Characterization and Antimicrobial Property of Poly(Acrylic Acid) Nanogel Containing Silver Particle Prepared by Electron Beam
Abstract
:1. Introduction
2. Results and Discussion
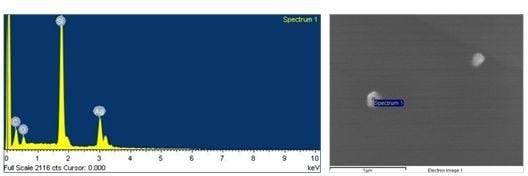
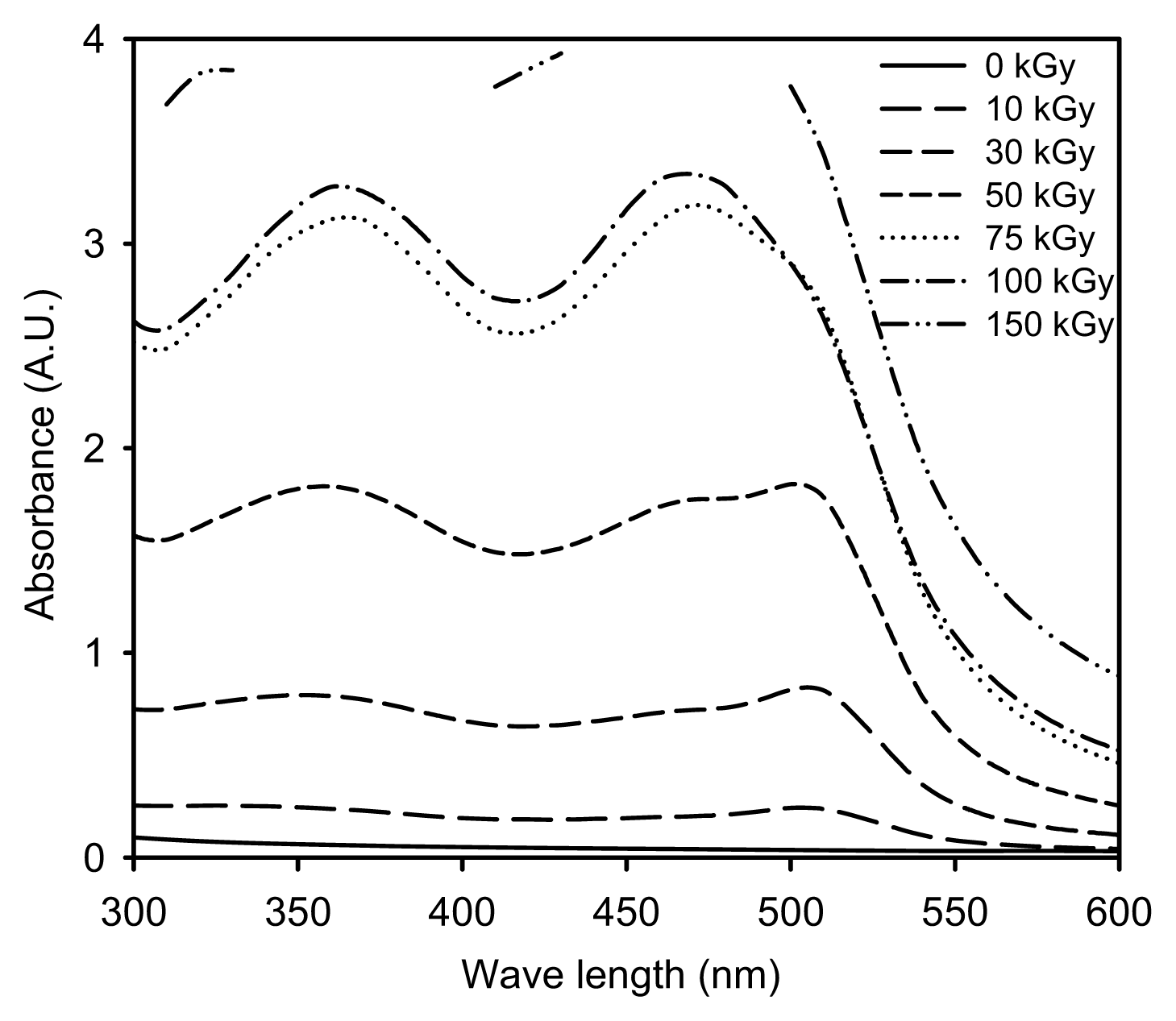
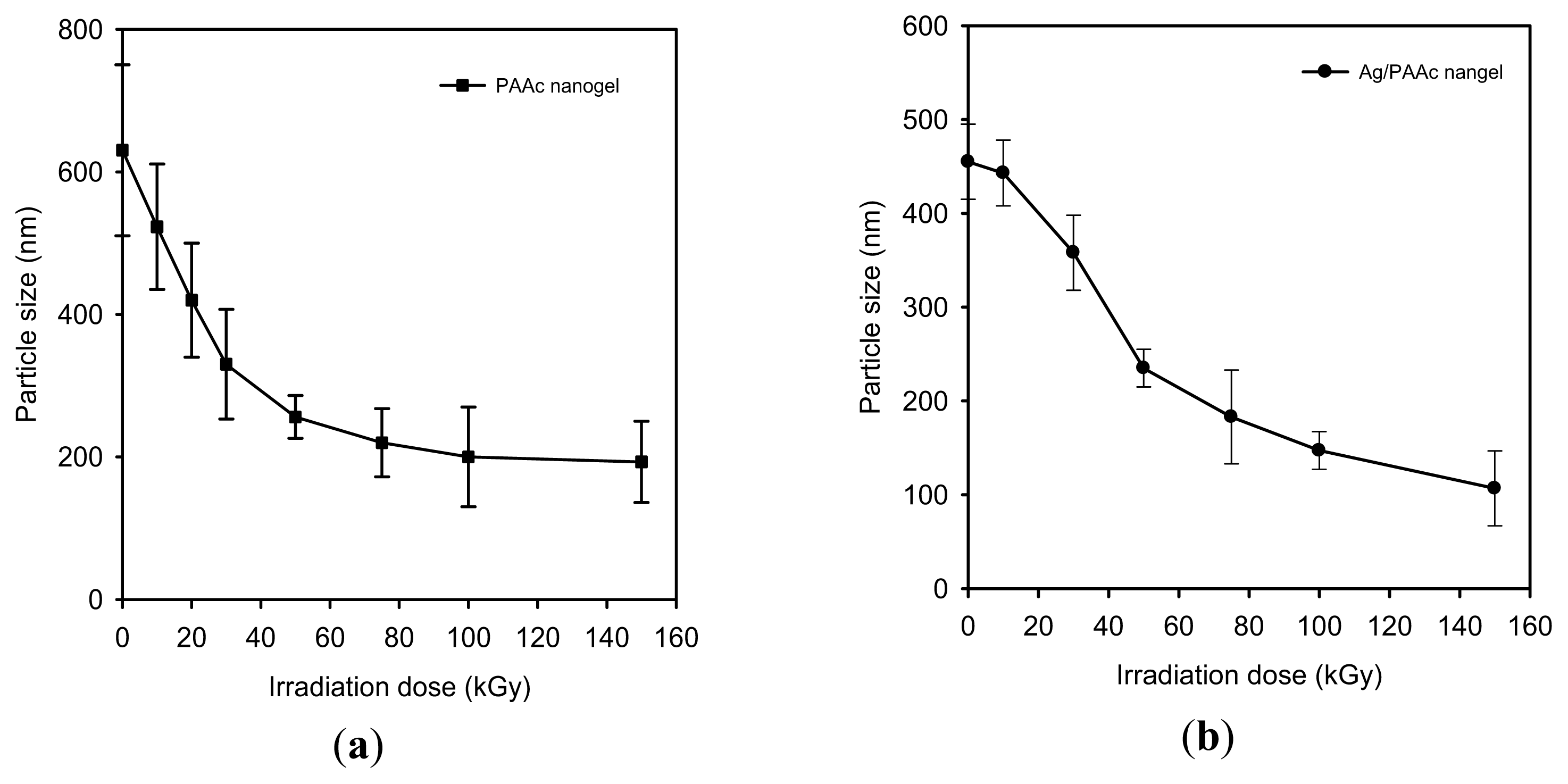
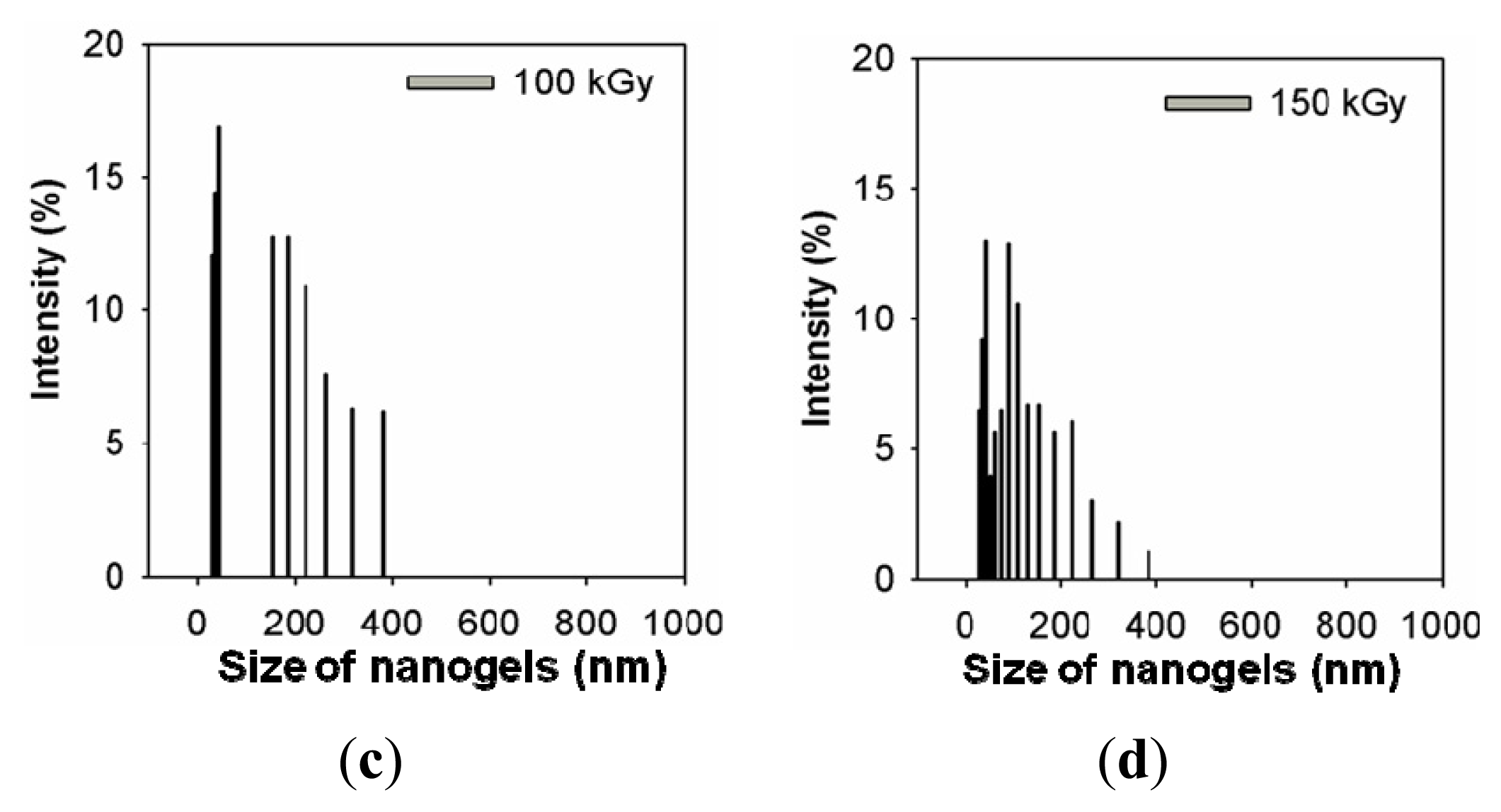
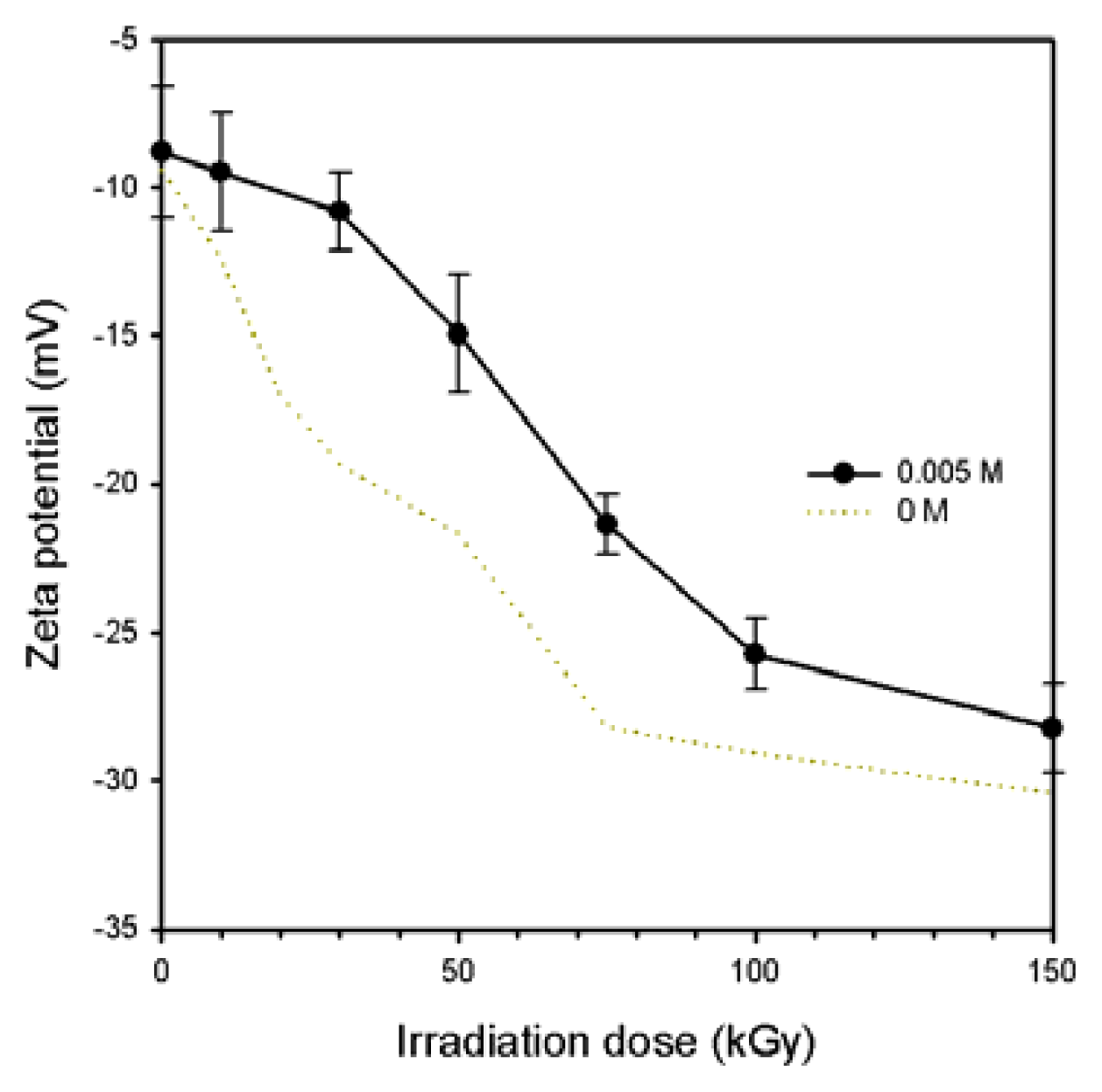
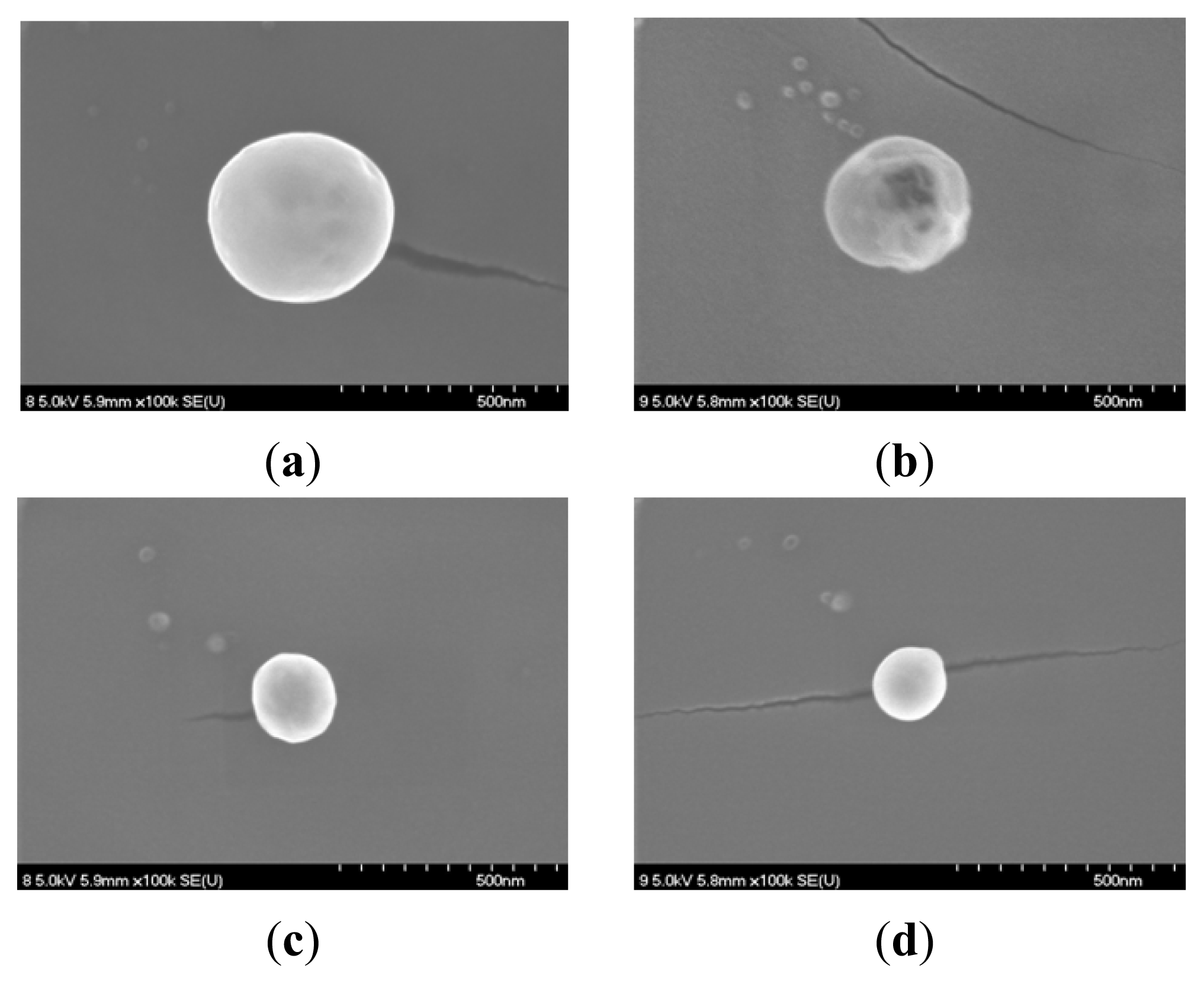
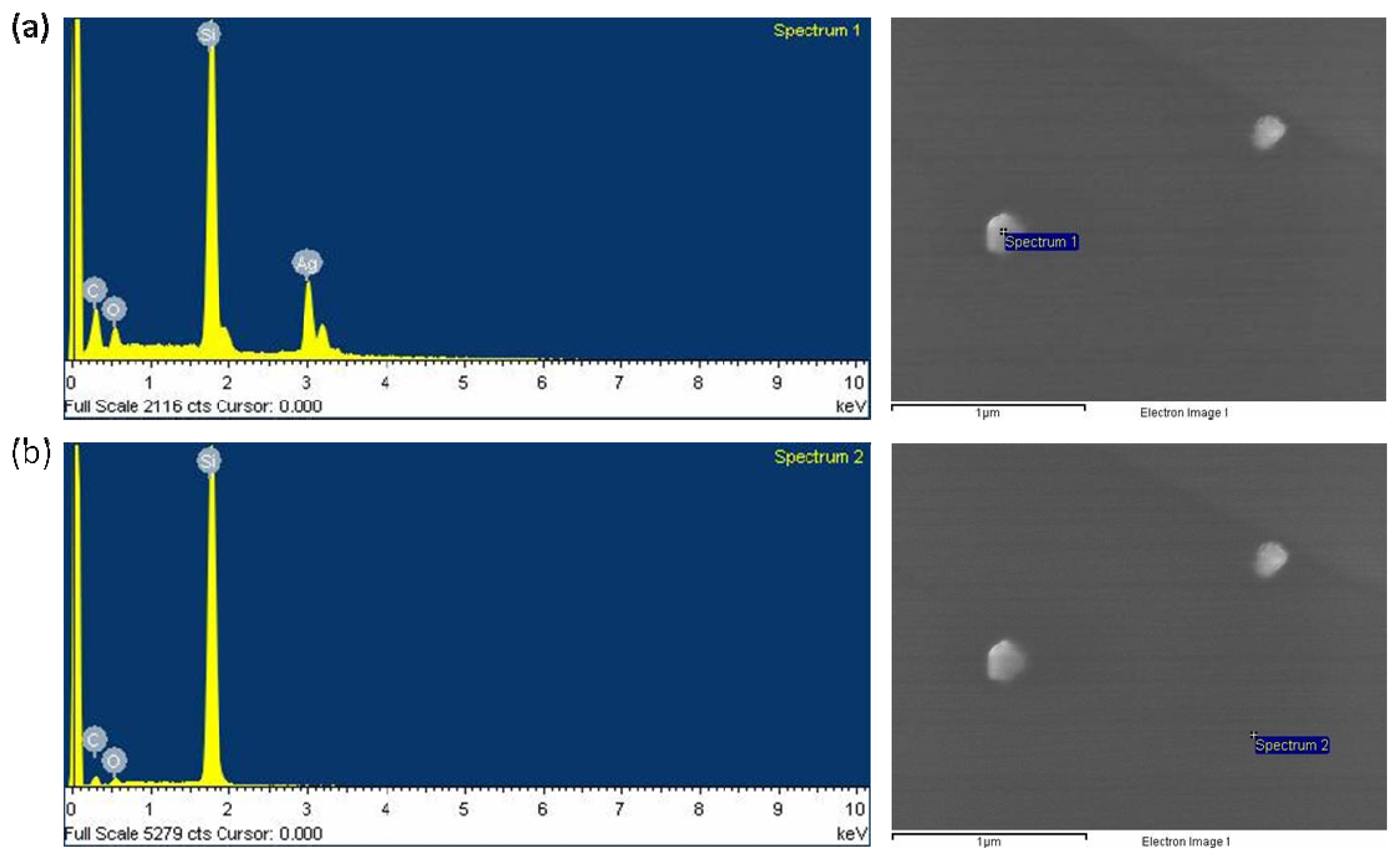
2.1. Characterization of the Prepared Ag/PAAc Nanogel
2.2. Antimicrobial Property of the Prepared Ag/PAAc Nanogel
2.3. In Vivo Wound Healing
3. Experimental Section
3.1. Materials
3.2. Preparation of PAAc Nanogels and Ag/PAAc Nanogels
3.3. UV-Vis Spectrophotometer
3.4. Particle Size Distribution and Zeta Potential Analysis
3.5. FE-SEM and EDX Analysis
3.6. Antibacterial Test
3.7. In Vivo Wound Healing
