Enhanced Inhibition of Bladder Cancer Cell Growth by Simultaneous Knockdown of Antiapoptotic Bcl-xL and Survivin in Combination with Chemotherapy
Abstract
:1. Introduction
2. Results and Discussion
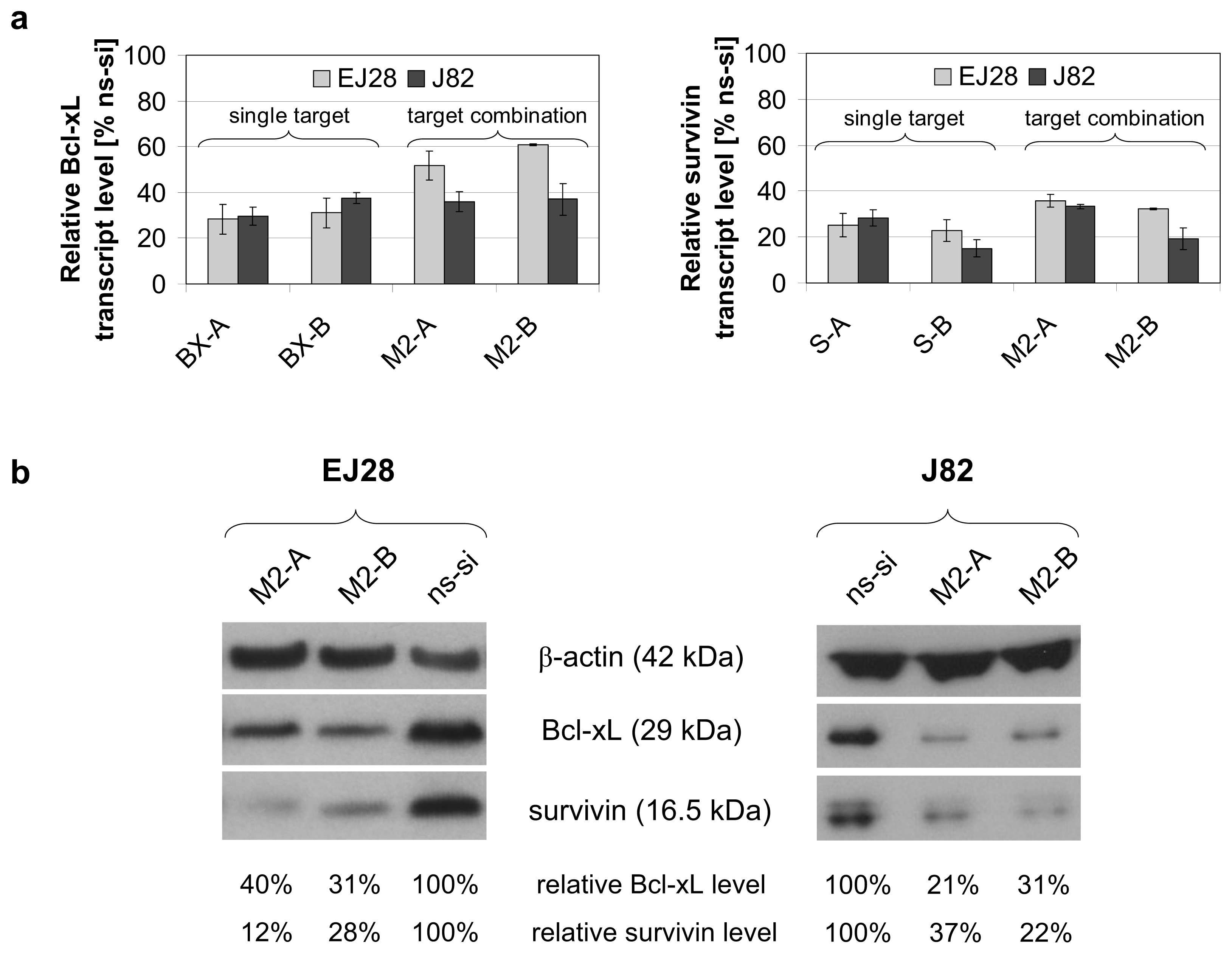
2.1. Reduction of Bcl-xL and Survivin Expression after siRNA Transfection
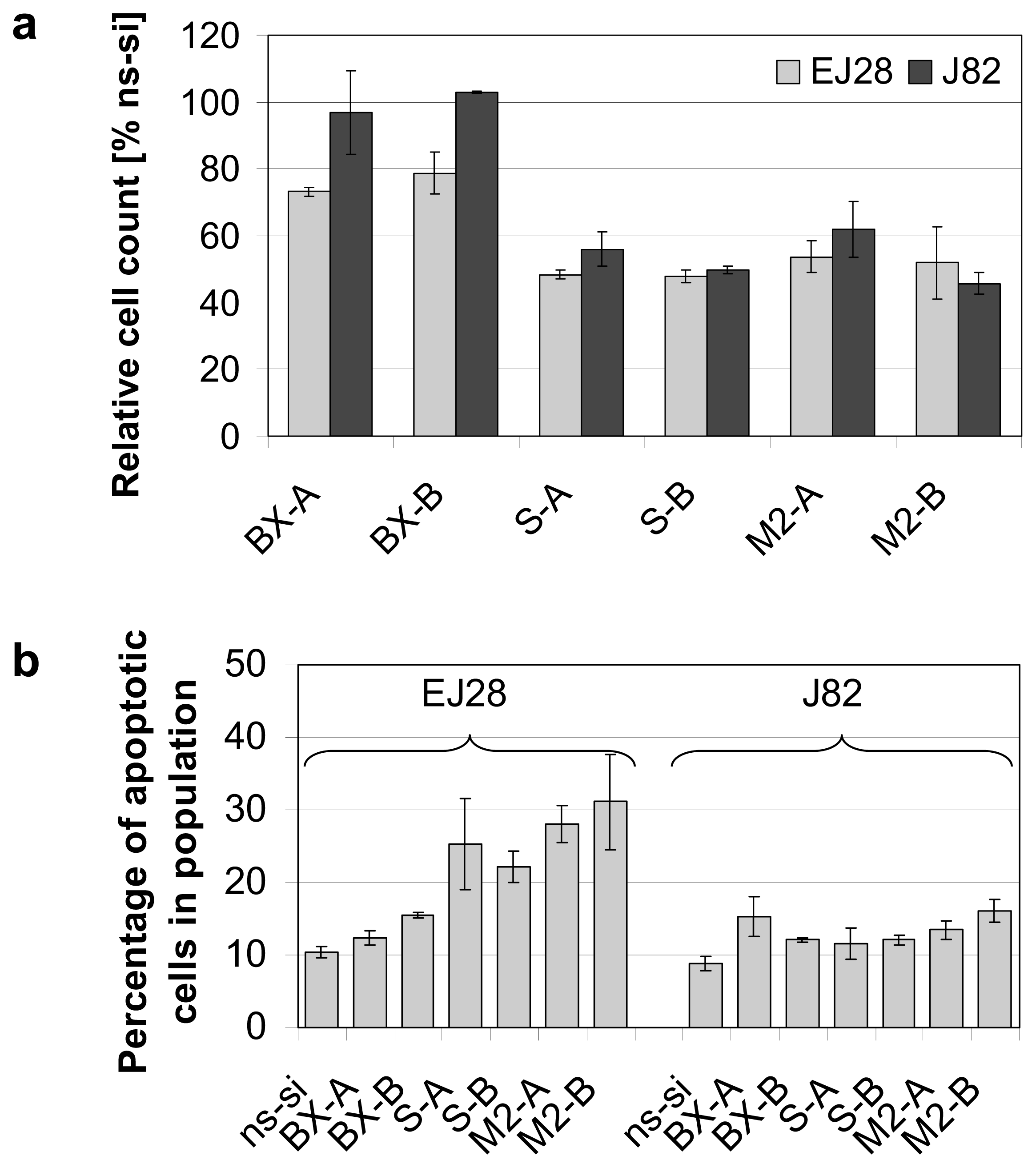
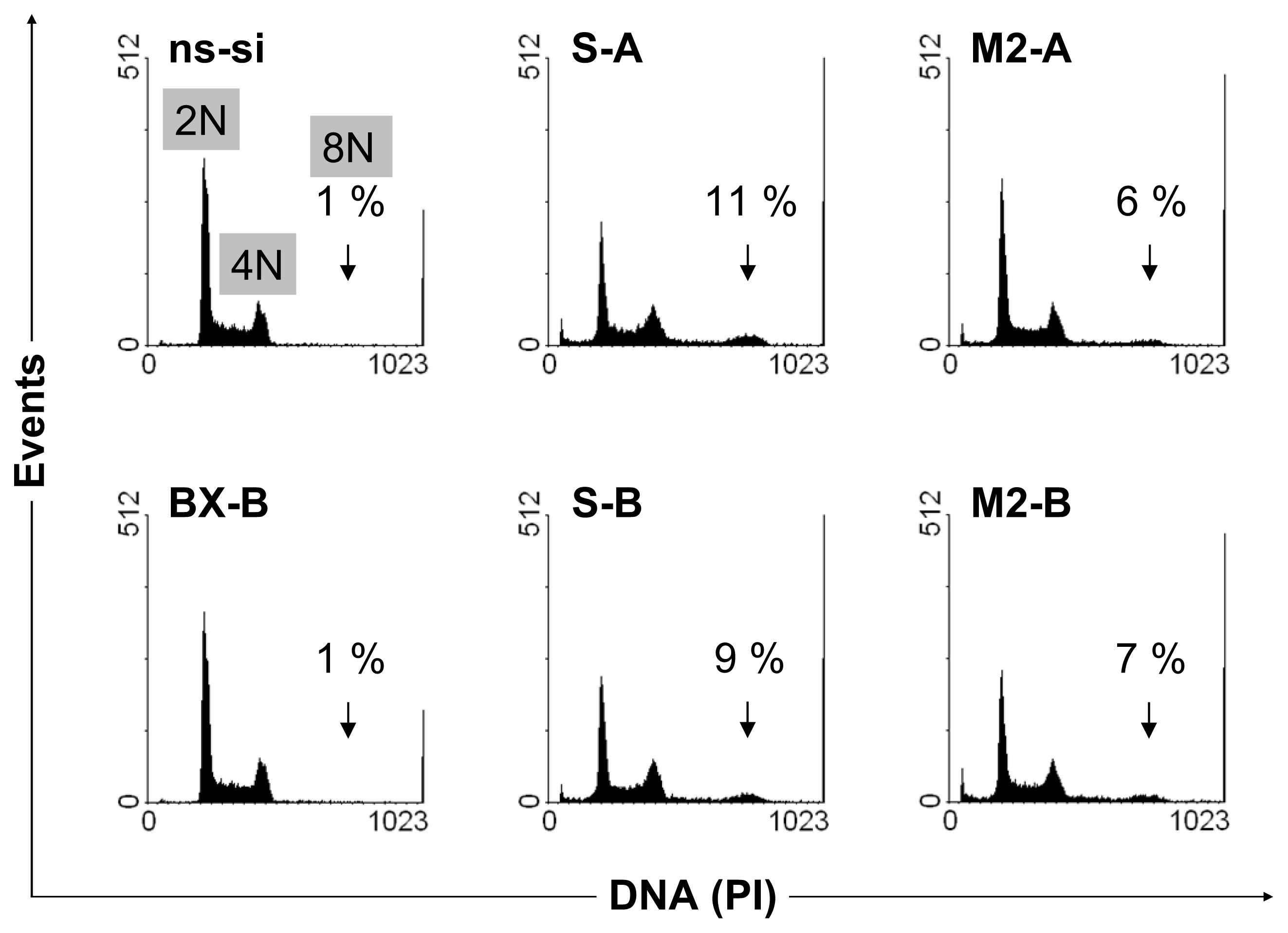
2.2. Cellular Effects of siRNA-Mediated Inhibition of Bcl-xL and Survivin
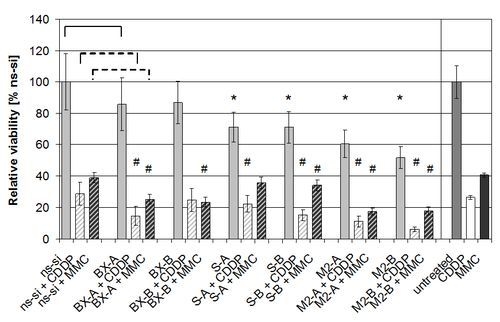
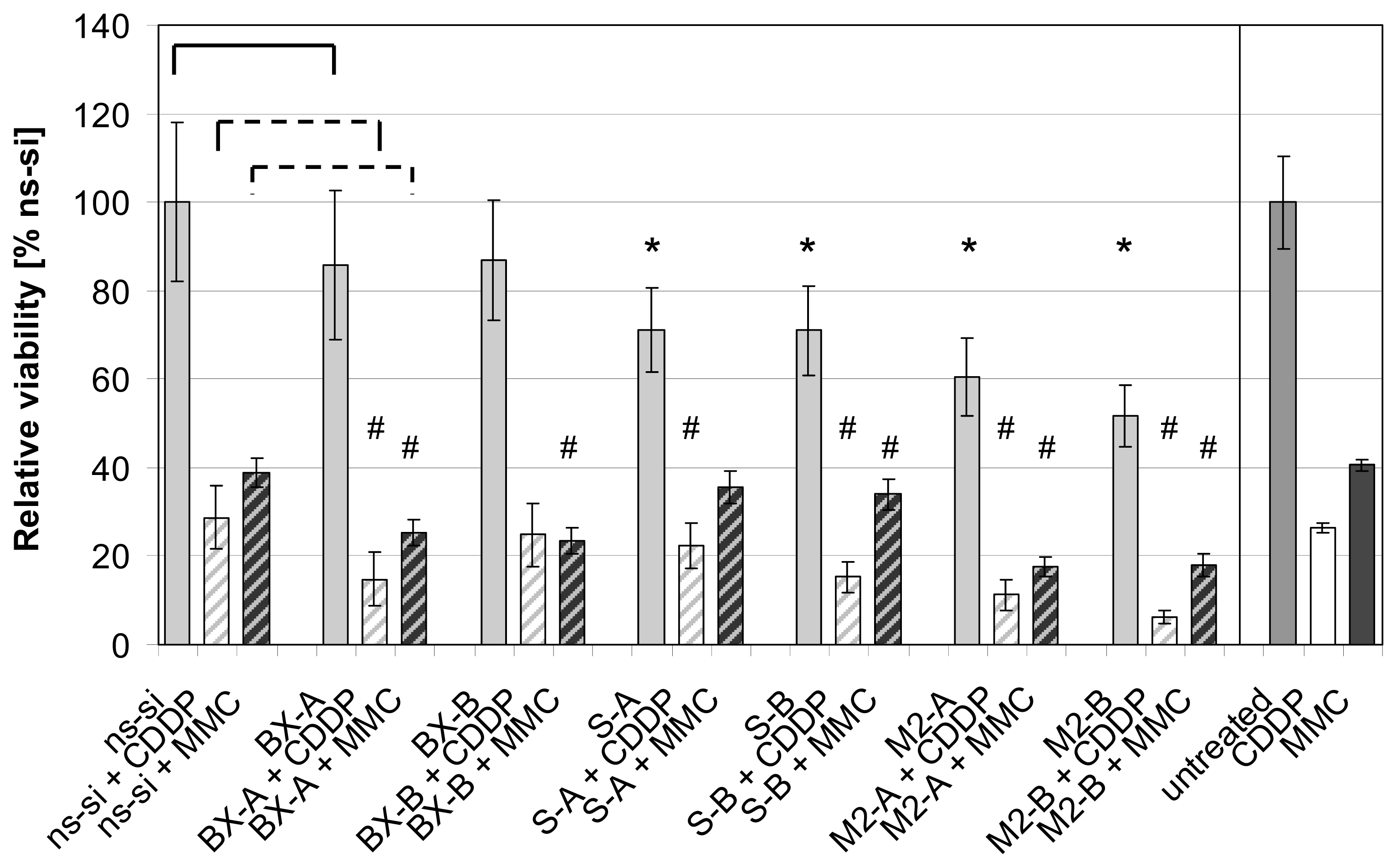
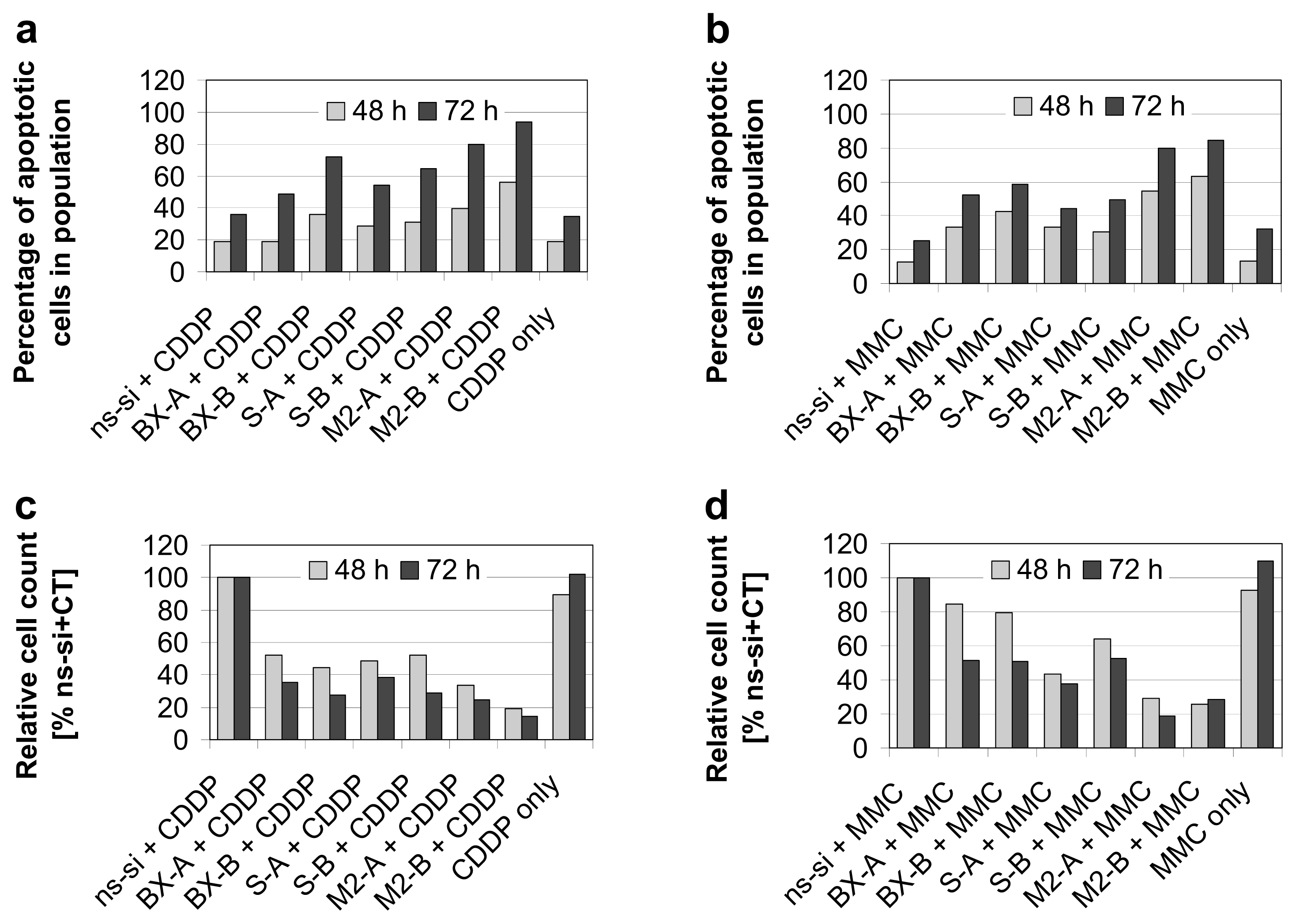
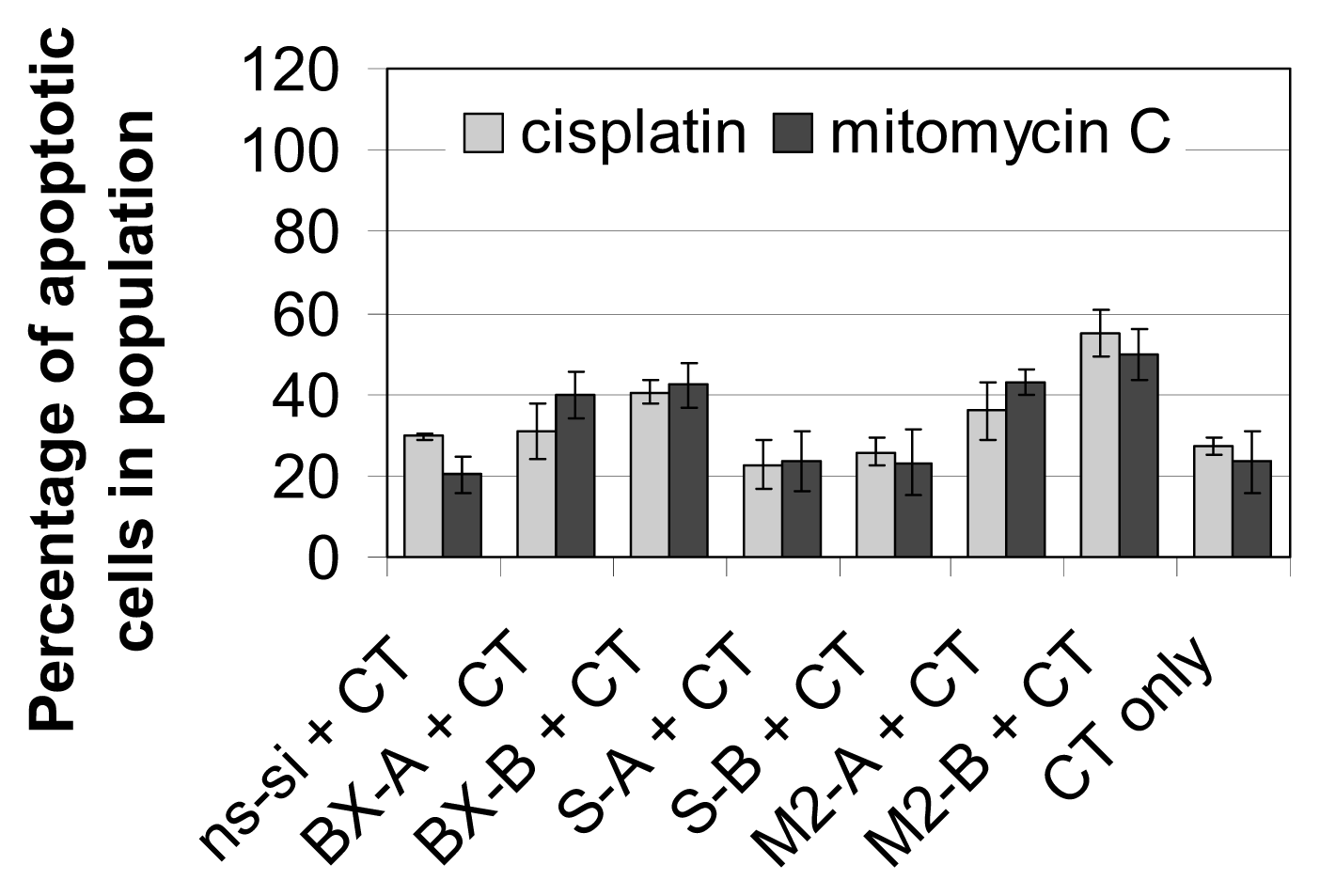
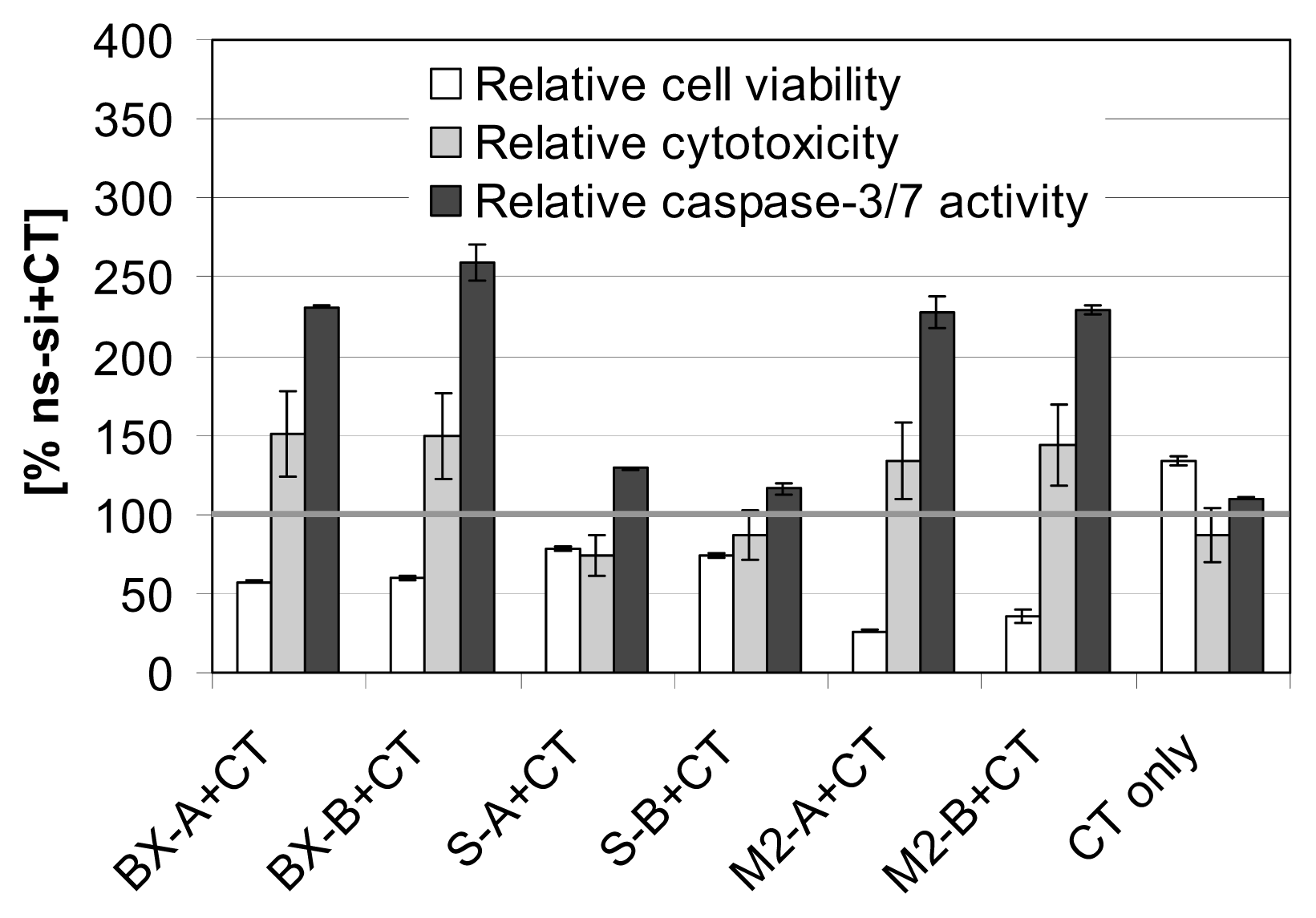
2.3. Effects of Combined Treatment with siRNAs and Chemotherapy
2.4. Discussion
3. Experimental Section
3.1. Cell Culture
3.2. siRNA Transfection
3.3. Treatment with Chemotherapy
3.4. Cell Counts and Cell Viability
3.5. Apoptosis Detection and Cell Cycle Analyses
3.6. ApoTox-Glo Triplex Assay
3.7. RNA Isolation, cDNA Synthesis, and Quantitative PCR
3.8. Western Blot
3.9. Statistics
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Ferlay, J.; Parkin, D.M.; Steliarova-Foucher, E. Estimates of cancer incidence and mortality in Europe in 2008. Eur. J. Cancer 2010, 46, 765–781. [Google Scholar]
