Synthesis, Cytotoxicity, DNA Binding and Apoptosis of Rhein-Phosphonate Derivatives as Antitumor Agents
Abstract
:1. Introduction
2. Results and Discussion
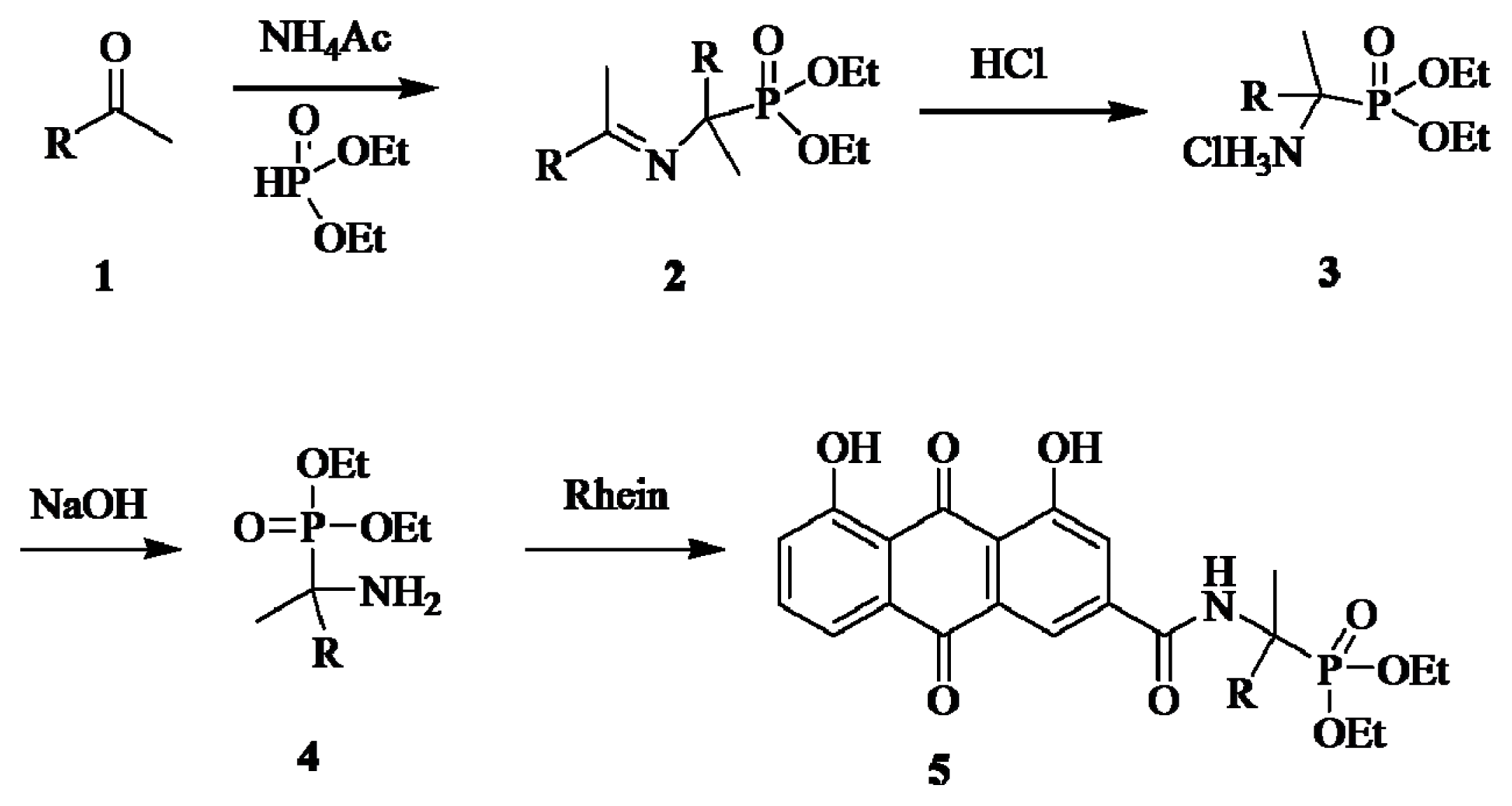
2.1. Chemistry
2.2. Cytotoxicity Effects
2.3. DNA Binding
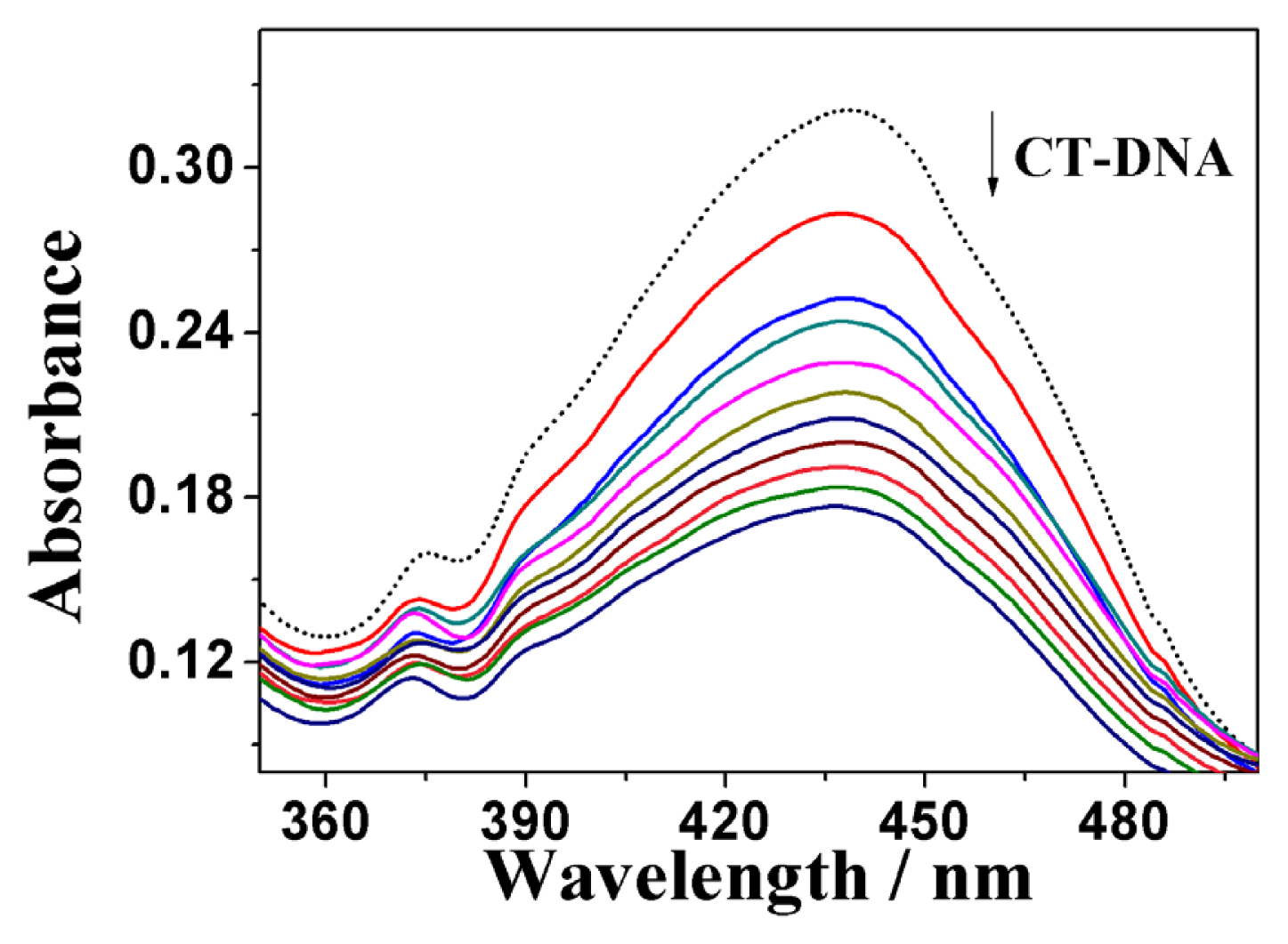
2.3.1. UV-Vis Absorption Spectral Analysis
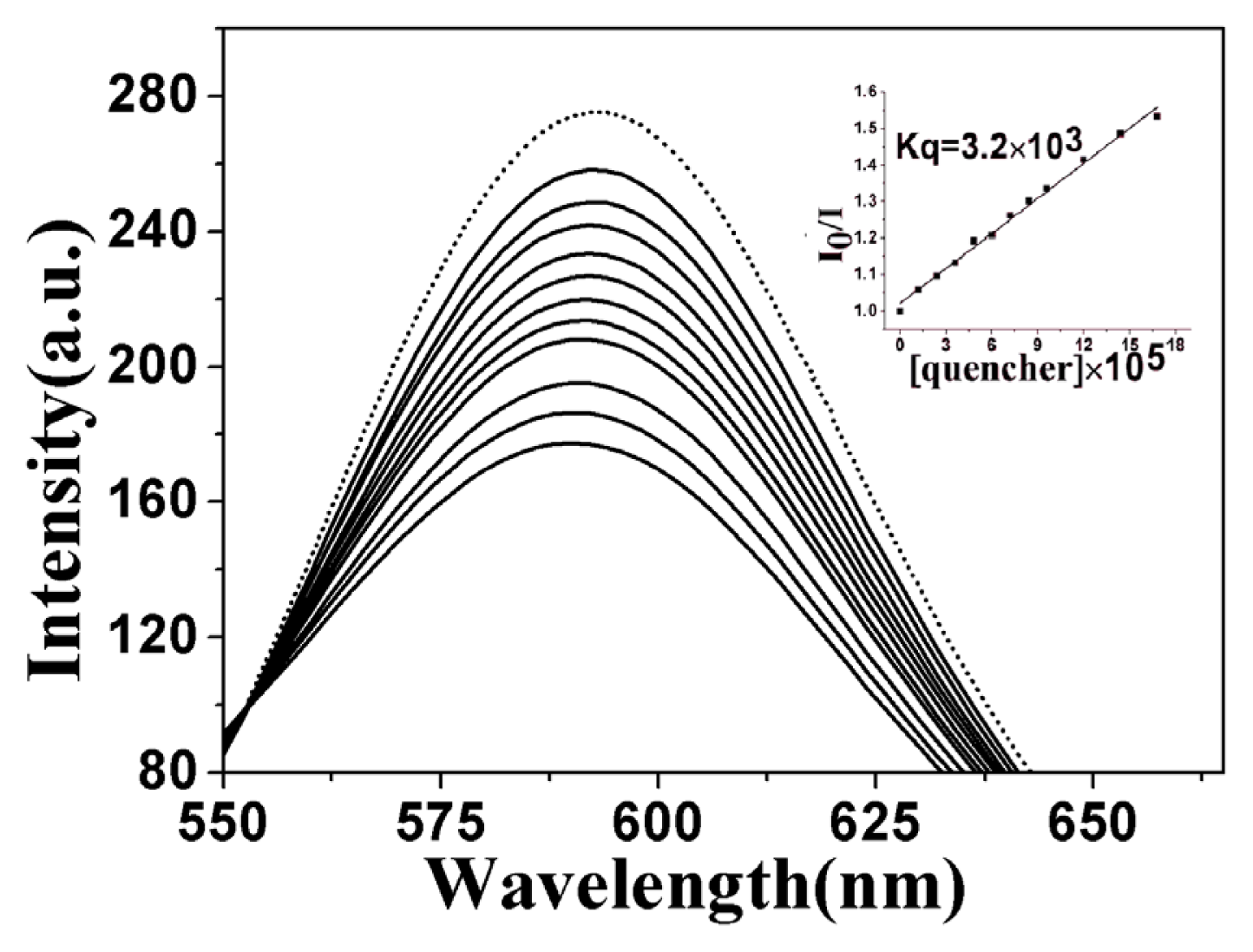
2.3.2. Fluorescence Emission Titration
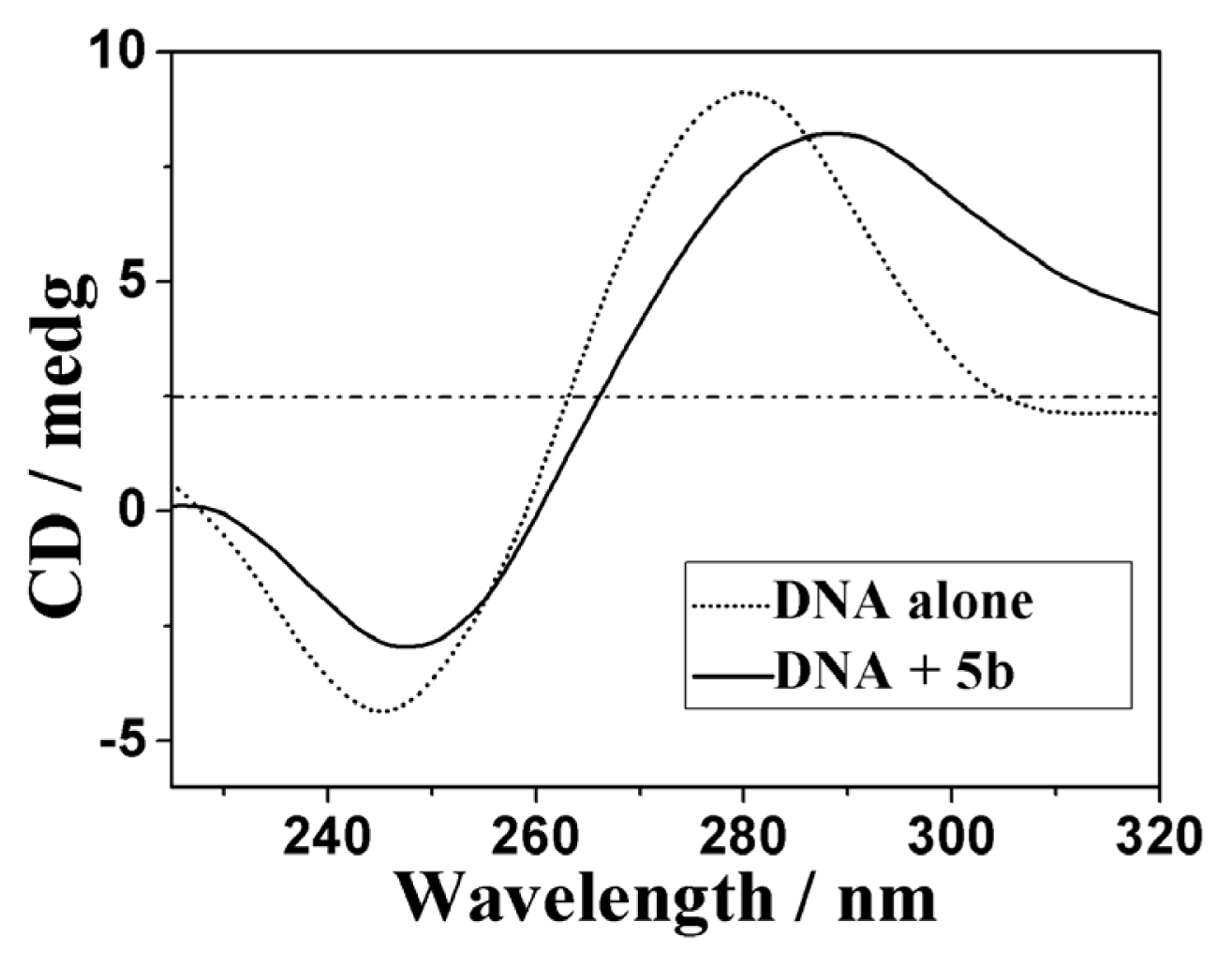
2.3.3. Circular Dichroism Spectra
2.4. Apoptosis and Cell Cycle Analysis
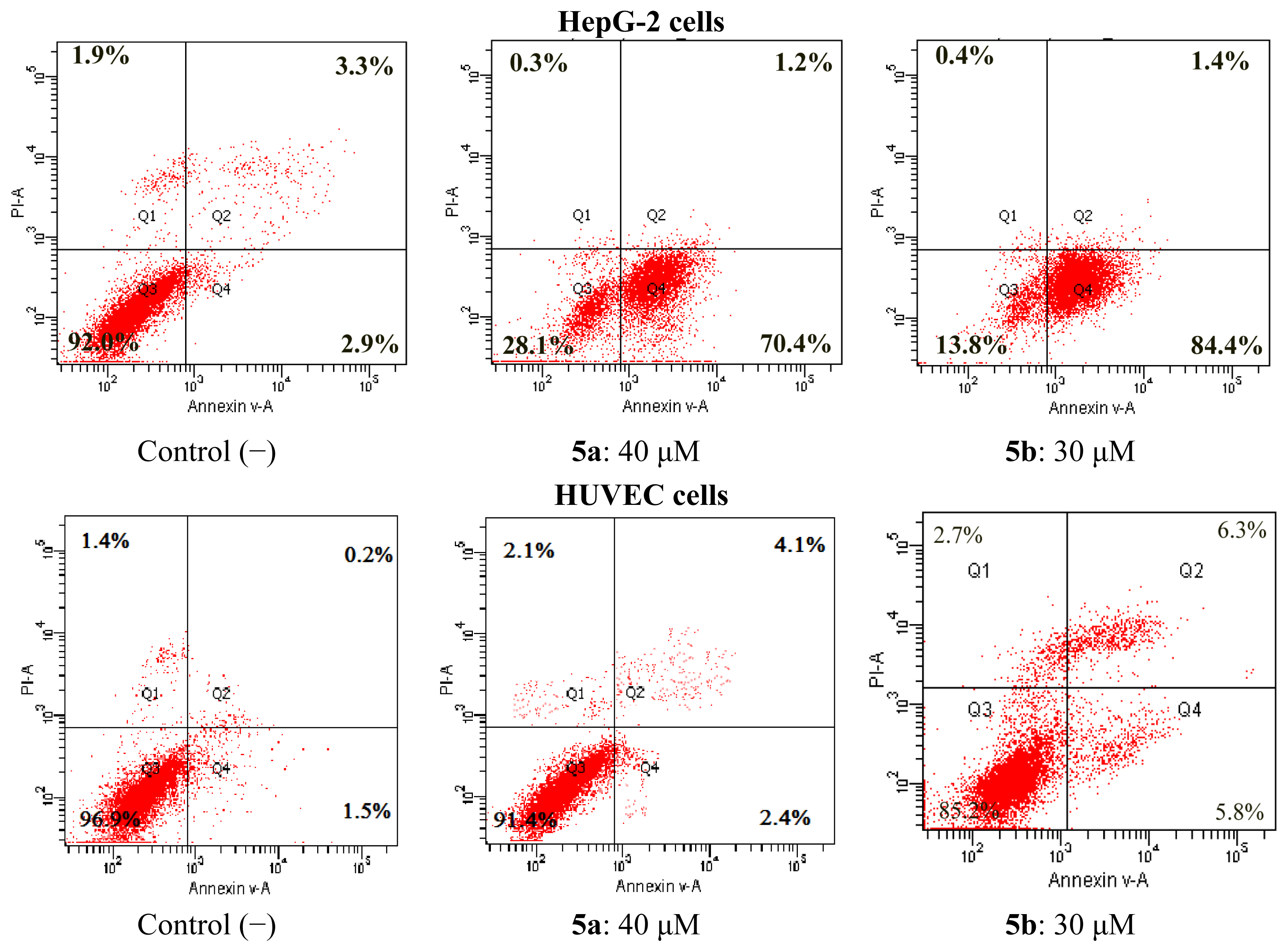
2.4.1. Apoptosis
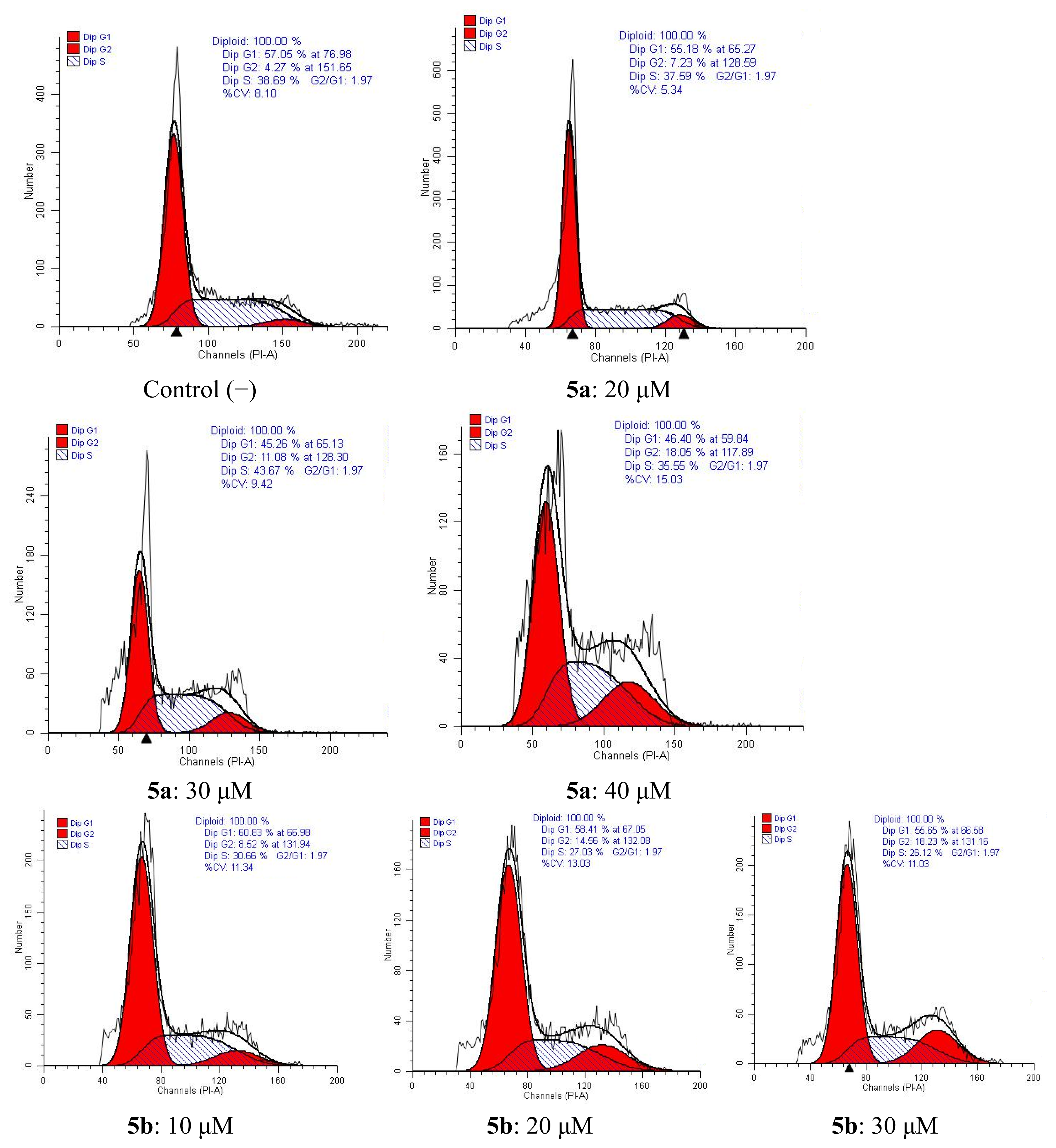
2.4.2. Cell Cycle Analysis
3. Experimental Section
3.1. Chemistry
3.2. Biological Assays
3.2.1. Cytotoxicity of Rhein Derivatives
3.2.1.1. Cell Lines
3.2.1.2. Cell Culture
3.2.1.3. MTT Assay
3.2.2. Determination of Apoptosis and Cell Cycle Analysis
3.2.2.1. Apoptosis Analysis
3.2.2.2. Cell Cycle Analysis
3.2.3. Spectroscopic Studies on DNA Interaction
3.3. Statistics
4. Conclusions
Supplementary Information
ijms-14-09424-s001.pdfAcknowledgments
Conflict of Interest
References
- Mandhanea, P.G.; Joshia, R.S.; Nagargojea, D.R.; Chatea, A.V.; Gilla, C.H. Ultrasonic promoted synthesis and antibacterial screening of some novel piperidine incorporated α-aminophosphonates. Phosphorus Sulfur Silicon Relat. Elem 2010, 186, 149–158. [Google Scholar]
- Reddy, S.S.; Rao, V.K.; Krishna, B.S.; Reddy, C.S.; Visweswara, P.R.; Raju, C.N. Synthesis, antimicrobial, and antioxidant activity of new α-aminophosphonates. Phosphorus Sulfur Silicon Relat. Elem 2011, 186, 1411–1421. [Google Scholar]
- Liu, W.; Rogers, C.J.; Fisher, A.J.; Toney, M.D. Aminophosphonate inhibitors of dialkylg-lycine decarboxylase: Structural basis for slow binding inhibition. Biochemistry 2002, 41, 12320–12328. [Google Scholar]
- Kafarski, P.; Lejczak, B. Aminophosphonic acids of potential medical importance. Curr. Med. Chem. Anticancer Agents 2001, 1, 301–312. [Google Scholar]