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Kratochvil, P.; Suter, U.W. Definitions of terms relating to individual macromolecules, their assemblies, and dilute polymer solutions. Pure Appl. Chem 1989, 61, 211–241. [Google Scholar]
- Funke, W.; Okay, O.; Joos-Muller, B. Microgels–Intra-molecularly crosslinked macromolecules with a globular structure. Adv. Polym. Sci 1998, 136, 139–234. [Google Scholar]
- Peppas, N.A. Hydrogels in Medicine and Pharmacy; CRC Press Inc: Boca Raton, FL, USA, 1986–1987. [Google Scholar]
- Oh, J.K.; Drumright, R.; Siegwart, D.J.; Matyjaszewski, K. The development of microgels/nanogels for drug delivery applications. Prog. Polym. Sci 2008, 33, 448–477. [Google Scholar]
- Van Thienen, T.G.; Lucas, F.B.; Flesch, M.; van Nostrum, J.; Demeester, C.; de Smedt, C. On the synthesis and characterization of biodegradable dextran nanogels with tunable degradation properties. Macromolucules 2005, 38, 8503–8511. [Google Scholar]
- Kadlubowski, S.; Ulanski, P.; Rosiak, J.M. Synthesis of tailored nanogels by means of two-stage irradiation. Polymer 2012, 53, 1985–1991. [Google Scholar]
- An, J.C.; Weaver, A.; Kim, B N.; Barkatt, A.; Poster, D.; Vreeland, W.N.; Silverman, J.; Al-Sheikhly, M. Radiation-induced synthesis of poly(vinylpyrrolidone) nanogel. Polymer 2011, 52, 5746–5755. [Google Scholar]
- Ulański, P.; Kadłubowski, S.; Rosiak, J.M. Synthesis of poly(acrylic acid) nanogels by preparative pulse radiolysis. Radiat. Phys. Chem 2002, 63, 533–537. [Google Scholar]
- Nurkeeva, Z.S.; Khutoryanskiy, V.V.; Mun, G.A.; Bitekenova, A.B.; Kadlubowski, S.; Shilina, Y.A.; Ulanski, P.; Rosiak, J.M. Interpolymer complexes of poly(acrylic acid) nanogels with some non-ionic polymers in aqueous solutions. Colloids Surfaces A 2002, 236, 141–146. [Google Scholar]
- Yang, Z.; Zhang, Y.; Markland, P.; Yang, V.C. Poly(glutamic acid) poly(ethylene glycol) hydrogels prepared by photoinduced polymerization: Synthesis, characterization, and preliminary release studies of protein drugs. J. Biomed. Mater. Res 2002, 62, 14–21. [Google Scholar]
