Contribution of the Tyr-1 in Plantaricin149a to Disrupt Phospholipid Model Membranes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Peptide Synthesis, Purification, and Characterization
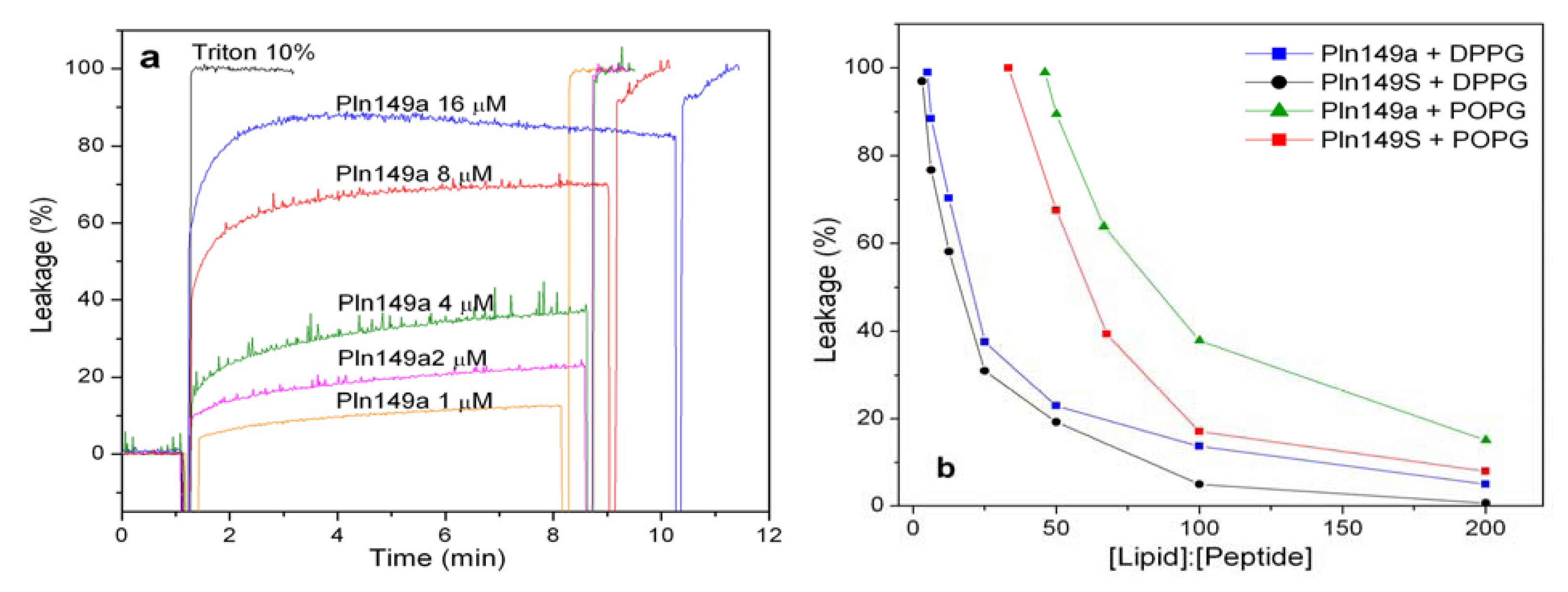
2.2. 8-Aminonaphthalene-1,3,6-Trisulfonic Acid (ANTS)-P-Xylene-Bis-Pyridinium Bromide (DPX) Leakage Assays
2.3. Far-UV Circular Dichroism Analyses
2.4. Liposome Titration into Pln149-Analogs with a Trp Residue
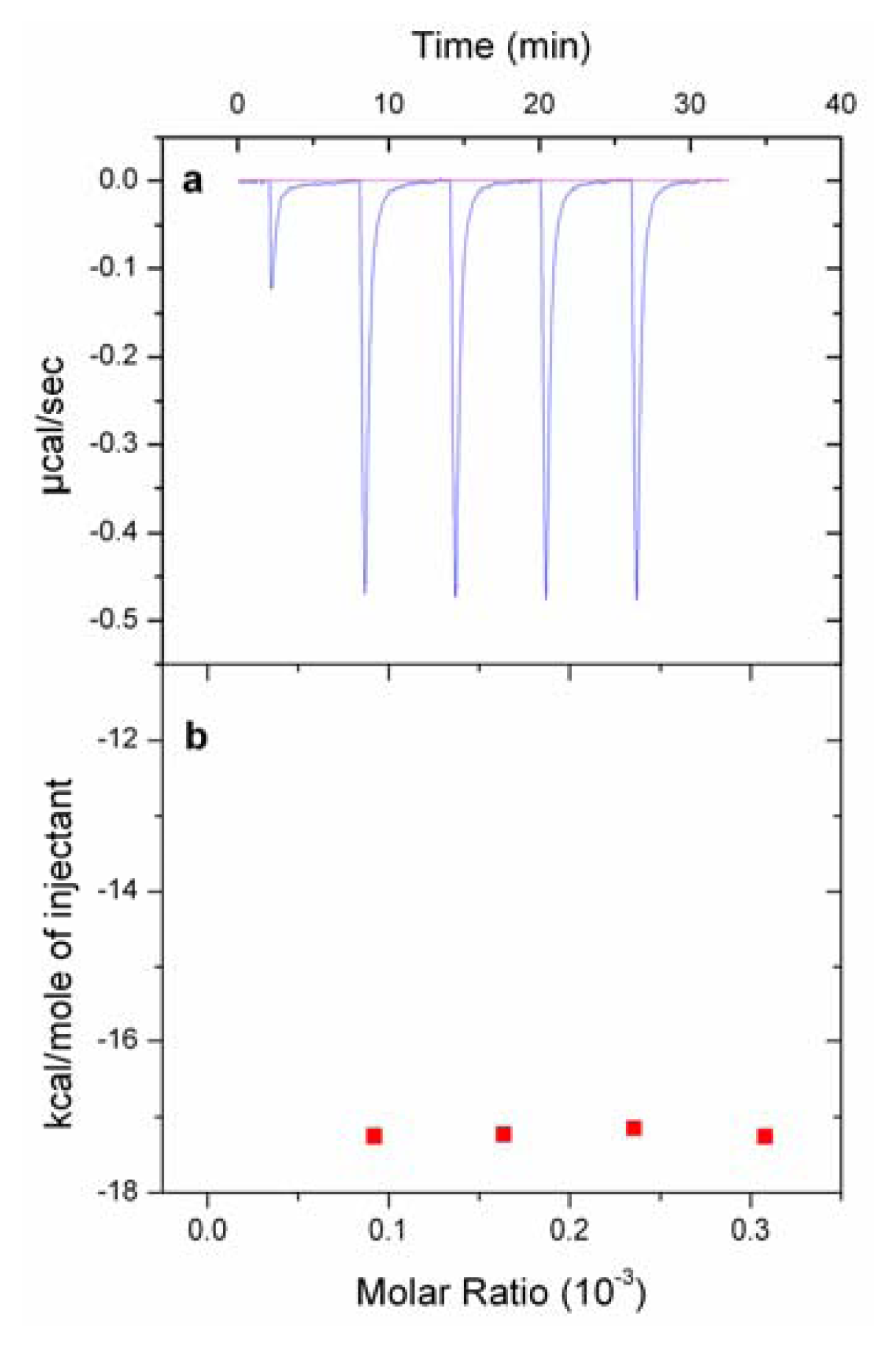
2.5. Isothermal Titration Calorimetry (ITC) Experiments
2.6. Antimicrobial and Hemolytic Activities
3. Experimental Section
3.1. Peptide Synthesis and Purification
3.2. Liposome Preparation
3.3. ANTS/DPX Leakage Assays
3.4. Far-UV Circular Dichroism (CD)
3.5. Tryptophan Fluorescence Titrations
3.6. Isothermal Titration Calorimetry (ITC)
3.7. Hemolytic Activity
3.8. Antibacterial Activity and MIC Determination
4. Conclusions
Supplementary Information
ijms-14-12313-s001.pdfAcknowledgments
Conflict of Interest
References
- Kościuczuk, E.M.; Lisowski, P.; Jarczak, J.; Strzałkowska, N.; JóŸwik, A.; Horbańczuk, J.; Krzyżewski, J.; Zwierzchowski, L.; Bagnicka, E. Cathelicidins: Family of antimicrobial peptides. A review. Mol. Biol. Rep 2012, 39, 10957–10970. [Google Scholar]
- Bergaoui, I.; Zairi, A.; Tangy, F.; Aouni, M.; Selmi, B.; Hani, K. In vitro antiviral activity of dermaseptin S(4) and derivatives from amphibian skin against herpes simplex virus type 2. J. Med. Virol. 2012. [Google Scholar] [CrossRef]
- Pálffy, R.; Gardlík, R.; Behuliak, M.; Kadasi, L.; Turna, J.; Celec, P. On the physiology and pathophysiology of antimicrobial peptides. Mol. Med 2009, 15, 51–59. [Google Scholar]
- Mills, S.; Stanton, C.; Hill, C.; Ross, R.P. New developments and applications of bacteriocins and peptides in foods. Annu. Rev. Food Sci. Technol 2011, 2, 299–329. [Google Scholar]
- Ray, B.; Daeschel, M. Food Biopreservatives of Microbial Origin, 1st ed.; CRC Press: Boca Raton, FL, USA, 1992; p. 386. [Google Scholar]
- Toke, O. Antimicrobial peptides: New candidates in the fight against bacterial infections. Biopolymers 2005, 80, 717–735. [Google Scholar]
- Cerovsky, V.; Slaninova, J.; Fucik, V. New potent antimicrobial peptides from the venom of Polistinae wasps and their analogs. Peptides 2008, 29, 992–1003. [Google Scholar]
