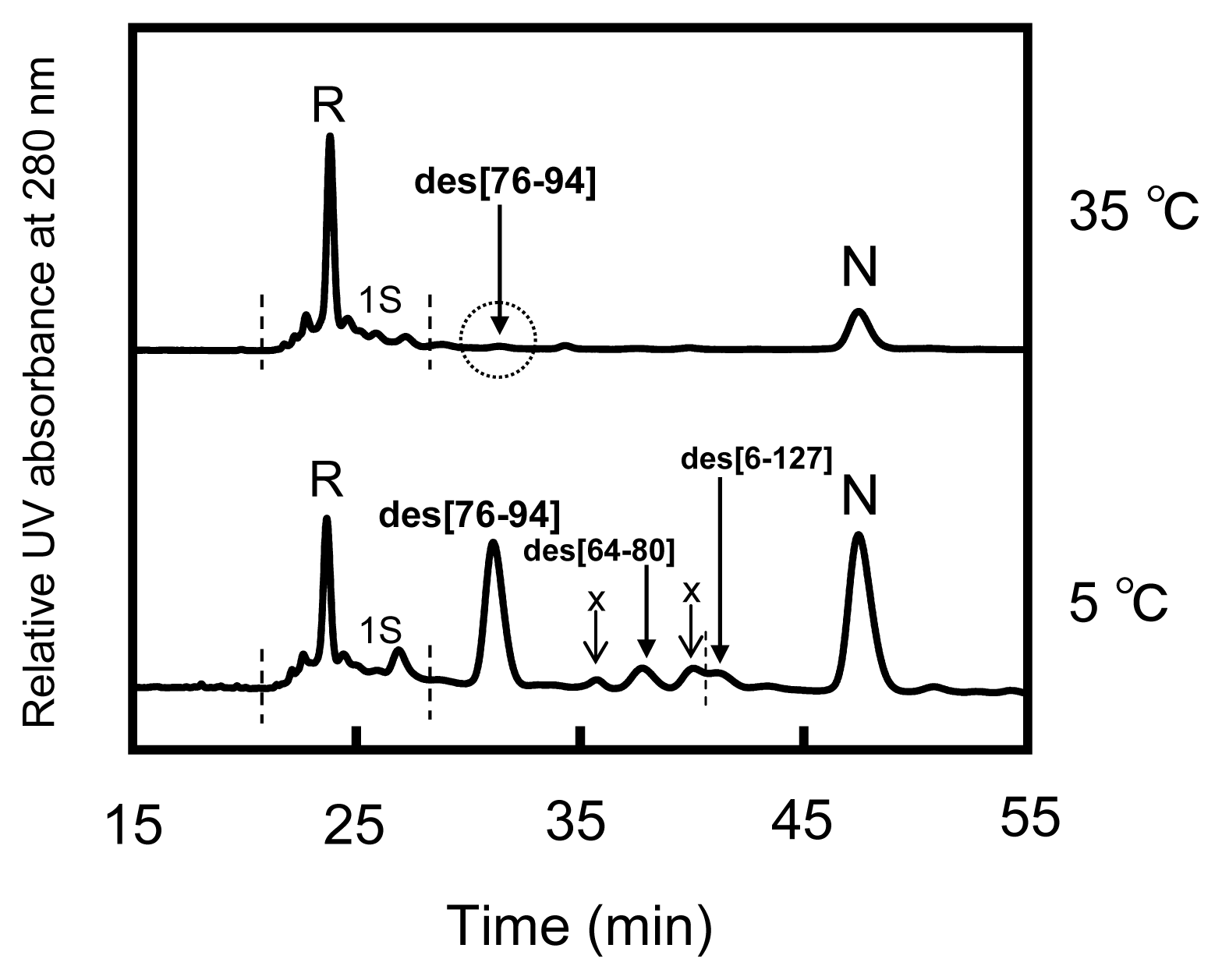
2.2. Long-Term Folding of HEL Using DHSox
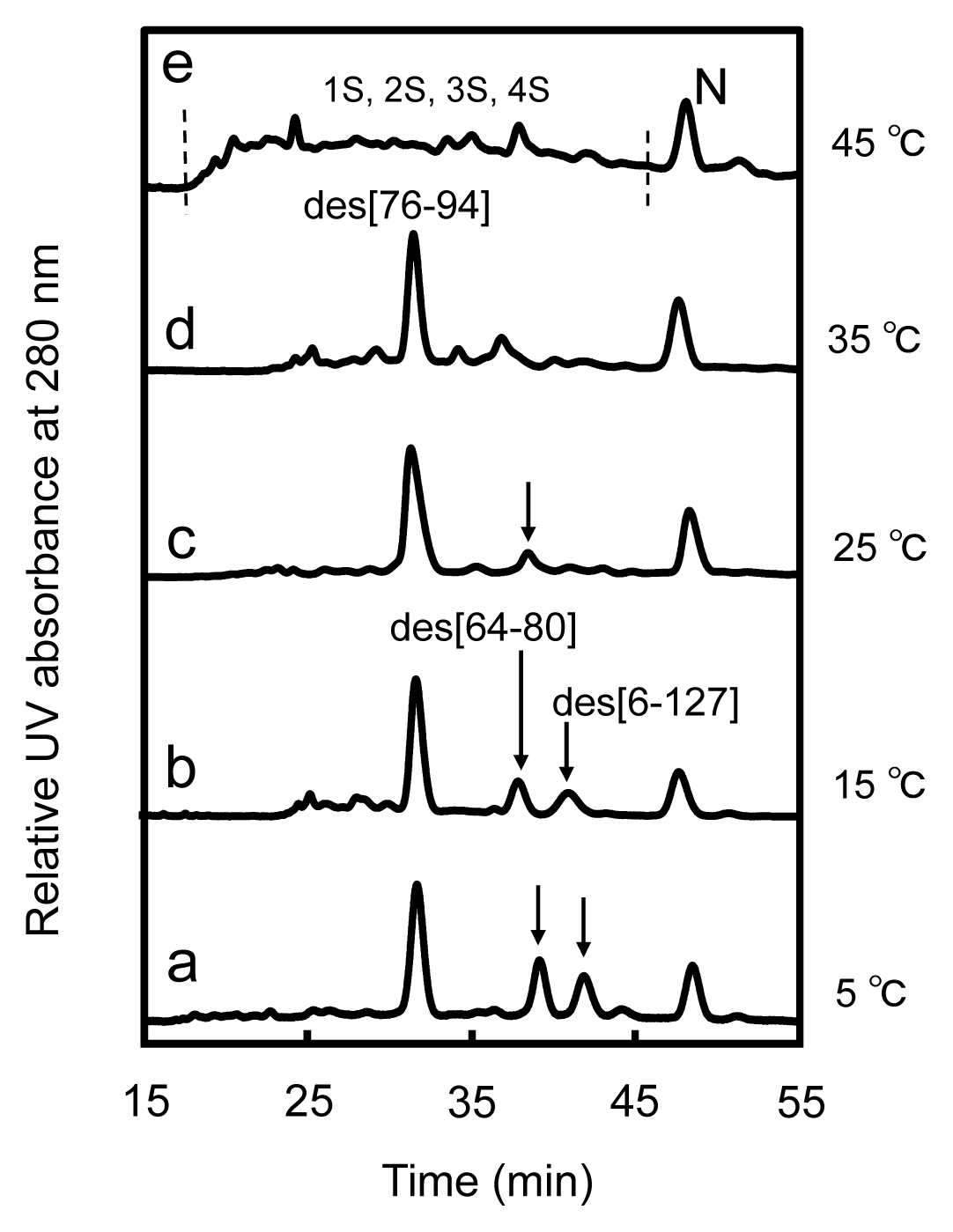
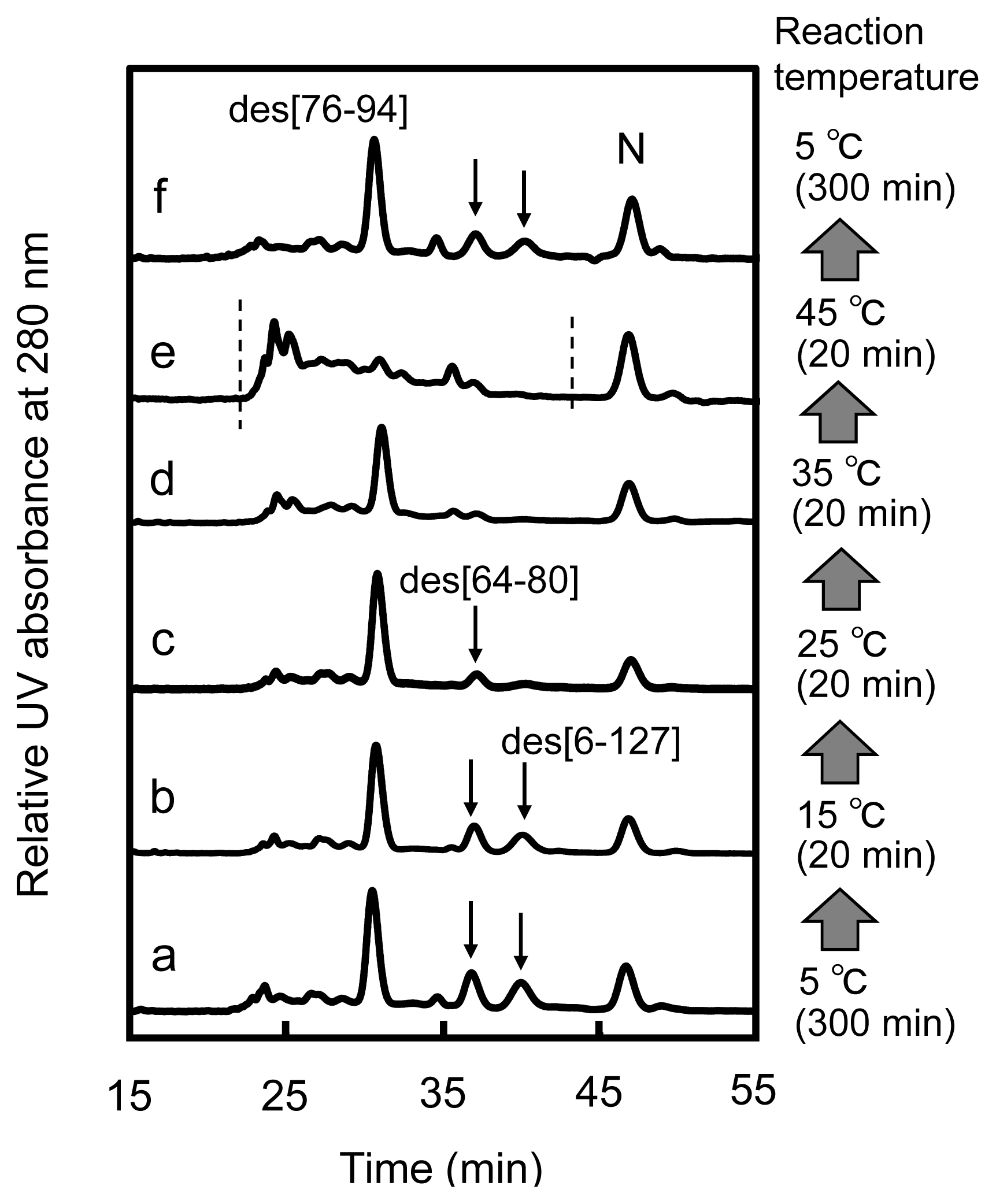
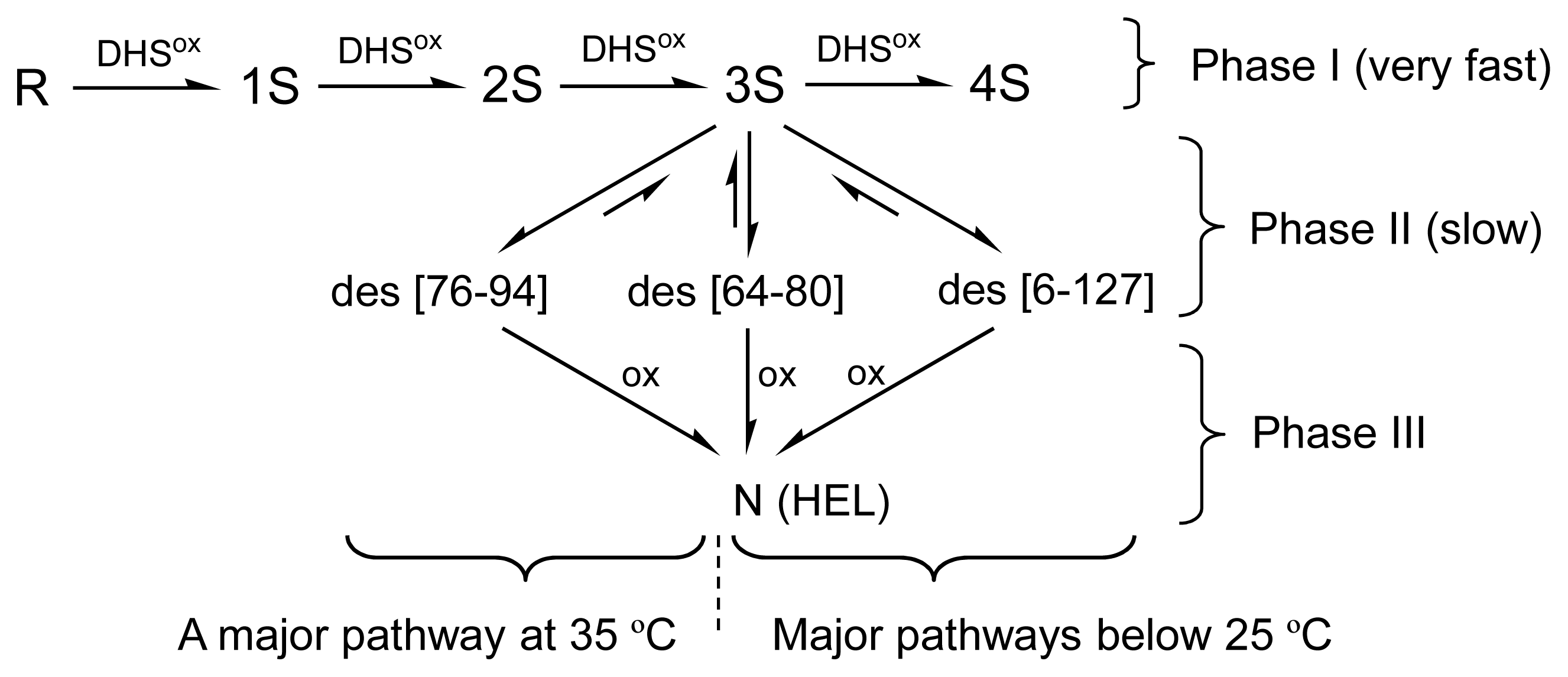
2.3. Equilibria between 3S and Des Intermediates
2.4. Reduction Pulse
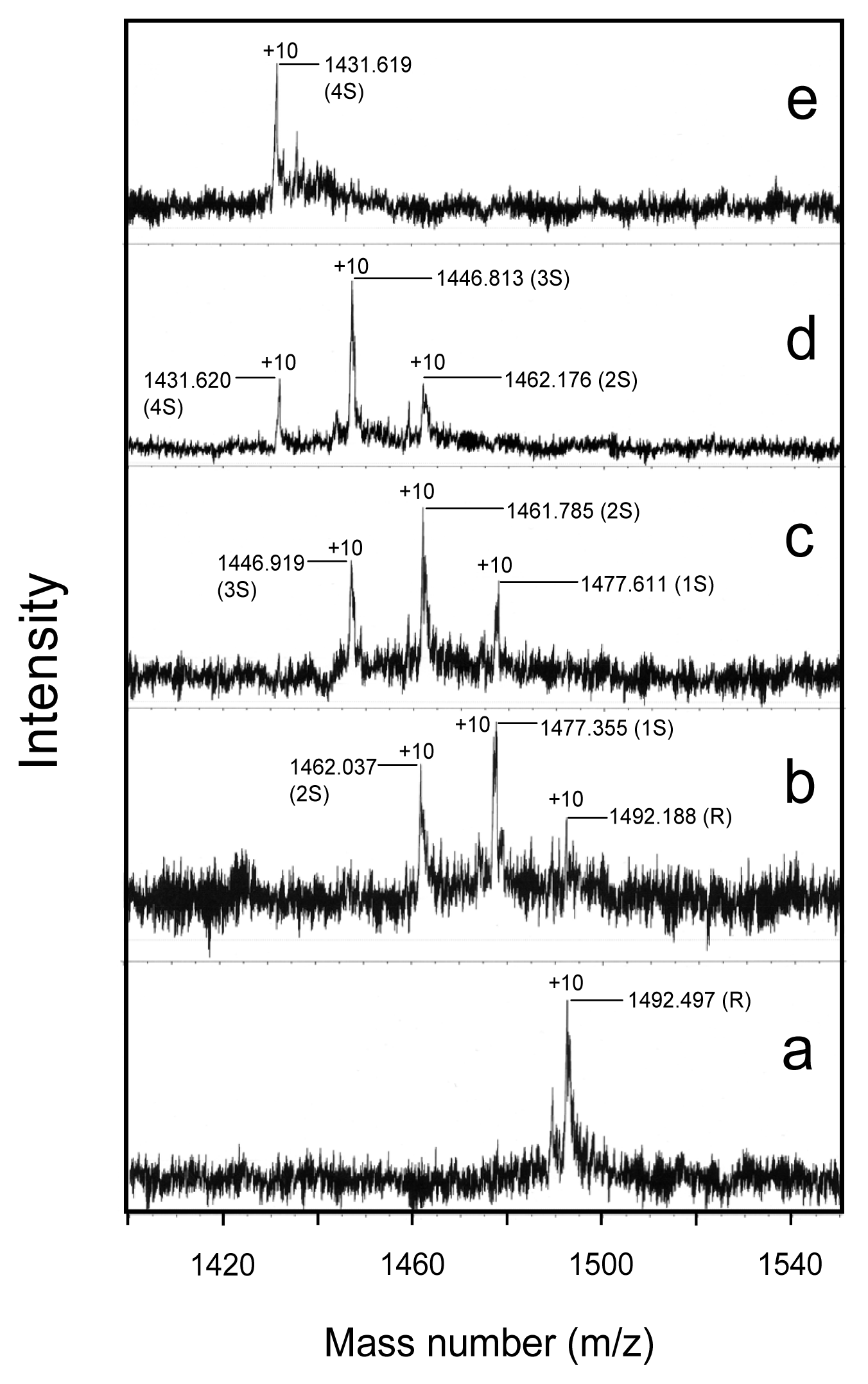
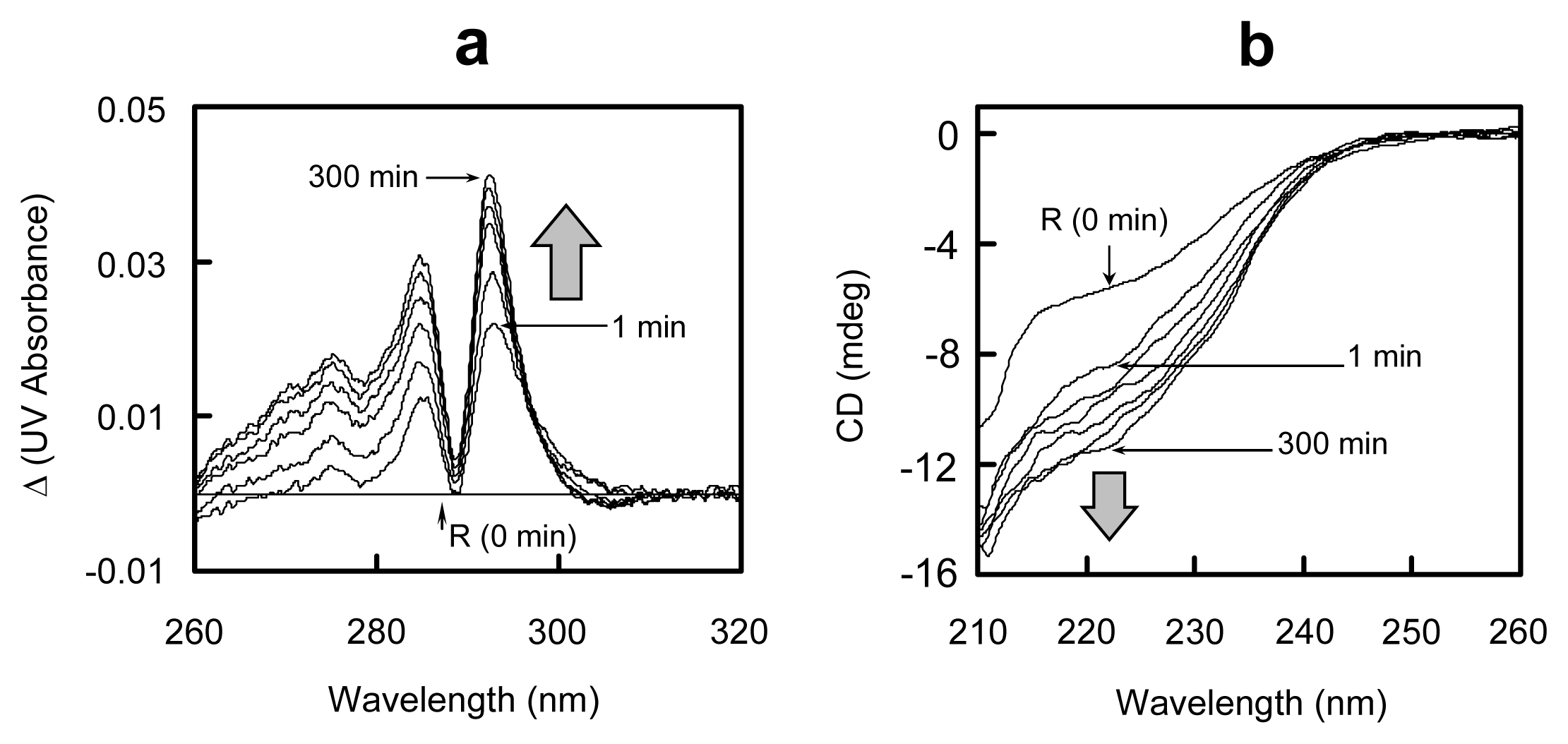
2.5. Conformational Changes during the Oxidative Folding of HEL
2.6. Oxidation Pulse
3. Discussion
3.1. Precursors of the Des Intermediates
3.2. Relative Stability of the Des Intermediates
3.3. Switching the Major Pathways toward N by Temperature Control
4. Experimental Section
4.1. Materials
4.2. Preparation of Reduced HEL (R)
4.3. Oxidative Folding Experiment
4.4. Temperature-Jump Experiments
4.5. HPLC Analysis
4.6. Characterization of the Folding Intermediates
5. Conclusions
Supplementary Information
ijms-14-13194-s001.pdfAcknowledgments
Abbreviations
| 1S, 2S, 3S, and 4S | ensembles of folding intermediates of HEL with one, two, three, and four SS linkages, respectively |
| AEMTS | 2-aminoethyl methanethiosulfonate |
| CD | circular dichroism |
| des[76–94], des[6–127], and des[64–80] | structured 3S intermediates of HEL having three native SS bonds but lacking one native SS bond specified |
| DHSox | trans-3,4-dihydroxyselenolane oxide |
| DTTred | dithiothreitol |
| EDTA | ethylenediaminetetraacetic acid |