- Hu, Y.; Jiang, X.; Ding, Y.; Ge, H.; Yuan, Y.; Yang, C. Synthesis and characterization of chitosan–Poly(acrylic acid) nanoparticles. Biomaterials 2002, 23, 3193–3201. [Google Scholar]
- Changez, M.; Koul, V.; Dinda, A.K. Efficacy of antibiotics-loaded interpenetrating network (IPNs) hydrogel based on poly(acrylic acid) and gelatin for treatment of experimental osteomyelitis: In vivo study. Biomaterials 2005, 26, 2095–2104. [Google Scholar]
- Yan, X.; Gemeinhart, R.A. Cisplatin delivery from poly(acrylic acid-co-methyl methacrylate) microparticles. J. Control Release 2005, 106, 198–208. [Google Scholar]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci 2009, 145, 83–96. [Google Scholar]
- Tolaymat, T.M.; El Badawy, A.M.; Genaidy, A.; Scheckel, K.G.; Luxton, T.P.; Suidan, M. An evidence-based environmental perspective of manufactured silver nanoparticle in syntheses and applications: A systematic review and critical appraisal of peer-reviewed scientific papers. Sci. Total Environ 2010, 408, 999–1006. [Google Scholar]
- Huang, N.M.; Lim, H.N.; Radiman, S.; Khiew, P.S.; Chiu, W.S.; Hashim, R.; Chia, C.H. Sucrose ester micellar-mediated synthesis of Ag nanoparticles and the antibacterial properties. Colloid Surfaces A 2010, 353, 69–76. [Google Scholar]
- Liu, F.K.; Hsu, Y.C.; Tsai, M.H.; Chu, T.C. Using γ-irradiation to synthesize Ag nanoparticles. Mater. Lett 2007, 61, 2402–2405. [Google Scholar]
- Park, J.S.; Kuang, J.; Lim, Y.M.; Gwon, H.J.; Nho, Y.C. Characterization of silver nanoparticle in the carboxymethyl cellulose hydrogel prepared by a gamma ray irradiation. J. Nanosci. Nanotechnol 2012, 12, 742–747. [Google Scholar]
- Bhui, D.K.; Bar, H.; Sarkar, P.; Sahoo, G.P.; De, S.P.; Misra, A. Sythesis and UV-vis spectroscopic of silver nanoparticle in aqueous SDS solution. J. Mol. Liq 2009, 145, 33–37. [Google Scholar]