- Marshall, S.H.; Arenas, G. Antimicrobial peptides: A natural alternative to chemical antibiotics and a potential for applied biotechnology. Electron. J. Biotech 2003, 6, 271–284. [Google Scholar]
- Hancock, R.E.; Patrzykat, A. Clinical development of cationic antimicrobial peptides: From natural to novel antibiotics. Curr. Drug Targets Infect. Disord 2002, 2, 79–83. [Google Scholar]
- Finlay, B.B.; Hancock, R.E. Can innate immunity be enhanced to treat infections? Nat. Microbiol 2004, 2, 497–504. [Google Scholar]
- Bowdish, D.M.E.; Davidson, D.J.; Scott, M.G.; Hancock, R.E. Immunomodulatory activities of small host defense peptides. Antimicrob. Agents Chemother 2005, 49, 1727–1732. [Google Scholar]
- Marr, A.K.; Gooderham, W.J.; Hancock, R.E. Antibacterial peptides for therapeutic use: Obstacles and realistic outlook. Curr. Opin. Pharmacol 2006, 6, 468–472. [Google Scholar]
- Osrin, D.; Vergnano, S.; Costello, A. Serious bacterial infections in newborn infants in developing countries. Curr. Opin. Infect. Dis 2004, 17, 217–224. [Google Scholar]
- Schaller-Bals, S.; Schulze, A.; Bals, R. Increased levels of antimicrobial peptides in tracheal aspirates of newborn infants during infection. Am. J. Respir. Crit. Care Med 2002, 165, 992–995. [Google Scholar]
- Iley, M.A.; Wertz, J.E. Bacteriocin diversity: Ecological and evolutionary perspectives. Biochimie 2002, 84, 357–364. [Google Scholar]
- De Vuyst, L.; Vandamme, E.J. Bacteriocins of Lactic Acid Bacteria; Academic & Professional: London, UK, 1994; p. 409. [Google Scholar]
- Kelly, W.J.; Asmundson, R.V.; Huang, C.M. Characterization of plantaricin kw30, a bacteriocin produced by Lactobacillus plantarum. J. Appl. Bacteriol 1996, 81, 657–662. [Google Scholar]
- Bodaszewska-Lubas, M.; Brzychczy-Wloch, M.; Gosiewski, T.; Heczko, P.B. Antibacterial activity of selected standard strains of lactic acid bacteria producing bacteriocins—Pilot study. Postepy Hig. Med. Dosw 2012, 25, 787–794. [Google Scholar]
- Nes, I.F.; Holo, H. Class II antimicrobial peptides from lactic acid bacteria. Biopolymers 2000, 55, 50–61. [Google Scholar]
- Matsuzaki, K. Why and how are peptide-lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim. Biophys. Acta 1999, 1462, 1–10. [Google Scholar]
