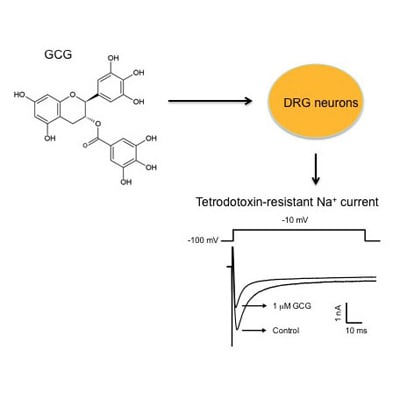
Effects of (−)-Gallocatechin-3-Gallate on Tetrodotoxin-Resistant Voltage-Gated Sodium Channels in Rat Dorsal Root Ganglion Neurons
Abstract
:1. Introduction
2. Results and Discussion
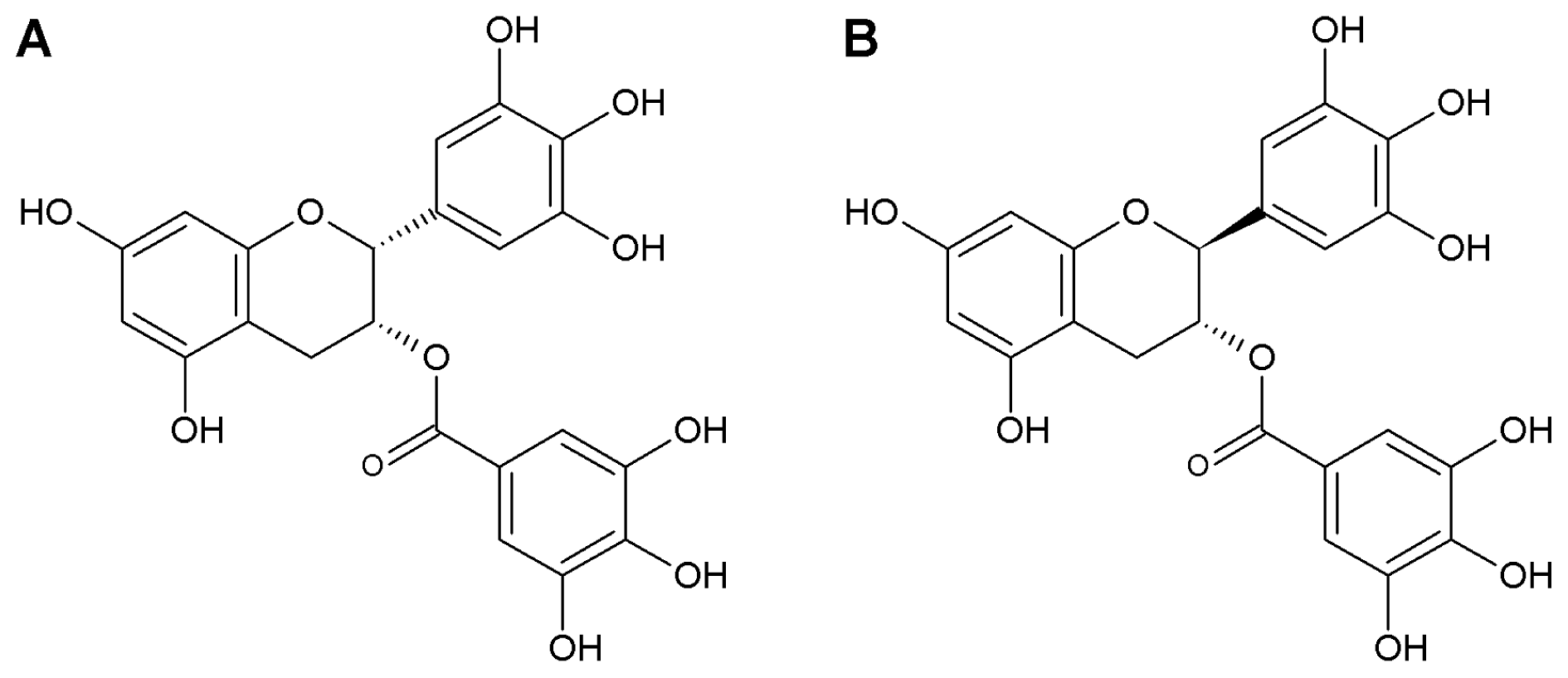
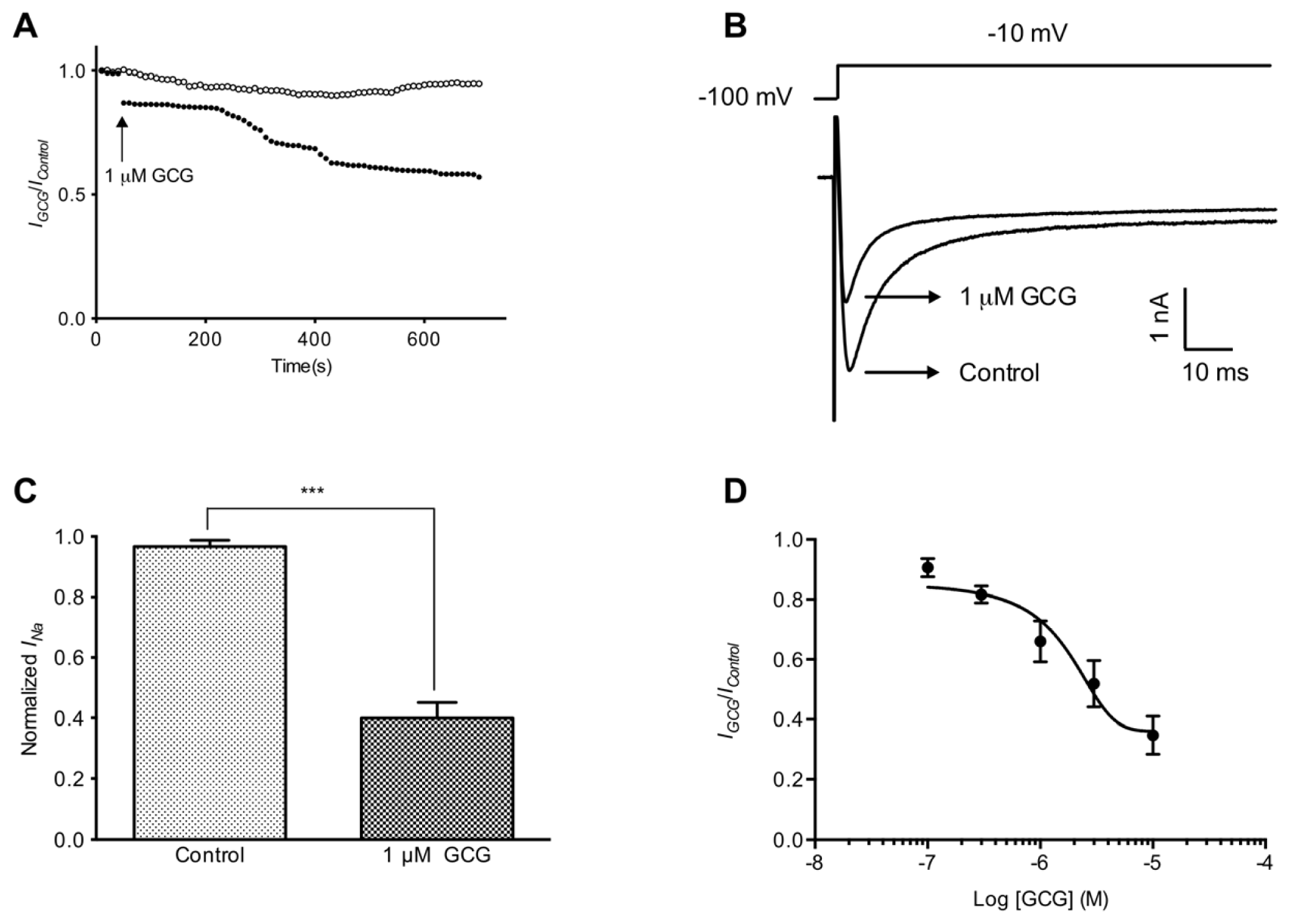
2.1. GCG Inhibits Tetrodotoxin-Resistant Na+ Currents in Dorsal Root Ganglion Neurons
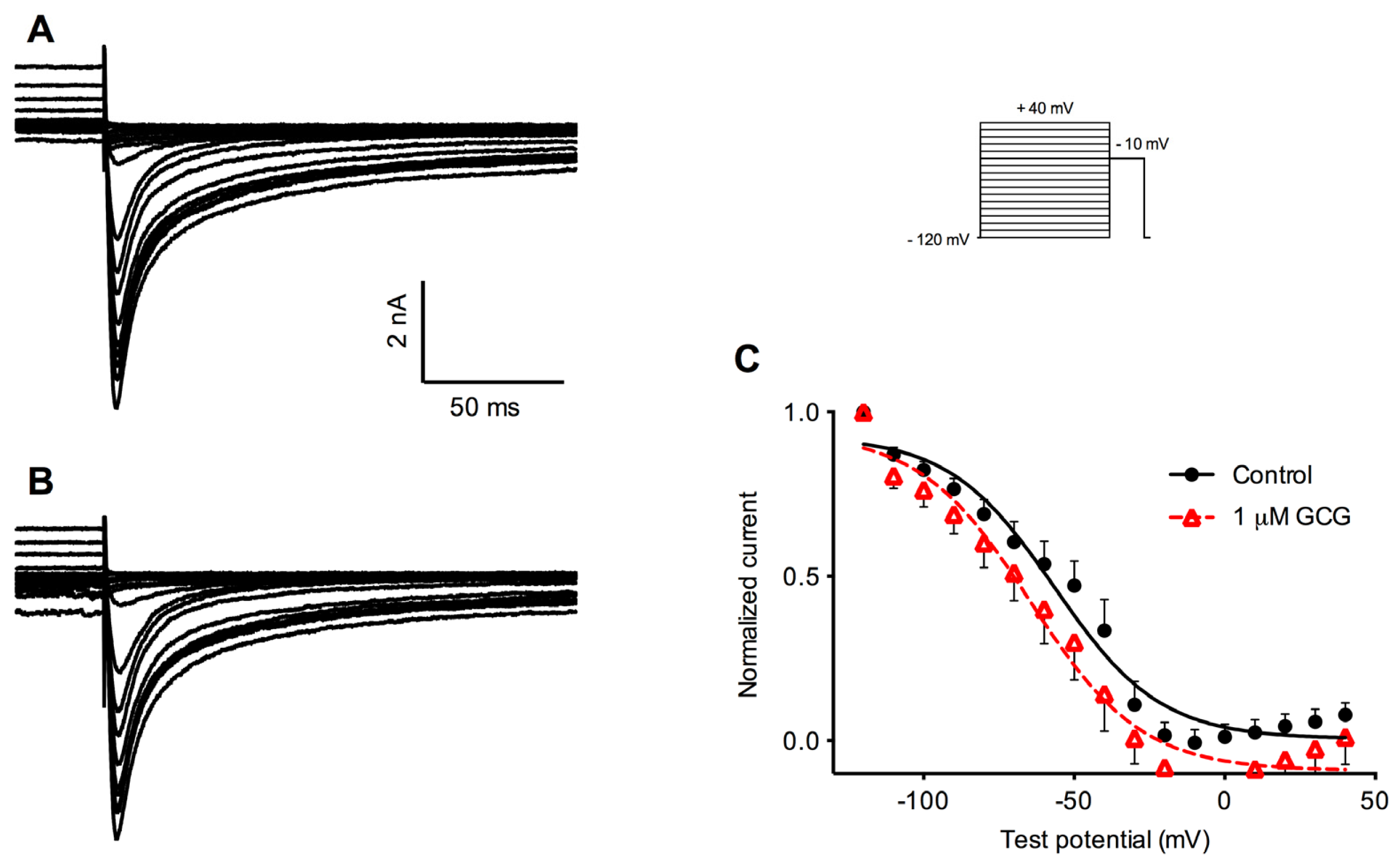
2.2. Effects of GCG on Tetrodotoxin-Resistant Na+ Current Activation
2.3. Effects of GCG on Steady-State Inactivation Tetrodotoxin-Resistant Na+ Currents
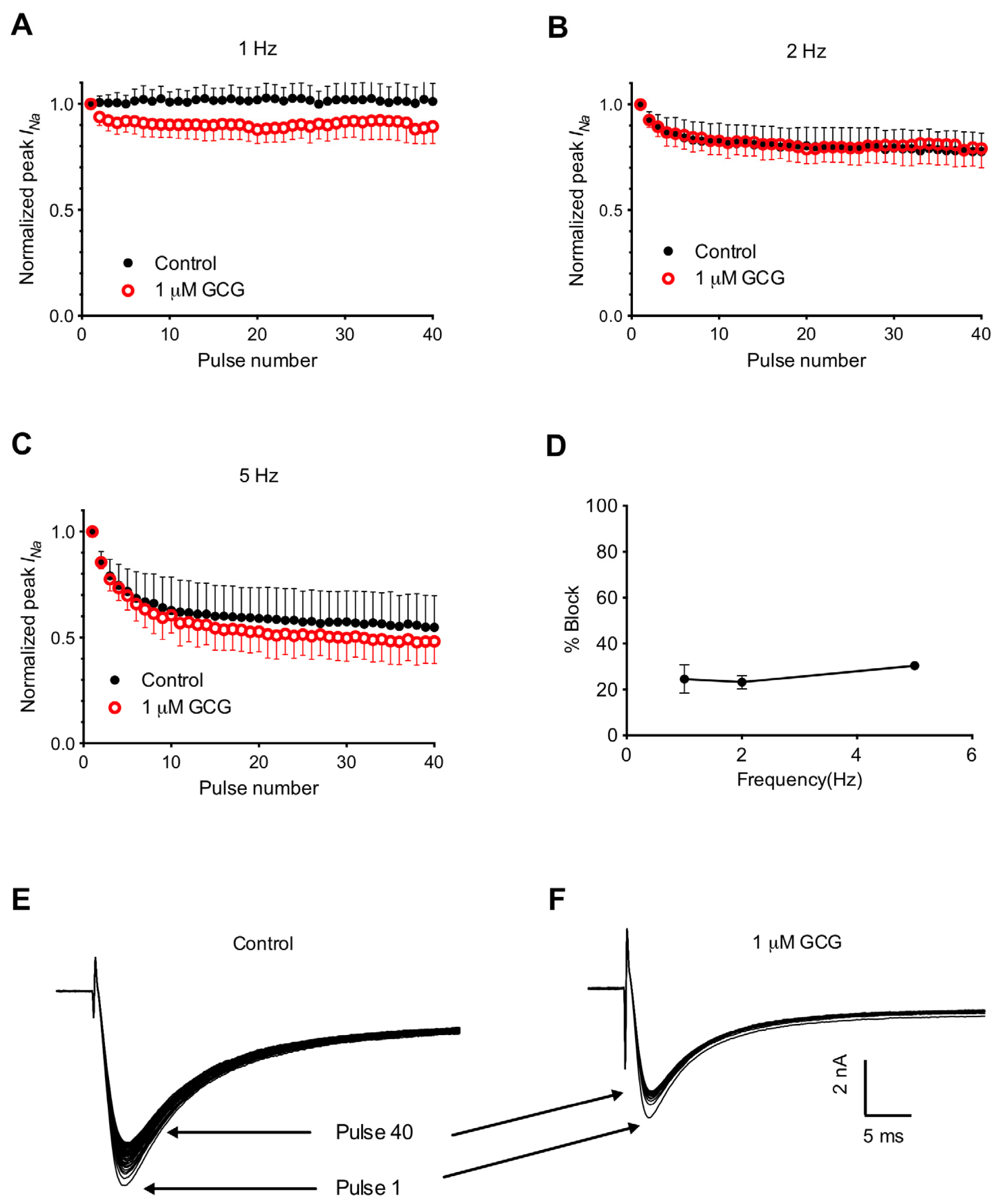
2.4. Frequency-Dependent Block of Tetrodotoxin-Resistant Na+ Currents
2.5. Discussion
3. Experimental Section
3.1. Dissociation and Culture of Dorsal Root Ganglion Neurons
3.2. Electrophysiology
3.3. Statistical Analysis
4. Conclusions
Acknowledgements
Conflict of Interest
References
- Kaur, S.; Anurag, A.; Tirkey, N.; Chopra, K. Reversal of LPS-induced central and peripheral hyperalgesia by green tea extract. Phytother. Res 2005, 19, 39–43. [Google Scholar]