- Jin, L.H.; Song, B.A.; Zhang, G.P.; Xu, R.Q.; Zhang, S.M.; Gao, X.W.; Hu, D.Y.; Yang, S. Synthesis, X-ray crystallographic analysis, and antitumor activity of N-(benzothiazole-2-yl)-1-(fluorophenyl)-O,O-dialkyl-α-aminophosphonates. Bioorg. Med. Chem. Lett 2006, 16, 1537–1543. [Google Scholar]
- Kudzin, Z.H.; Kudzin, M.H.; Drabowicz, J.; Stevens, C.V. Aminophosphonic acids—Phosphorus analogues of natural amino acids. Part 1: Syntheses of α-aminophosphonic acids. Curr. Org. Chem 2011, 15, 2015–2071. [Google Scholar]
- Orsini, F.; Sello, G.; Sisti, M. Aminophosphonic acids and derivatives. Synthesis and biological applications. Curr. Med. Chem 2010, 17, 264–289. [Google Scholar]
- Kukhar, V.P.; Hudson, H.R. Aminophosphonic and Aminophosphinic Acids: Chemistry and Biological Activity. In Aminophosphonic and Aminophosphinic Acids: Chemistry and Biological Activity; John Wiley: New York, NY, USA, 2000. [Google Scholar]
- Naydenova, E.; Troev, K.; Topashka-Ancheva, M.; Hägele, G.; Ivanov, I.; Kril, A. Synthesis, cytotoxicity and clastogenicity of novel α-aminophosphonic acids. Amino Acids 2007, 33, 695–702. [Google Scholar]
- Naydenova, E.D.; Todorov, P.T.; Troev, K.D. Recent synthesis of aminophosphonic acids as potential biological importance. Amino Acids 2010, 38, 23–30. [Google Scholar]
- Flors, V.; Miralles, C.; Gonzalez-Bosch, C.; Carda, M.; Garcia-Agustin, P. Molecular cloning and characterization of an intestinal cathepsin L protease from the plant-parasitic nematode Meloidogyne incognita. Physiol. Mol. Plant Pathol 2003, 63, 151–158. [Google Scholar]
- Paula, V.F.; Barbosa, L.C.; Demuner, A.J.; Piló-Veloso, D.; Picanço, M.C. Synthesis and insecticidal activity of new amide derivatives of piperine. Pest Manag. Sci 2000, 56, 168–174. [Google Scholar]
- Lamberth, C.; Kempf, H.J.; Kriz, M. Synthesis and fungicidal activity of N-2-(3-methoxy-4-propargyloxy) phenethyl amides. Part 3: Stretched and heterocyclic mandelamide oomyceticides. Pest Manag. Sci 2007, 63, 57–62. [Google Scholar]