- Nicolas, P.; Mor, A. Peptides as weapons against microorganisms in the chemical defense system of vertebrates. Annu. Rev. Microbiol 1995, 49, 277–304. [Google Scholar]
- Hancock, R.E.; Falla, T.J.; Brown, M. Cationic Bactericidal Peptides. In Advances in Microbial Physiology; Poole, R.K., Ed.; Academic Press: London, UK, 1995; Volume 37, pp. 136–175. [Google Scholar]
- Zhao, H.; Sood, R.; Jutila, A.; Bose, S.; Fimland, G.; Nissen-Meyer, J.; Kinnunen, P.K. Interaction of the antimicrobial peptide pheromone Plantaricin A with model membranes: Implications for a novel mechanism of action. Biochim. Biophys. Acta 2006, 1758, 1461–1474. [Google Scholar]
- Hauge, H.H.; Mantzilas, D.; Moll, G.N.; Konings, W.N.; Driessen, A.J.; Eijsink, V.G.; Nissen-Meyer, J. Plantaricin A is an amphiphilic alpha-helical bacteriocin-like pheromone which exerts antimicrobial and pheromone activities through different mechanisms. Biochemistry 1998, 37, 16026–16032. [Google Scholar]
- Kristiansen, P.E.; Fimland, G.; Mantzilas, D.; Nissen-Meyer, J. Structure and mode of action of the membrane-permeabilizing antimicrobial peptide pheromone plantaricin A. J. Biol. Chem 2005, 280, 22945–22450. [Google Scholar]
- Anderssen, E.L.; Diep, D.B.; Nes, I.F.; Eijsink, V.G.; Nissen-Meyer, J. Antagonistic activity of Lactobacillus plantarum C11: Two new two-peptide bacteriocins, plantaricins EF and JK, and the induction factor plantaricin A. J. Appl. Environ. Microbiol 1998, 64, 2269–2272. [Google Scholar]
- Kato, T.; Matsuda, T.; Ogawa, E.; Ogawa, H.; Kato, H.; Doi, U.; Nakamura, R. Plantaricin-149, a bacteriocin produced by Lactobacillus plantarum NRIC 149. J. Ferment. Bioeng 1994, 77, 277–282. [Google Scholar]
- Müller, D.M.; Carrasco, M.S.; Simonetta, A.C.; Beltramini, L.M.; Tonarelli, G.G. A synthetic analog of plantaricin 149 inhibiting food-borne pathogenic bacteria: Evidence for α-helical conformation involved in bacteria-membrane interaction. J. Pept. Sci 2007, 13, 171–178. [Google Scholar]
- Lopes, J.L.S.; Nobre, T.M.; Siano, A.; Humpola, V.M.; Bossolan, N.R.S.; Zaniquelli, M.E.D.; Tonarelli, G.; Beltramini, L.M. Disruption of Saccharomyces cerevisiae by Plantaricin 149 and investigation of its mechanism of action with biomembrane model systems. Biochim. Biophys. Acta Biomembr 2009, 1788, 2252–2258. [Google Scholar]