- Sylvester, R.J.; Brausi, M.A.; Kirkels, W.J.; Hoeltl, W.; Calais Da Silva, F.; Powell, P.H.; Prescott, S.; Kirkali, Z.; van de Beek, C.; Gorlia, T.; et al. Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guerin, and bacillus Calmette-Guerin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur. Urol 2010, 57, 766–773. [Google Scholar]
- Witjes, J.A.; Compérat, E.; Cowan, N.C.; de Santis, M.; Gakis, G.; Lebret, T.; Ribal, M.J.; Sherif, A. Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Uroweb. 2013. Available online: http://www.uroweb.org/gls/pdf/07_Bladder%20Cancer_LRV2.pdf (accessed on 17 April 2013).
- Babjuk, M.; Burger, M.; Zigeuner, R.; Shariat, S.F.; van Rhijn, B.; Compérat, E.; Sylvester, R.J.; Kaasinen, E.; Böhle, A.; Palou, J.; et al. Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and CIS). Uroweb. 2013. Available online: http://www.uroweb.org/gls/pdf/05_TaT1_Bladder_Cancer_LR.pdf (accessed on 17 April 2013).
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: the next generation. Cell 2011, 144, 646–674. [Google Scholar]
- Kunze, D.; Kraemer, K.; Erdmann, K.; Froehner, M.; Wirth, M.P.; Fuessel, S. Simultaneous siRNA-mediated knockdown of antiapoptotic BCL2, Bcl-xL, XIAP and survivin in bladder cancer cells. Int. J. Oncol 2012, 41, 1271–1277. [Google Scholar]
- Ning, S.; Fuessel, S.; Kotzsch, M.; Kraemer, K.; Kappler, M.; Schmidt, U.; Taubert, H.; Wirth, M.P.; Meye, A. siRNA-mediated down-regulation of survivin inhibits bladder cancer cell growth. Int. J. Oncol 2004, 25, 1065–1071. [Google Scholar]