- Jennings, L.D.; Rayner, D.R.; Jordan, D.B.; Okonys, J.F.; Basarab, G.S.; Amorose, D.K.; Anaclerio, B.M.; Lee, J.K. Cyclobutane carboxamide inhibitors of fungal melanin: Biosynthesis and their evaluation as fungicides. Bioorg. Med. Chem 2000, 8, 897–907. [Google Scholar]
- Navickiene, H.M.D.; Miranda, J.E.; Bortoli, S.A.; Kato, M.J.; Bolzani, V.S.; Furlan, M. Toxicity of extracts and isobutyl amides from Piper tuberculatum: Potent compounds with potential for the control of the velvetbean caterpillar, Anticarsia gemmatalis. Pest Manag. Sci 2007, 63, 399–403. [Google Scholar]
- Caldweu, C.G.; Sahoo, S.P.; Polo, S.A.; Eversole, R.R.; Lanza, T.J.; Mills, S.G.; Niedzwiecki, L.M.; Izquierdo-Martin, M.; Chang, B.C.; Harrison, R.K.; et al. Phosphinic acid inhibitors of matrix metalloproteinases. Bioorg. Med. Chem. Lett 1996, 6, 323–328. [Google Scholar]
- Bianchini, G.; Aschi, M.; Cavicchio, G.; Crucianelli, M.; Preziuso, S.; Gallina, C.; Nastari, A.; Gavuzzo, E.; Mazza, F. Design, modelling, synthesis and biological evaluation of peptidomi-metic phosphinates as inhibitors of matrix metalloproteinases MMP-2 and MMP-8. Bioorg. Med. Chem 2005, 13, 4740–4749. [Google Scholar]
- Pochetti, G.; Gavuzzo, E.; Campestre, C.; Agamennone, M.; Tortorella, P.; Consalvi, V.; Carlo, G.; Hiller, O.; Tschesche, H.; Tucker, P.A.; et al. Structural insight into the stereo-selective inhibition of MMP-8 by enantiomeric sulfonamide phosphonates. J. Med. Chem 2006, 49, 923–931. [Google Scholar]
- Naydenova, E.; Topashka-Ancheva, M.; Todorov, P.; Yordanova, T.; Troev, K. Novel α-aminophosphonic acids. Design, characterization, and biological activity. Bioorg. Med. Chem 2006, 14, 2190–2196. [Google Scholar]
- Naydenova, E.; Vassilev, A.; Popova, Y.; Troev, K. Phosphonylmethylaminocyclopentane-1-carboxylic acid. Heteroat. Chem 2003, 14, 229–230. [Google Scholar]
- Naydenova, E.; Todorov, P.; Topashka-Ancheva, M.; Momekov, G.; Yordanova, T.; Konstantinov, S.; Troev, K. Novel N-(phosphonomethyl) glycine derivatives: Design, characterization and biological activity. Eur. J. Med. Chem 2008, 43, 1199–1205. [Google Scholar]