| ESI | electron spray ionization |
| GSH | glutathione |
| GSSG | oxidized glutathione |
| HEL | hen egg white lysozyme |
| HPLC | high performance liquid chromatography |
| N | native HEL |
| R | reduced HEL |
| RNase A | ribonuclease A |
| SH | thiol |
| SS | disulfide |
| TFA | trifluoroacetic acid |
| UV | ultraviolet. |
Conflict of Interest
References
- Tu, B.P.; Weissman, J.S. The FAD- and O2-dependent reaction cycle of Ero1-mediated oxidative protein folding in the endoplasmic reticulum. Mol. Cell 2002, 10, 983–994. [Google Scholar]
- Sato, Y.; Inaba, K. Disulfide bond formation network in the three biological kingdoms, bacteria, fungi and mammals. FEBS J 2012, 279, 2262–2271. [Google Scholar]
- Antonio-Pérez, A.; Ramón-Luing, L.A.; Ortega-López, J. Chromatographic refolding of rhodanese and lysozyme assisted by the GroEL apical domain, DsbA and DsbC immobilized on cellulose. J. Chromatogr. A 2012, 1248, 122–129. [Google Scholar]
- Benham, A.M. The protein disulfide isomerase family: Key players in health and disease. Antioxid. Redox Sign 2012, 16, 781–789. [Google Scholar]
- Narayan, M. Disulfide bonds: Protein folding and subcellular protein trafficking. FEBS J 2012, 279, 2272–2282. [Google Scholar]
- Dobson, C.M.; Evans, P.A.; Radford, S.E. Understanding how proteins fold: The lysozyme story so far. Trends Biochem. Sci 1994, 19, 31–37. [Google Scholar]