- Renno, W.M.; Saleh, F.; Klepacek, I.; Al-Khaledi, G.; Ismael, H.; Asfar, S. Green tea pain modulating effect in sciatic nerve chronic constriction injury rat model. Nutr. Neurosci 2006, 9, 41–47. [Google Scholar]
- Choi, J.I.; Kim, W.M.; Lee, H.G.; Kim, Y.O.; Yoon, M.H. Role of neuronal nitric oxide synthase in the antiallodynic effects of intrathecal EGCG in a neuropathic pain rat model. Neurosci. Lett 2012, 510, 53–57. [Google Scholar]
- Kuang, X.; Huang, Y.; Gu, H.F.; Zu, X.Y.; Zou, W.Y.; Song, Z.B.; Guo, Q.L. Effects of intrathecal epigallocatechin gallate, an inhibitor of Toll-like receptor 4, on chronic neuropathic pain in rats. Eur. J. Pharmacol 2012, 676, 51–56. [Google Scholar]
- Hotta, Y.; Huang, L.; Muto, T.; Yajima, M.; Miyazeki, K.; Ishikawa, N.; Fukuzawa, Y.; Wakida, Y.; Tushima, H.; Ando, H.; et al. Positive inotropic effect of purified green tea catechin derivative in guinea pig hearts: The measurements of cellular Ca2+ and nitric oxide release. Eur. J. Pharmacol 2006, 552, 123–130. [Google Scholar]
- Ikeda, I.; Kobayashi, M.; Hamada, T.; Tsuda, K.; Goto, H.; Imaizumi, K.; Nozawa, A.; Sugimoto, A.; Kakuda, T. Heat-epimerized tea catechins rich in gallocatechin gallate and catechin gallate are more