- Kunze, D.; Erdmann, K.; Froehner, M.; Wirth, M.P.; Fuessel, S. siRNA-mediated inhibition of antiapoptotic genes enhances chemotherapy efficacy in bladder cancer cells. Anticancer Res 2012, 32, 4313–4318. [Google Scholar]
- Ryan, B.M.; O’Donovan, N.; Duffy, M.J. Survivin: A new target for anti-cancer therapy. Cancer Treat. Rev 2009, 35, 553–562. [Google Scholar]
- Shariat, S.F.; Ashfaq, R.; Karakiewicz, P.I.; Saeedi, O.; Sagalowsky, A.I.; Lotan, Y. Survivin expression is associated with bladder cancer presence, stage, progression, and mortality. Cancer 2007, 109, 1106–1113. [Google Scholar]
- Dohi, T.; Okada, K.; Xia, F.; Wilford, C.E.; Samuel, T.; Welsh, K.; Marusawa, H.; Zou, H.; Armstrong, R.; Matsuzawa, S.; et al. An IAP-IAP complex inhibits apoptosis. J. Biol. Chem 2004, 279, 34087–34090. [Google Scholar]
- Brunelle, J.K.; Letai, A. Control of mitochondrial apoptosis by the Bcl-2 family. J. Cell Sci 2009, 122, 437–441. [Google Scholar]
- Korkolopoulou, P.; Lazaris, A.; Konstantinidou, A.E.; Kavantzas, N.; Patsouris, E.; Christodoulou, P.; Thomas-Tsagli, E.; Davaris, P. Differential expression of bcl-2 family proteins in bladder carcinomas. Relationship with apoptotic rate and survival. Eur. Urol 2002, 41, 274–283. [Google Scholar]
- Hunter, A.M.; LaCasse, E.C.; Korneluk, R.G. The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis 2007, 12, 1543–1568. [Google Scholar]