- Van den Berg, B.; Chung, E.W.; Robinson, C.V.; Dobson, C.M. Characterisation of the dominant oxidative folding intermediate of hen lysozyme. J. Mol. Biol 1999, 290, 781–796. [Google Scholar]
- Van den Berg, B.; Chung, E.W.; Robinson, C.V.; Mateo, P.L.; Dobson, C.M. The oxidative refolding of hen lysozyme and its catalysis by protein disulfide isomerase. EMBO J 1999, 18, 4794–4803. [Google Scholar]
- Iwaoka, M.; Kumakura, F.; Yoneda, M.; Nakahara, T.; Henmi, K.; Aonuma, H.; Nakatani, H.; Tomoda, S. Direct observation of conformational folding coupled with disulphide rearrangement by using a water-soluble selenoxide reagent—A case of oxidative regeneration of ribonuclease A under weakly basic conditions. J. Biochem 2008, 144, 121–130. [Google Scholar]
- Arai, K.; Noguchi, M.; Singh, B.G.; Priyadarsini, K.I.; Fujio, K.; Kubo, Y.; Takayama, K.; Ando, S.; Iwaoka, M. A water-soluble selenoxide reagent as a useful probe for the reactivity and folding of polythiol peptides. FEBS Open Bio 2013, 3, 55–64. [Google Scholar]
- Arai, K.; Kumakura, F.; Iwaoka, M. Kinetic and thermodynamic analysis of the conformational folding process of SS-reduced bovine pancreatic ribonuclease A using a selenoxide reagent with high oxidizing ability. FEBS Open Bio 2012, 2, 60–70. [Google Scholar]