effective to inhibit cholesterol absorption than tea catechins rich in epigallocatechin gallate and epicatechin gallate. J. Agric. Food Chem 2003, 51, 7303–7307. [Google Scholar]
- Peng, L.; Khan, N.; Afaq, F.; Ye, C.; Mukhtar, H. In vitro and in vivo effects of water extract of white cocoa tea (Camellia ptilophylla) against human prostate cancer. Pharm. Res 2010, 27, 1128–1137. [Google Scholar]
- Waxman, S.G.; Cummins, T.R.; Dib-Hajj, S.; Fjell, J.; Black, J.A. Sodium channels, excitability of primary sensory neurons, and the molecular basis of pain. Muscle Nerve 1999, 22, 1177–1187. [Google Scholar]
- Leo, S.; D’Hooge, R.; Meert, T. Exploring the role of nociceptor-specific sodium channels in pain transmission using Nav1.8 and Nav1.9 knockout mice. Behav. Brain Res 2010, 208, 149–157. [Google Scholar]
- Kim, T.H.; Lim, J.M.; Kim, S.S.; Kim, J.; Park, M.; Song, J.H. Effects of (−)-epigallocatechin-3-gallate on Na(+) currents in rat dorsal root ganglion neurons. Eur. J. Pharmacol 2009, 604, 20–26. [Google Scholar]
- Deng, H.M.; Yin, S.T.; Yan, D.; Tang, M.L.; Li, C.C.; Chen, J.T.; Wang, M.; Ruan, D.Y. Effects of EGCG on voltage-gated sodium channels in primary cultures of rat hippocampal CA1 neurons. Toxicology 2008, 252, 1–8. [Google Scholar]