- Shai, Y. From innate immunity to de novo designed antimicrobial peptides. Curr. Pharm. Des 2002, 8, 715–725. [Google Scholar]
- Lopes, J.L.S.; Hissa, D.; Melo, V.M.M.; Beltramini, L.M. Interaction of antimicrobial peptide Plantaricin149a and four analogs with lipid bilayers and bacterial membranes. Braz. J. Microbiol 2013, in press. [Google Scholar]
- Chou, H.T.; Wen, H.W.; Kuo, T.Y.; Lin, C.C.; Chen, W.J. Interaction of cationic antimicrobial peptides with phospholipid vesicles and their antibacterial activity. Peptides 2010, 1, 1811–1820. [Google Scholar]
- Fasman, G.D. Circular Dichroism and the Conformational Analysis of Biomolecules; Springer: New York, NY, USA, 1996; p. 738. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 2nd ed.; Springer: New York, NY, USA, 1999; p. 954. [Google Scholar]
- Wieprecht, T.; Apostolov, O.; Seelig, J. Binding of the antibacterial peptide magainin 2 amide to small and large unilamellar vesicles. Biophys. Chem 2000, 85, 187–198. [Google Scholar]
- Terzi, E.; Holzemann, G.; Seelig, J. Self-association of β-amyloid peptide (1–40) in solution and binding to lipid membranes. J. Mol. Biol 1995, 252, 633–642. [Google Scholar]
- Ulmschneider, M.B.; Sansom, M.S. Amino acid distributions in integral membrane protein structures. Biochim. Biophys. Acta 2001, 1512, 1–14. [Google Scholar]
- Sanderson, J.M.; Whelan, E.J. Characterization of the interactions of aromatic amino acids with diacetyl phosphatidylcholine. Phys. Chem. Chem. Phys 2004, 6, 1012–1017. [Google Scholar]
- Landolt-Marticorena, C.; Williams, K.A.; Deber, C.M.; Reithmeier, R.A. Non-random distribution of amino acids in the transmembrane segments of human type I single span membrane proteins. J. Mol. Biol 1993, 229, 602–608. [Google Scholar]
- Sanderson, J.M. Peptide-lipid interactions: Insights and perspectives. Org. Biomol. Chem 2005, 3, 201–212. [Google Scholar]
- Wang, G.; Sparrow, J.T.; Cushley, R.J. The helix-hinge-helix structural motif in human apolipoprotein A-I determined by NMR spectroscopy. Biochemistry 1997, 36, 13657–13666. [Google Scholar]
- Kaiser, E.; Colescott, R.L.; Bossinger, C.D.; Cook, P.I. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal. Biochem 1970, 34, 595–598. [Google Scholar]