- Arai, K.; Kumakura, F.; Iwaoka, M. Characterization of kinetic and thermodynamic phases in the prefolding process of bovine pancreatic ribonuclease A coupled with fast SS formation and SS reshuffling. Biochemistry 2010, 49, 10535–10542. [Google Scholar]
- Welker, E.; Hathaway, L.; Scheraga, H.A. A new method for rapid characterization of the folding pathways of multidisulfide-containing proteins. J. Am. Chem. Soc 2004, 126, 3720–3721. [Google Scholar]
- Radford, S.E.; Woolfson, D.N.; Martin, S.R.; Lowe, G.; Dobson, C.M. A three-disulphide derivative of hen lysozyme. Structure, dynamics and stability. Biochem. J 1991, 273, 211–217. [Google Scholar]
- Eyles, S.J.; Radford, S.E.; Robinson, C.V.; Dobson, C.M. Kinetic consequences of the removal of a disulfide bridge on the folding of hen lysozyme. Biochemistry 1994, 33, 13038–13048. [Google Scholar]
- Ueda, T.; Ohkuri, T.; Imoto, T. Identification of the peptide region that folds native conformation in the early stage of the renaturation of reduced lysozyme. Biochem. Biophys. Res. Commun 1996, 228, 203–208. [Google Scholar]
- Ohkuri, T.; Shioi, S.; Imoto, T.; Ueda, T. Effect of the structure of the denatured state of lysozyme on the aggregation reaction at the early stages of folding from the reduced form. J. Mol. Biol 2005, 347, 159–168. [Google Scholar]
- Tachibana, H. Propensities for the formation of individual disulfide bonds in hen lysozyme and the size and stability of disulfide-associated submolecular structures. FEBS Lett 2000, 480, 175–178. [Google Scholar]