- Weiser, T.; Wilson, N. Inhibition of tetrodotoxin (TTX)-resistant and TTX-sensitive neuronal Na(+) channels by the secretolytic ambroxol. Mol. Pharmacol 2002, 62, 433–438. [Google Scholar]
- Dessaint, J.; Yu, W.; Krause, J.E.; Yue, L. Yohimbine inhibits firing activities of rat dorsal root ganglion neurons by blocking Na+ channels and vanilloid VR1 receptors. Eur. J. Pharmacol 2004, 485, 11–20. [Google Scholar]
- Kelemen, K.; Kiesecker, C.; Zitron, E.; Bauer, A.; Scholz, E.; Bloehs, R.; Thomas, D.; Greten, J.; Remppis, A.; Schoels, W.; et al. Green tea flavonoid epigallocatechin-3-gallate (EGCG) inhibits cardiac hERG potassium channels. Biochem. Biophys. Res. Commun 2007, 364, 429–435. [Google Scholar]
- Kang, J.; Cheng, H.; Ji, J.; Incardona, J.; Rampe, D. In vitro electrocardiographic and cardiac ion channel effects of (−)-epigallocatechin-3-gallate, the main catechin of green tea. J. Pharmacol. Exp. Ther 2010, 334, 619–626. [Google Scholar]