- Jeyaprakash, A.A.; Klein, U.R.; Lindner, D.; Ebert, J.; Nigg, E.A.; Conti, E. Structure of a Survivin-Borealin-INCENP core complex reveals how chromosomal passengers travel together. Cell 2007, 131, 271–285. [Google Scholar]
- Fuessel, S.; Herrmann, J.; Ning, S.; Kotzsch, M.; Kraemer, K.; Schmidt, U.; Hakenberg, O.W.; Wirth, M.P.; Meye, A. Chemosensitization of bladder cancer cells by survivin-directed antisense oligodeoxynucleotides and siRNA. Cancer Lett 2006, 232, 243–254. [Google Scholar]
- Kappler, M.; Bache, M.; Bartel, F.; Kotzsch, M.; Panian, M.; Wurl, P.; Blumke, K.; Schmidt, H.; Meye, A.; Taubert, H. Knockdown of survivin expression by small interfering RNA reduces the clonogenic survival of human sarcoma cell lines independently of p53. Cancer Gene Ther 2004, 11, 186–193. [Google Scholar]
- Saito, T.; Hama, S.; Izumi, H.; Yamasaki, F.; Kajiwara, Y.; Matsuura, S.; Morishima, K.; Hidaka, T.; Shrestha, P.; Sugiyama, K.; et al. Centrosome amplification induced by survivin suppression enhances both chromosome instability and radiosensitivity in glioma cells. Br. J. Cancer 2008, 98, 345–355. [Google Scholar]
- Ramachandran, P.V.; Ignacimuthu, S. RNA interference as a plausible anticancer therapeutic tool. Asian Pac. J. Cancer Prev 2012, 13, 2445–2452. [Google Scholar]
- Fuessel, S.; Kunze, D.; Kraemer, K.; Schwenzer, B.; Meye, A.; Wirth, M.P. Chemosensitization of Human Bladder Cancer Cells by Pre-Treatment with Nucleic Acid Inhibitors: Antiproliferative Effects and Specificity. In Chemo-Immunosensitization of Resistant Tumor Cells to Apoptosis; Bonavida, B., Ed.; Transworld Research Network: Kerala, Indien, 2006; pp. 13–36. [Google Scholar]
