The Role of Strigolactones in Nutrient-Stress Responses in Plants
Abstract
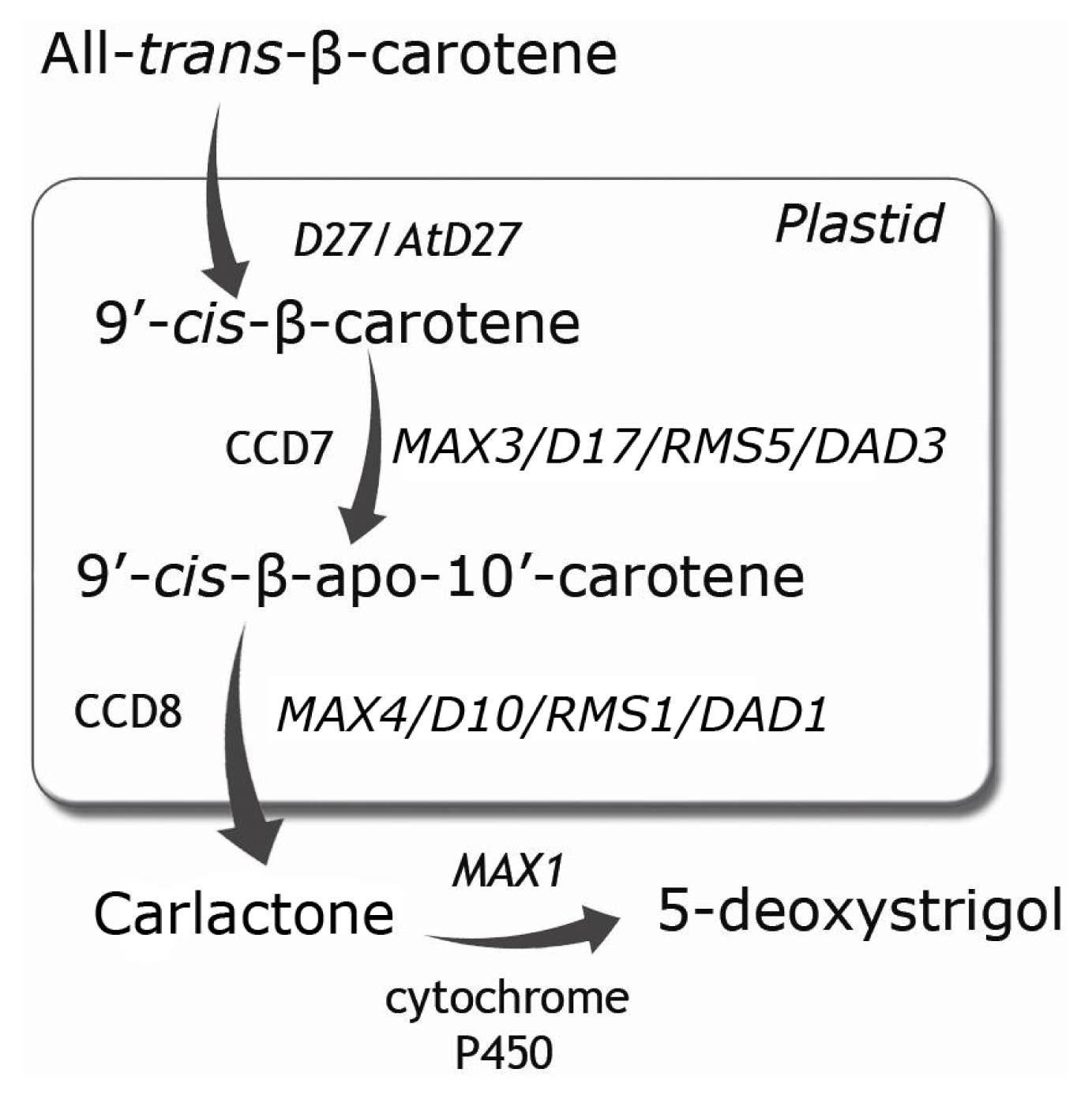
:1. Introduction
2. SLs Biosynthesis and Exudation under Nutrient Stress Conditions
3. SLs Regulate the Above-Ground Architecture of Plants during a Response to Nutrient Stress Conditions
4. SLs Regulate Root Development under Nutrient Stress Conditions
5. SLs Regulate the Expression of PSI Genes
6. SLs Secretion in Interactions with Fungi in the Nutrient Stress Response
7. SLs Secretion in Interactions with Bacteria in the Nutrient Stress Response
8. SLs in Responses to Other Stresses
9. Conclusions and Perspectives
Acknowledgments
Conflict of Interest
References
- Cook, C.E.; Whichard, L.P.; Turner, B.; Wall, M.E.; Egley, G.H. Germination of witchweed (Striga luteaLour.): Isolation and properties of a potent stimulant. Science 1966, 23753, 1189–1190. [Google Scholar]
- Akiyama, K.; Matsuzaki, K.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005, 7043, 824–827. [Google Scholar]
- Yoneyama, K.; Xie, X.; Sekimoto, H.; Takeuchi, Y.; Ogasawara, S.; Akiyama, K.; Hayashi, H.; Yoneyama, K. Strigolactones, host recognition signals for root parasitic plants and arbuscular mycorrhizal fungi, from Fabaceae plants. New Phytol 2008, 179, 484–494. [Google Scholar]
- Gomez-Roldan, V.; Fermas, S.; Brewer, P.B.; Puech-Pagès, V.; Dun, E.A.; Pillot, J.P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.C.; et al. Strigolactone inhibition of shoot branching. Nature 2008, 455, 189–194. [Google Scholar]
- Umehara, M.; Hanada, A.; Yoshida, S.; Akiyama, K.; Arite, T.; Takeda-Kamiya, N.; Magome, H.; Kamiya, Y.; Shirasu, K.; Yoneyama, K.; et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature 2008, 455, 195–200. [Google Scholar]
- Brewer, P.B.; Dun, E.A.; Ferguson, B.J.; Rameau, C.; Beveridge, C.A. Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol 2009, 150, 482–493. [Google Scholar]
- Ferguson, B.J.; Beveridge, C.A. Roles for auxin, cytokinin and strigolactone in regulating shoot branching. Plant Physiol 2009, 149, 1929–1944. [Google Scholar]
- Hayward, A.; Stirnberg, P.; Beveridge, C.; Leyser, O. Interactions between auxin and strigolactone in shoot branching control. Plant Physiol 2009, 151, 400–412. [Google Scholar]
- Koltai, H.; Dor, E.; Hershenhorn, J.; Joel, D.M.; Weininger, S.; Lekalla, S.; Shealtiel, H.; Bhattacharya, C.; Eliahu, E.; Resnick, N.; et al. Strigolactones’ effect on root growth and root-hair elongation may be mediated by auxin-efflux carriers. J. Plant Growth Regul 2010, 29, 129–136. [Google Scholar]