- Meek, T.D.; Villafranca, J.J. Kinetic mechanism of Escherichia coli glutamine synthetase. Biochemistry 1980, 19, 5513–5519. [Google Scholar]
- Oleksyszyn, J.; Boduszek, B.; Kam, C.M.; Powers, J.C. Novel amidine-containing peptidyl phosphonates as irreversible inhibitors for blood coagulation and related serine proteases. J. Med. Chem 1994, 37, 226–231. [Google Scholar]
- Logusch, E.W.; Walker, D.M.; McDonald, J.F.; Franz, J.E.; Villafranca, J.J.; DiIanni, C.L.; Colanduoni, J.A.; Schineller, J.B. Inhibition of Escherichia coli glutamine synthetase by alpha- and gamma-substituted phosphinothricins. Biochemistry 1990, 29, 366–372. [Google Scholar]
- Kuo, R.Y.; Qian, K.; Morris-Natschke, S.L.; Lees, K.H. Plant-derived triterpenoids and analogues as antitumor and anti-HIV agents. Nat. Prod. Rep 2009, 26, 1321–1344. [Google Scholar]
- Deng, S.L.; Baglin, I.; Nour, M.; Cavé, C. Synthesis of phosphonodipeptide conjugates of ursolic acid and their homologs. Heteroat. Chem 2008, 19, 55–65. [Google Scholar]
- Deng, S.L.; Baglin, I.; Nour, M.; Flekhter, O.; Vita, C.; Cavé, C. Synthesis of ursolic phosphonate derivatives as potential anti-HIV agents. Phosphorus Sulfur Silicon Relat. Elem 2007, 182, 951–967. [Google Scholar]
- Cai, Y.; Sun, M.; Xing, J.; Corke, H. Antioxidant phenolic constituents in roots of Rheum officinale and Rubia cordifolia: Structure-radical scavenging activity relationships. J. Agric. Food Chem 2004, 52, 7884–7890. [Google Scholar]
- Gao, Q.; Qin, W.S.; Jia, Z.H.; Zheng, J.M.; Zeng, C.H.; Li, L.S.; Liu, Z.H. Rhein improves renal lesion and ameliorates dyslipidemia in db/db mice with diabetic nephropathy. Planta Med 2010, 76, 27–33. [Google Scholar]
- Tamura, T.; Shirai, T.; Kosaka, N.; Ohmori, K.; Takafumi, N. Pharmacological studies of diacerein in animal models of inflammation, arthritis and bone resorption. Eur. J. Pharmacol 2002, 448, 81–87. [Google Scholar]