- Lonning, P.E.; Knappskog, S. Mapping genetic alterations causing chemoresistance in cancer: identifying the roads by tracking the drivers. Oncogene 2013. [Google Scholar] [CrossRef]
- Fulda, S. Inhibitor of Apoptosis (IAP) proteins as therapeutic targets for radiosensitization of human cancers. Cancer Treat. Rev 2012, 38, 760–766. [Google Scholar]
- Shimura, T. Acquired radioresistance of cancer and the AKT/GSK3beta/cyclin D1 overexpression cycle. J. Radiat. Res 2011, 52, 539–544. [Google Scholar]
- Hao, J.H.; Gu, Q.L.; Liu, B.Y.; Li, J.F.; Chen, X.H.; Ji, Y.B.; Zhu, Z.G.; Lin, Y.Z. Inhibition of the proliferation of human gastric cancer cells SGC-7901 in vitro and in vivo using Bcl-2 siRNA. Chin. Med. J 2007, 120, 2105–2111. [Google Scholar]
- Liu, Y.; Wu, X.; Sun, Y.; Chen, F. Silencing of X-linked inhibitor of apoptosis decreases resistance to cisplatin and paclitaxel but not gemcitabine in non-small cell lung cancer. J. Int. Med. Res 2011, 39, 1682–1692. [Google Scholar]
- Jeong, J.Y.; Kim, K.S.; Moon, J.S.; Song, J.A.; Choi, S.H.; Kim, K.I.; Kim, T.H.; An, H.J. Targeted inhibition of phosphatidyl inositol-3-kinase p110beta, but not p110alpha, enhances apoptosis and sensitivity to paclitaxel in chemoresistant ovarian cancers. Apoptosis 2013, 18, 509–520. [Google Scholar]