- Noda, Y.; Yokota, A.; Horii, D.; Tominaga, T.; Tanisaka, Y.; Tachibana, H.; Segawa, S. NMR structural study of two-disulfide variant of hen lysozyme: 2SS[6–127,30–115]—A disulfide intermediate with a partly unfolded structure. Biochemistry 2002, 41, 2130–2139. [Google Scholar]
- Jarrett, N.M.; Djavadi-Ohaniance, L.; Willson, R.C.; Tachibana, H.; Goldberg, M.E. Immunochemical pulsed-labeling characterization of intermediates during hen lysozyme oxidative folding. Protein Sci 2002, 11, 2584–2595. [Google Scholar]
- Ohkuri, T.; Imoto, T.; Ueda, T. Characterization of refolded hen lysozyme variant lacking two outside disulfide bonds. Biosci. Biotechnol. Biochem 2005, 69, 1206–1208. [Google Scholar]
- Collins, E.S.; Wirmer, J.; Hirai, K.; Tachibana, H.; Segawa, S.; Dobson, C.M.; Schwalbe, H. Characterisation of disulfide-bond dynamics in non-native states of lysozyme and its disulfide deletion mutants by NMR. ChemBioChem 2005, 6, 1619–1627. [Google Scholar]
- Noda, Y.; Narama, K.; Kasai, K.; Tachibana, H.; Segawa, S. Glycerol-enhanced detection of a preferential structure latent in unstructured 1SS-variants of lysozyme. Biopolymers 2012, 97, 539–549. [Google Scholar]