- Cai, J.; Duan, Y.B.; Yu, J.; Chen, J.Q.; Chao, M.; Ji, M. Bone-targeting glycol and NSAIDS ester prodrugs of rhein: Synthesis, hydroxyapatite affinity, stability, anti-inflammatory, ulcerogenicity index and pharmacokinetics studies. Eur. J. Med. Chem 2012, 55, 409–419. [Google Scholar]
- Lin, M.L.; Chung, J.G.; Lu, Y.C.; Yang, C.Y.; Chen, S.S. Rhein inhibits invasion and migration of human nasopharyngeal carcinoma cells in vitro by down-regulation of matrix metalloproteinases-9 and vascular endothelial growth factor. Oral Oncol 2009, 45, 531–537. [Google Scholar]
- Raimondi, F.; Santoro, P.; Maiuri, L.; Londei, M.; Annunziata, S.; Ciccimarra, F. Reactive nitrogen species modulate the effects of rhein, an active component of senna laxatives, on human epithelium in vitro. J. Pediatr. Gastroenterol. Nutr 2002, 34, 529–534. [Google Scholar]
- Lin, S.; Fu, J.M.; Hou, D.X. Rhein induces apoptosis in HL-60 cells via reactive oxygen species-independent mitochondrial death pathway. Arch. Biochem. Biophys 2003, 418, 99–107. [Google Scholar]
- Ip, S.W.; Weng, Y.S.; Lin, S.Y.; Mei, D.; Tang, N.Y.; Su, C.C. The role of Ca2+ on rhein induced apoptosis in human cervical cancer Ca ski cells. Anticancer Res 2007, 27, 379–389. [Google Scholar]
- Lin, M.L.; Chen, S.S.; Lu, Y.C.; Liang, R.Y.; Ho, Y.T.; Yang, C.Y. Rhein induces apoptosis through induction of endoplasmic reticulum stress and Ca2+-dependent mitochondrial death pathway in human nasopharyngeal carcinoma cells. Anticancer Res 2007, 27, 3313–3322. [Google Scholar]
- Tarasiuk, J.; Mazerski, J.; Tkaczyk-Gobis, K.; Borowski, E. Molecular basis of the low activity of antitumor anthracenediones, mitoxantrone and ametantrone, in oxygen radical generation catalyzed by NADH dehydrogenase. Enzymatic and molecular modelling studies. Eur. J. Med. Chem 2005, 40, 321–328. [Google Scholar]
- Shchekotikhin, A.E.; Glazunova, V.A.; Dezhenkova, L.G.; Luzikov, Y.N.; Sinkevich, Y.B.; Kovalenko, L.V.; Buyanov, V.N.; Balzarini, J.; Huang, F.C.; Lin, J.J.; et al. Synthesis and cytotoxic properties of 4,11-bis(aminoethyl)aminoanthra[2,3-b]thiophene-5,10-diones, novel analogues of antitumor anthracene-9,10-diones. Bioorg. Med. Chem 2009, 17, 1861–1869. [Google Scholar]