- Han, Z.; Chatterjee, D.; Early, J.; Pantazis, P.; Hendrickson, E.A.; Wyche, J.H. Isolation and characterization of an apoptosis-resistant variant of human leukemia HL-60 cells that has switched expression from Bcl-2 to Bcl-xL. Cancer Res 1996, 56, 1621–1628. [Google Scholar]
- Wang, Q.; Shu, R.; He, H.; Wang, L.; Ma, Y.; Zhu, H.; Wang, Z.; Wang, S.; Shen, G.; Lei, P. Co-silencing of Birc5 (survivin) and Hspa5 (Grp78) induces apoptosis in hepatoma cells more efficiently than single gene interference. Int. J. Oncol 2012, 41, 652–660. [Google Scholar]
- Yang, D.; Song, X.; Zhang, J.; Ye, L.; Wang, S.; Che, X.; Wang, J.; Zhang, Z.; Wang, L.; Shi, W. Therapeutic potential of siRNA-mediated combined knockdown of the IAP genes (Livin, XIAP, and Survivin) on human bladder cancer T24 cells. Acta Biochim. Biophys. Sin 2010, 42, 137–144. [Google Scholar]
- Kim, D.W.; Seo, S.W.; Cho, S.K.; Chang, S.S.; Lee, H.W.; Lee, S.E.; Block, J.A.; Hei, T.K.; Lee, F.Y. Targeting of cell survival genes using small interfering RNAs (siRNAs) enhances radiosensitivity of Grade II chondrosarcoma cells. J. Orthop. Res 2007, 25, 820–828. [Google Scholar]
- Di Cresce, C.; Figueredo, R.; Ferguson, P.J.; Vincent, M.D.; Koropatnick, J. Combining small interfering RNAs targeting thymidylate synthase and thymidine kinase 1 or 2 sensitizes human tumor cells to 5-fluorodeoxyuridine and pemetrexed. J. Pharmacol. Exp. Ther 2011, 338, 952–963. [Google Scholar]