- Silvers, R.; Sziegat, F.; Tachibana, H.; Segawa, S.; Whittaker, S.; Günther, U.L.; Gabel, F.; Huang, J.-R.; Blackledge, M.; Wirmer-Bartoschek, J.; et al. Modulation of structure and dynamics by disulfide bond formation in unfolded states. J. Am. Chem. Soc 2012, 134, 6846–6854. [Google Scholar]
- Radford, S.E.; Dobson, C.M.; Evans, P.A. The folding of hen lysozyme involves partially structured intermediates and multiple pathways. Nature 1992, 358, 302–307. [Google Scholar]
- Yokota, A.; Izutani, K.; Takai, M.; Kubo, Y.; Noda, Y.; Koumoto, Y.; Tachibana, H.; Segawa, S.-I. The transition state in the folding-unfolding reaction of four species of three-disulfide variant of hen lysozyme: The role of each disulfide bridge. J. Mol. Biol 2000, 295, 1275–1288. [Google Scholar]
- Ohkuri, T.; Ueda, T.; Tsurumaru, M.; Imoto, T. Evidence for an initiation site for hen lysozyme folding from the reduced form using its dissected peptide fragments. Protein Engineering 2001, 14, 829–833. [Google Scholar]
- Guez, V.; Roux, P.; Navon, A.; Goldberg, M.E. Role of individual disulfide bonds in hen lysozyme early folding steps. Protein Sci 2002, 11, 1136–1151. [Google Scholar]
- Yokota, A.; Hirai, K.; Miyauchi, H.; Iimura, S.; Noda, Y.; Inoue, K.; Akasaka, K.; Tachibana, H.; Segawa, S.-I. NMR characterization of three-disulfide variants of lysozyme, C64A/C80A, C76A/C94A, and C30A/C115A - A marginally stable state in folded proteins. Biochemistry 2004, 43, 6663–6669. [Google Scholar]