- Mucha, A.; Kafarski, P.; Berlicki, Ł. Remarkable potential of the α-aminophosphonate/phosphinate structural motif in medicinal chemistry. J. Med. Chem 2011, 54, 5955–5980. [Google Scholar]
- Jolly, S.R.; Kane, W.J.; Bailie, M.B.; Abrams, G.D.; Lucchesiet, B.R. Canine myocardial reperfusion injury: Its reduction by the combined administration of superoxide dismutase and catalase. Circ. Res 1984, 54, 277–285. [Google Scholar]
- Omar, B.A.; Flores, S.C.; McCord, J.M. Superoxide dismutase: Pharmacological develop-ments and applications. Adv. Pharmacol 1992, 21, 109–161. [Google Scholar]
- Kaboudin, B.; Moradi, K. A simple and convenient procedure for the synthesis of 1-amino-phosphonates from aromatic aldehydes. Tetrahedron Lett 2005, 46, 2989–2991. [Google Scholar]
- Sara, S.; Zahra, T. Al(OTf)3 as an efficient catalyst for one-pot synthesis of primary diethyl 1-aminophosphonates under solvent-free conditions. Synth. Commun 2008, 39, 120–131. [Google Scholar]
- Sharma, R.I.; Smith, T.A.D. Colorectal tumor cells treated with 5-FU, oxaliplatin, irinotecan, and cetuximab exhibit changes in 18F-FDG incorporation corresponding to hexokinase activity and glucose transport. J. Nucl. Med 2008, 49, 1386–1394. [Google Scholar]
- Cashman, D.J.; Kellogg, G.E. A computational model for anthracycline binding to DNA: Tuning groove-binding intercalators for specific sequences. J. Med. Chem 2004, 47, 1360–1374. [Google Scholar]
- Tan, J.H.; Zhang, Q.X.; Huang, Z.S.; Chen, Y.; Wang, X.D.; Gu, L.Q.; Wu, J.Y. Synthesis, DNA binding and cytotoxicity of new pyrazole emodin derivatives. Eur. J. Med. Chem 2006, 41, 1041–1047. [Google Scholar]
- Dutta, S.; Abe, H.; Aoyagi, S.; Kibayashi, C.; Gates, K.S. DNA damage by fasicularin. J. Am. Chem. Soc 2005, 127, 15004–15005. [Google Scholar]
- Tanaka, K.; Yamada, Y.; Shionoya, M. Formation of silver(I)-mediated DNA duplex and triplex through an alternative base pair of pyridine nucleobases. J. Am. Chem. Soc 2002, 124, 8802–8803. [Google Scholar]