- Prasad Rao, J.; Geckeler, K.E. Polymer nanoparticles: Preparation techniques and size-control parameters. Prog. Polym. Sci 2011, 36, 887–913. [Google Scholar]
- Akman, E.; Genc Oztoprak, B.; Gunes, M.; Kacar, E.; Demir, A. Effect of femtosecond Ti: Sapphire laser wavelengths on plasmonic behaviour and size evolution of silver nanoparticles. Photonic. Nanostruct 2011, 9, 276–286. [Google Scholar]
- Shrivastava, S.; Bera, T.; Singh, S.K.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of antiplatelet properties of silver nanoparticles. ACS Nano 2009, 3, 1357–1364. [Google Scholar]
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique cellular interaction of silver nanoparticles: Size-dependent generation of reactive oxygen species. J. Phys. Chem. B 2008, 118, 13608–13619. [Google Scholar]
- Singh, M.; Singh, S.; Prasad, S.; Gambhir, I.S. Nanotechnology in medicine and antibacterial effect of silver nanoparticles. Dig. J. Nanomater. Biostruct 2008, 3, 115–122. [Google Scholar]
- Sondia, I.; Salopek-Sondib, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci 2004, 275, 177–182. [Google Scholar]
- Mishra, M.; Kumar, H.; Tripathi, K. Daibetic delayed wound healing and the role of silver nanoparticles. Dig. J. Nanomater. Biostruct 2008, 3, 49–54. [Google Scholar]


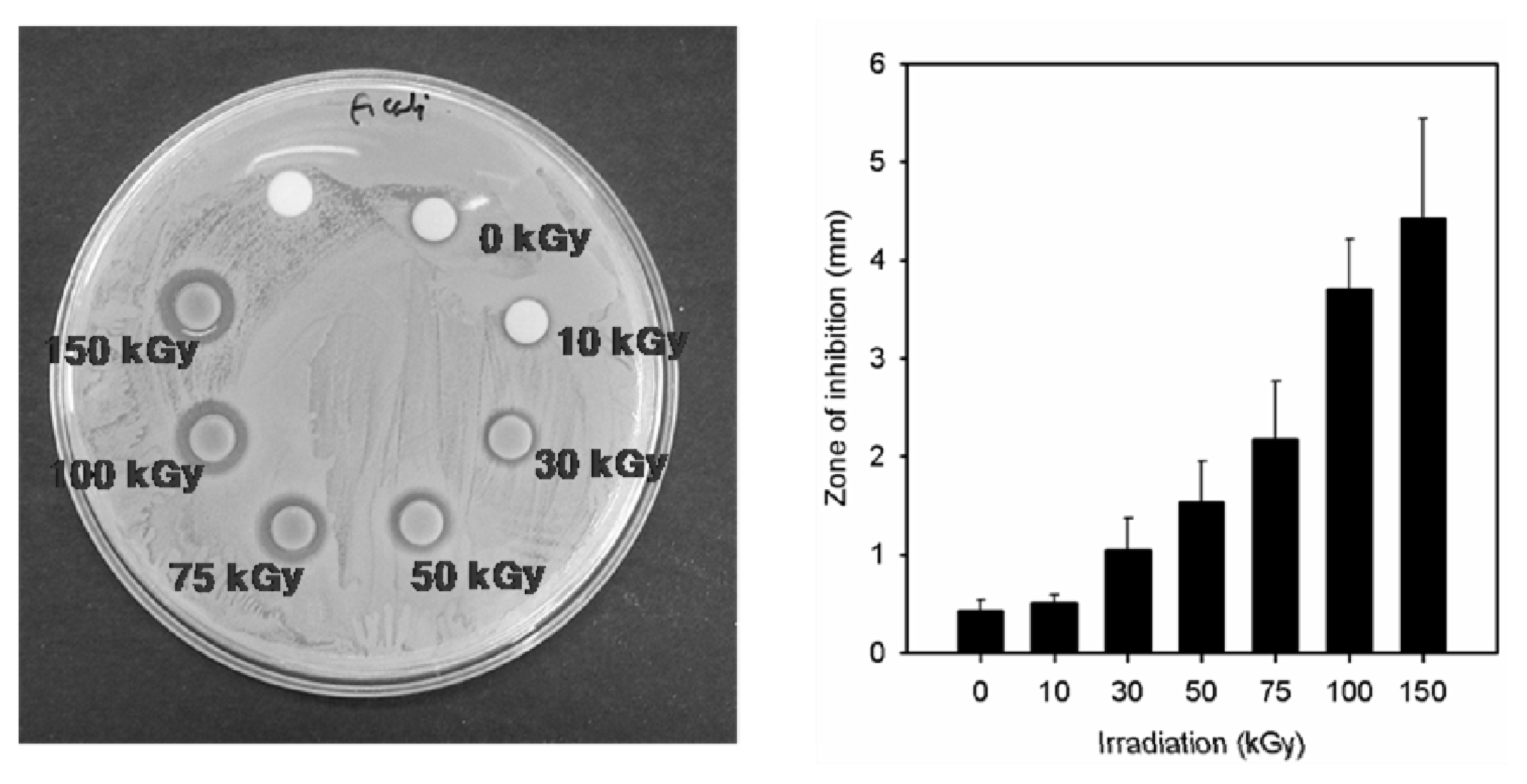
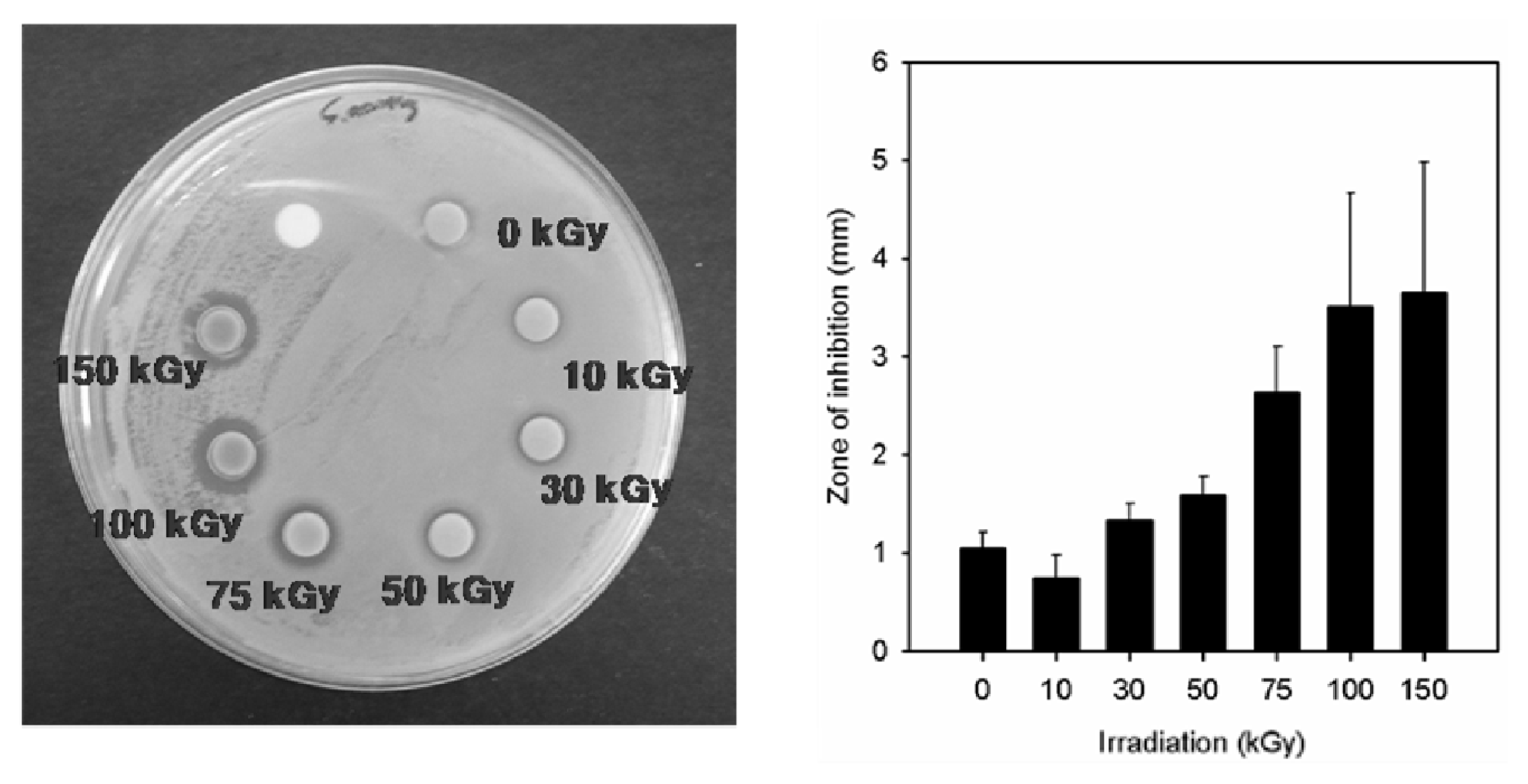

| Radiation dose (kGy) | Gram Negative | Gram Positive |
|---|---|---|
| E. coli | S. aureus | |
| Inhibition zone (mm) | ||
| 0 | 0.4 ± 0.1 | 1.1 ± 0.2 |
| 10 | 0.5 ± 0.1 | 0.7 ± 0.2 |
| 30 | 1.1 ± 0.3 | 1.3 ± 0.2 |
| 50 | 1.5 ± 0.4 | 1.6 ± 0.2 |
| 75 | 2.2 ± 0.6 | 2.6 ± 0.4 |
| 100 | 3.7 ± 0.5 | 3.5 ± 1.2 |
| 150 | 4.4 ± 1.0 | 3.7 ± 1.3 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Choi, J.-B.; Park, J.-S.; Khil, M.-S.; Gwon, H.-J.; Lim, Y.-M.; Jeong, S.-I.; Shin, Y.-M.; Nho, Y.-C. Characterization and Antimicrobial Property of Poly(Acrylic Acid) Nanogel Containing Silver Particle Prepared by Electron Beam. Int. J. Mol. Sci. 2013, 14, 11011-11023. https://doi.org/10.3390/ijms140611011
Choi J-B, Park J-S, Khil M-S, Gwon H-J, Lim Y-M, Jeong S-I, Shin Y-M, Nho Y-C. Characterization and Antimicrobial Property of Poly(Acrylic Acid) Nanogel Containing Silver Particle Prepared by Electron Beam. International Journal of Molecular Sciences. 2013; 14(6):11011-11023. https://doi.org/10.3390/ijms140611011
Chicago/Turabian StyleChoi, Jong-Bae, Jong-Seok Park, Myung-Seob Khil, Hui-Jeong Gwon, Youn-Mook Lim, Sung-In Jeong, Young-Min Shin, and Young-Chang Nho. 2013. "Characterization and Antimicrobial Property of Poly(Acrylic Acid) Nanogel Containing Silver Particle Prepared by Electron Beam" International Journal of Molecular Sciences 14, no. 6: 11011-11023. https://doi.org/10.3390/ijms140611011