- Hirai, M.; Hotta, Y.; Ishikawa, N.; Wakida, Y.; Fukuzawa, Y.; Isobe, F.; Nakano, A.; Chiba, T.; Kawamura, N. Protective effects of EGCg or GCg, a green tea catechin epimer, against postischemic myocardial dysfunction in guinea-pig hearts. Life Sci 2007, 80, 1020–1032. [Google Scholar]
- Lee, S.M.; Kim, C.W.; Kim, J.K.; Shin, H.J.; Baik, J.H. GCG-rich tea catechins are effective in lowering cholesterol and triglyceride concentrations in hyperlipidemic rats. Lipids 2008, 43, 419–429. [Google Scholar]
- Masukawa, Y.; Matsui, Y.; Shimizu, N.; Kondou, N.; Endou, H.; Kuzukawa, M.; Hase, T. Determination of green tea catechins in human plasma using liquid chromatography-electrospray ionization mass spectrometry. J. Chromatogr. B 2006, 834, 26–34. [Google Scholar]
- Kotani, A.; Miyashita, N.; Kusu, F. Determination of catechins in human plasma after commercial canned green tea ingestion by high-performance liquid chromatography with electrochemical detection using a microbore column. J. Chromatogr. B 2003, 788, 269–275. [Google Scholar]
- Chow, H.H.; Hakim, I.A.; Vining, D.R.; Crowell, J.A.; Ranger-Moore, J.; Chew, W.M.; Celaya, C.A.; Rodney, S.R.; Hara, Y.; Alberts, D.S. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals. Clin. Cancer Res 2005, 11, 4627–4633. [Google Scholar]