- Monneret, C. Recent developments in the field of antitumour anthracyclines. Eur. J. Med. Chem 2001, 36, 483–493. [Google Scholar]
- Carter, M.T.; Rodriguez, M.; Bard, A.J. Voltammetric studies of the interaction of metal chelates with DNA. 2. Tris-chelated complexes of cobalt(III) and iron(II) with 1,10-phenanthroline and 2,2′-bipyridine. J. Am. Chem. Soc 1989, 111, 8901–8911. [Google Scholar]
- Liu, X.W.; Li, J.; Li, H.; Zheng, K.C.; Chao, H.; Ji, L.N. Synthesis, characterization, DNA-binding and photocleavage of complexes Ru(phen)2(6-OH-dppz)2+ and Ru(phen)2(6-NO2-dppz)2+. J. Inorg. Biochem 2005, 99, 2372–2380. [Google Scholar]
- Liu, M.; Zhao, H.M.; Chen, S.; Yu, H.T.; Zhang, Y.B.; Quan, X. Label-free fluorescent detection of Cu(II) ions based on DNA cleavage-dependent graphene-quenched DNAzymes. Chem. Commun 2011, 47, 7749–7751. [Google Scholar]
- Zhang, G.; Guo, J.; Zhao, N.; Wang, J. Study of interaction between kaempferol-Eu3+ complex and DNA with the use of the neutral red dye as a fluorescence probe. Sens. Actuators B Chem 2010, 144, 239–246. [Google Scholar]
- Mahadevan, S.; Palaniandavar, M. Spectroscopic and voltammetric studies on copper complexes of 2,9-dimethyl-1,10-phenanthrolines bound to calf thymus DNA. Inorg. Chem 1998, 37, 693–700. [Google Scholar]
- Rajendiran, V.; Murali, M.; Suresh, E.; Palaniandavar, M.; Periasamy, V.S.; Akbarsha, M.A. Non-covalent DNA binding and cytotoxicity of certain mixed-ligand ruthenium(II) complexes of 2,2-dipyridylamine and diimines. Dalton Trans. 2008, 2157–2170. [Google Scholar]
- Schäfe, S.; Gust, I.; Ott, R.; Sheldrick, W.S. Influence of the polypyridyl (pp) ligand size on the DNA binding properties, cytotoxicity and cellular uptake of organoruthenium(II) complexes of the type (η6-C6Me6) Ru(L)(pp)n+ L = Cl, n = 1; L = (NH2)2CS, n = 2. Eur. J. Inorg. Chem 2007, 19, 3034–3046. [Google Scholar]
- Xu, H.; Zheng, K.C.; Chen, Y.; Li, Y.Z.; Lin, L.J.; Li, H.; Zhang, P.X.; Ji, L.N. Effects of ligand planarity on the interaction of polypyridyl Ru(II) complexes with DNA. Dalton Trans. 2003, 2260–2268. [Google Scholar]