- Gill, C.; Dowling, C.; O’Neill, A.J.; Watson, R.W. Effects of cIAP-1, cIAP-2 and XIAP triple knockdown on prostate cancer cell susceptibility to apoptosis, cell survival and proliferation. Mol. Cancer 2009, 8, 39. [Google Scholar]
- Gavrilov, K.; Saltzman, W.M. Therapeutic siRNA: principles, challenges, and strategies. Yale J. Biol. Med 2012, 85, 187–200. [Google Scholar]
- Whitehead, K.A.; Langer, R.; Anderson, D.G. Knocking down barriers: Advances in siRNA delivery. Nat. Rev. Drug Discov 2009, 8, 129–138. [Google Scholar]
- Davis, M.E.; Zuckerman, J.E.; Choi, C.H.; Seligson, D.; Tolcher, A.; Alabi, C.A.; Yen, Y.; Heidel, J.D.; Ribas, A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 2010, 464, 1067–1070. [Google Scholar]
- Seth, S.; Matsui, Y.; Fosnaugh, K.; Liu, Y.; Vaish, N.; Adami, R.; Harvie, P.; Johns, R.; Severson, G.; Brown, T.; et al. RNAi-based therapeutics targeting survivin and PLK1 for treatment of bladder cancer. Mol. Ther 2011, 19, 928–935. [Google Scholar]
- WinMDI2.8 software. Available online: http://facs.scripps.edu/software.html (accessed on 17 April 2013).
| Target | Sequence 5′→3′ |
|---|---|
| Bcl-xL 1 | target-specific Real-Time Reagent Mix (AJ Roboscreen, Leipzig, Germany) containing the appropriate primers and probes |
| survivin2 | Primers: for: GAACTGGCCCTTCTTGGAG, rev: AAGTCTGGCTCGTTCTCAGTG Probe: Universal ProbeLibrary Probe #86 (Roche, Germany, cat.no. 04689119001) |