- Xu, J.Z.; Yeung, S.Y.; Chang, Q.; Huang, Y.; Chen, Z.Y. Comparison of antioxidant activity and bioavailability of tea epicatechins with their epimers. Br. J. Nutr 2004, 91, 873–881. [Google Scholar]

© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, Y.; Jia, Y.-Y.; Guo, J.-L.; Liu, P.-Q.; Jiang, J.-M. Effects of (−)-Gallocatechin-3-Gallate on Tetrodotoxin-Resistant Voltage-Gated Sodium Channels in Rat Dorsal Root Ganglion Neurons. Int. J. Mol. Sci. 2013, 14, 9779-9789. https://doi.org/10.3390/ijms14059779
Zhang Y, Jia Y-Y, Guo J-L, Liu P-Q, Jiang J-M. Effects of (−)-Gallocatechin-3-Gallate on Tetrodotoxin-Resistant Voltage-Gated Sodium Channels in Rat Dorsal Root Ganglion Neurons. International Journal of Molecular Sciences. 2013; 14(5):9779-9789. https://doi.org/10.3390/ijms14059779
Chicago/Turabian StyleZhang, Yan, Yan-Yan Jia, Jin-Lei Guo, Pei-Qing Liu, and Jian-Min Jiang. 2013. "Effects of (−)-Gallocatechin-3-Gallate on Tetrodotoxin-Resistant Voltage-Gated Sodium Channels in Rat Dorsal Root Ganglion Neurons" International Journal of Molecular Sciences 14, no. 5: 9779-9789. https://doi.org/10.3390/ijms14059779