- Ghosh, S.; Barve, A.C.; Kumbhar, A.A.; Kumbhar, A.S.; Puranik, V.G.; Datar, P.A.; Sonawane, U.B.; Joshi, R.R. Synthesis, characterization, X-ray structure and DNA photocleavage by cis-dichloro bis(diimine) Co(III) complexes. J. Inorg. Biochem 2006, 100, 331–343. [Google Scholar]
| Compd. | R | HepG-2 | CNE | Spca-2 | Hela | Hct-116 | HUVEC |
|---|---|---|---|---|---|---|---|
| 5a | p-Ph-CH3 | 15.11 ± 1.54 | 33.75 ± 2.17 | 16.94 ± 1.32 | >50 | 30.64 ± 8.75 | >100 |
| 5b | Ethylbenzene | 8.82 ± 0.95 | 27.27 ± 3.78 | 9.01 ± 0.87 | 45.36 | 12.66 ± 1.50 | >100 |
| 5c | Ph | 18.23 ± 1.87 | 38.34 ± 8.23 | 25.12 ± 2.72 | >50 | 23.44 ± 3.22 | >100 |
| Rhein | 38.34 ± 6.34 | >50 | 28.31 ± 1.40 | >50 | >50 | 79.74 ± 5.40 | |
| 5-Fu | 20.30 ± 2.43 | >50 | No Date | >50 | 4.3 ± 0.52 [44] | No Date |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ye, M.-Y.; Yao, G.-Y.; Wei, J.-C.; Pan, Y.-M.; Liao, Z.-X.; Wang, H.-S. Synthesis, Cytotoxicity, DNA Binding and Apoptosis of Rhein-Phosphonate Derivatives as Antitumor Agents. Int. J. Mol. Sci. 2013, 14, 9424-9439. https://doi.org/10.3390/ijms14059424
Ye M-Y, Yao G-Y, Wei J-C, Pan Y-M, Liao Z-X, Wang H-S. Synthesis, Cytotoxicity, DNA Binding and Apoptosis of Rhein-Phosphonate Derivatives as Antitumor Agents. International Journal of Molecular Sciences. 2013; 14(5):9424-9439. https://doi.org/10.3390/ijms14059424
Chicago/Turabian StyleYe, Man-Yi, Gui-Yang Yao, Jing-Chen Wei, Ying-Ming Pan, Zhi-Xin Liao, and Heng-Shan Wang. 2013. "Synthesis, Cytotoxicity, DNA Binding and Apoptosis of Rhein-Phosphonate Derivatives as Antitumor Agents" International Journal of Molecular Sciences 14, no. 5: 9424-9439. https://doi.org/10.3390/ijms14059424
APA StyleYe, M.-Y., Yao, G.-Y., Wei, J.-C., Pan, Y.-M., Liao, Z.-X., & Wang, H.-S. (2013). Synthesis, Cytotoxicity, DNA Binding and Apoptosis of Rhein-Phosphonate Derivatives as Antitumor Agents. International Journal of Molecular Sciences, 14(5), 9424-9439. https://doi.org/10.3390/ijms14059424