- McClare, C.W. An accurate and convenient organic phosphorus assay. Anal. Biochem 1971, 39, 527–530. [Google Scholar]
- Fernández-Vidal, M.; Rojo, N.; Herrera, E.; Gómara, M.J.; Haro, I. Liposome destabilization induced by synthetic lipopeptides corresponding to envelope and non-structural domains of GBV-C/HGV virus. Conformational requirements for leakage. Biophys. Chem 2008, 132, 55–63. [Google Scholar]
- Ladokhin, A.S.; Jayasinghe, S.; White, S.H. How to measure and analyze tryptophan fluorescence in membranes properly, and why bother? Anal. Biochem 2000, 285, 235–245. [Google Scholar]
- Christiaens, B.; Symoens, S.; Verheyden, S.; Engelborghs, Y.; Joliot, A.; Prochiantz, A.; Vandekerckhove, J.; Rosseneu, M.; Vanloo, B.; Vanderheyden, S. Tryptophan fluorescence study of the interaction of penetrating peptides with model membranes. Eur. J. Biochem 2002, 269, 2918–2926. [Google Scholar]
- Blondelle, S.E.; Lohner, K.; Aguilar, M.I. Lipid-induced conformation and lipid-binding properties of cytolytic and antimicrobial peptides: Determination and biological specificity. Biochim. Biophys. Acta 1999, 1462, 89–108. [Google Scholar]


| Peptide/lipid | Pln149W (%) | Pln149SW (%) |
|---|---|---|
| (1:25) | 88.8 | 75.6 |
| (1:50) | 94.1 | 86.1 |
| (1:100) | 96.9 | 92.5 |
| (1:200) | 98.4 | 96.1 |
| (1:300) | 99.0 | 97.4 |
| (1:400) | 99.2 | 98.0 |
| (1:500) | 99.4 | 98.4 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lopes, J.L.S.; Gómara, M.J.; Haro, I.; Tonarelli, G.; Beltramini, L.M. Contribution of the Tyr-1 in Plantaricin149a to Disrupt Phospholipid Model Membranes. Int. J. Mol. Sci. 2013, 14, 12313-12328. https://doi.org/10.3390/ijms140612313
Lopes JLS, Gómara MJ, Haro I, Tonarelli G, Beltramini LM. Contribution of the Tyr-1 in Plantaricin149a to Disrupt Phospholipid Model Membranes. International Journal of Molecular Sciences. 2013; 14(6):12313-12328. https://doi.org/10.3390/ijms140612313
Chicago/Turabian StyleLopes, José L. S., Maria J. Gómara, Isabel Haro, Georgina Tonarelli, and Leila M. Beltramini. 2013. "Contribution of the Tyr-1 in Plantaricin149a to Disrupt Phospholipid Model Membranes" International Journal of Molecular Sciences 14, no. 6: 12313-12328. https://doi.org/10.3390/ijms140612313