- Narayan, M.; Welker, E.; Wedemeyer, W.J.; Scheraga, H.A. Oxidative folding of proteins. Acc. Chem. Res 2000, 33, 805–812. [Google Scholar]
- Iwaoka, M.; Takahashi, T.; Tomoda, S. Syntheses and structural characterization of water-soluble selenium reagents for the redox control of protein disulfide bonds. Heteroatom Chem 2001, 12, 293–299. [Google Scholar]
- Bruice, T.W.; Kenyon, G.L. Novel alkyl alkanethiolsulfonate sulfhydryl reagents. Modification of derivatives of l-cysteine. J. Protein Chem 1982, 1, 47–58. [Google Scholar]
- Takezawa, A.; Ohshima, Y.; Sudo, T.; Asami, O.; Nohara, D. Renaturation of lysozyme with a protein disulfide isomerase chaperone results in enzyme super activity. J. Biosci. Bioeng 2008, 106, 503–506. [Google Scholar]
- Saxena, V.P.; Wetlaufer, D.B. Formation of three-dimensional structure in proteins. I. Rapid nonenzymic reactivation of reduced lysozyme. Biochemistry 1970, 9, 5015–5023. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys 1959, 82, 70–77. [Google Scholar]
- Chang, J.-Y.; Li, L. The unfolding mechanism and the disulfide structures of denatured lysozyme. FEBS Lett 2002, 511, 73–78. [Google Scholar]


| Temperature (°C) | des[76–94]∞a (%) | des[64–80]∞a (%) | des[6–127]∞a (%) | Equilibria | Kb | ΔGc (kcal/mol) |
|---|---|---|---|---|---|---|
| 5 | 36 (±1) | 7 (±1) | 4 (±1) | des[76–94] ⇌ des[64–80] | 0.19 (±0.03) | 0.9 (±0.1) |