| TBP 1 | Primers: for: GAATATAATCCCAAGCGGTTTG, rev: ACTTCACATCACAGCTCCCC Probes: TTTCCCAGAACTGAAAATCAGTGCC-FL, LC-TGGTTCGTGGCTCTCTTATCCTCATG-PH |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kunze, D.; Erdmann, K.; Froehner, M.; Wirth, M.P.; Fuessel, S. Enhanced Inhibition of Bladder Cancer Cell Growth by Simultaneous Knockdown of Antiapoptotic Bcl-xL and Survivin in Combination with Chemotherapy. Int. J. Mol. Sci. 2013, 14, 12297-12312. https://doi.org/10.3390/ijms140612297
Kunze D, Erdmann K, Froehner M, Wirth MP, Fuessel S. Enhanced Inhibition of Bladder Cancer Cell Growth by Simultaneous Knockdown of Antiapoptotic Bcl-xL and Survivin in Combination with Chemotherapy. International Journal of Molecular Sciences. 2013; 14(6):12297-12312. https://doi.org/10.3390/ijms140612297
Chicago/Turabian StyleKunze, Doreen, Kati Erdmann, Michael Froehner, Manfred P. Wirth, and Susanne Fuessel. 2013. "Enhanced Inhibition of Bladder Cancer Cell Growth by Simultaneous Knockdown of Antiapoptotic Bcl-xL and Survivin in Combination with Chemotherapy" International Journal of Molecular Sciences 14, no. 6: 12297-12312. https://doi.org/10.3390/ijms140612297
APA StyleKunze, D., Erdmann, K., Froehner, M., Wirth, M. P., & Fuessel, S. (2013). Enhanced Inhibition of Bladder Cancer Cell Growth by Simultaneous Knockdown of Antiapoptotic Bcl-xL and Survivin in Combination with Chemotherapy. International Journal of Molecular Sciences, 14(6), 12297-12312. https://doi.org/10.3390/ijms140612297