| des[76–94] ⇌ des[6–127] | 0.11 (±0.03) | 1.2 (±0.1) | ||||
| 15 | 42 (±3) | 4 (±3) | 3 (±2) | des[76–94] ⇌ des[64–80] | 0.10 (±0.07) | 1.3 (±0.7) |
| des[76–94] ⇌ des[6–127] | 0.07 (±0.05) | 1.5 (±0.7) | ||||
| 25 | 51 (±3) | 2 (±1) | 1 (±1) | des[76–94] ⇌ des[64–80] | 0.04 (±0.02) | 1.9 (±0.4) |
| des[76–94] ⇌ des[6–127] | 0.02 (±0.02) | 2.3 (±0.4) | ||||
| 35 | 13 (±2) | NG d | NG | des[76–94] ⇌ des[64–80] | ND e | ND |
| des[76–94] ⇌ des[6–127] | ND | ND |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Arai, K.; Shibagaki, W.; Shinozaki, R.; Iwaoka, M. Reinvestigation of the Oxidative Folding Pathways of Hen Egg White Lysozyme: Switching of the Major Pathways by Temperature Control. Int. J. Mol. Sci. 2013, 14, 13194-13212. https://doi.org/10.3390/ijms140713194
Arai K, Shibagaki W, Shinozaki R, Iwaoka M. Reinvestigation of the Oxidative Folding Pathways of Hen Egg White Lysozyme: Switching of the Major Pathways by Temperature Control. International Journal of Molecular Sciences. 2013; 14(7):13194-13212. https://doi.org/10.3390/ijms140713194
Chicago/Turabian StyleArai, Kenta, Wataru Shibagaki, Reina Shinozaki, and Michio Iwaoka. 2013. "Reinvestigation of the Oxidative Folding Pathways of Hen Egg White Lysozyme: Switching of the Major Pathways by Temperature Control" International Journal of Molecular Sciences 14, no. 7: 13194-13212. https://doi.org/10.3390/ijms140713194