- Arite, T.; Kameoka, H.; Kyozuka, J. Strigolactone positively controls crown root elongation in rice. J. Plant Growth Regul 2012, 31, 165–172. [Google Scholar]
- Kapulnik, Y.; Delaux, P.M.; Resnick, N.; Mayzlish-Gati, E.; Wininger, S.; Bhattacharya, C.; Séjalon-Delmas, N.; Combier, J.P.; Bécard, G.; Belausov, E.; et al. Strigolactones affect lateral root formation and root-hair elongation in Arabidopsis. Planta 2011, 233, 209–216. [Google Scholar]
- Koltai, H. Strigolactones are regulators of root development. New Phytol 2011, 190, 545–549. [Google Scholar]
- Hu, Z.; Yan, H.; Yang, J.; Yamaguchi, S.; Maekawa, M.; Takamure, I.; Tsutsumi, N.; Kyozuka, J.; Nakazono, M. Strigolactones negatively regulate mesocotyl elongation in rice during germination and growth in darkness. Plant Cell Physiol 2010, 51, 1136–1142. [Google Scholar]
- Foo, E.; Davies, N.W. Strigolactones promote nodulation in pea. Planta 2011, 234, 1073–1081. [Google Scholar]
- Delaux, P.M.; Xie, X.; Timme, R.E.; Puech-Pages, V.; Dunand, C.; Lecompte, E.; Delwiche, C.F.; Yoneyama, K.; Bécard, G.; Séjalon-Delmas, N. Origin of strigolactones in the green lineage. New Phytol 2012, 195, 857–871. [Google Scholar]
- Nelson, D.R.; Schuler, M.A.; Paquette, S.M.; Werck-Reichhart, D.; Bak, S. Comparative genomics of rice and Arabidopsis. Analysis of 727 cytochrome P450 genes and pseudogenes from a monocot and a dicot. Plant Physiol 2004, 135, 756–772. [Google Scholar]
- Proust, H.; Hoffmann, B.; Xie, X.; Yoneyama, K.; Schaefer, D.G.; Yoneyama, K.; Nogué, F.; Rameau, C. Strigolactones regulate protonema branching and act as a quorum sensing-like signal in the moss Physcomitrella patens. Development 2011, 138, 1531–1539. [Google Scholar]
- Matusova, R.; Rani, K.; Verstappen, F.W.; Franssen, M.C.; Beale, M.H.; Bouwmeester, H.J. The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant Physiol 2005, 139, 920–934. [Google Scholar]
- Lin, H.; Wang, R.; Qian, Q.; Yan, M.; Meng, X.; Fu, Z.; Yan, C.; Jiang, B.; Su, Z.; Li, J.; et al. DWARF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell 2009, 21, 1512–1525. [Google Scholar]
- Alder, A.; Jamil, M.; Marzorati, M.; Bruno, M.; Vermathen, M.; Bigler, P.; Ghisla, S.; Bouwmeester, H.; Beyer, P.; Al-Babili, S. The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science 2012, 335, 1348–1351. [Google Scholar]
- Liu, J.; Lovisolo, C.; Schubert, A.; Cardinale, F. Signaling role of Strigolactones at the interface between plants, (micro)organisms and a changing environment. J. Plant Interact 2012, 8, 17–33. [Google Scholar]
- Booker, J.; Sieberer, T.; Wright, W.; Williamson, L.; Willett, B.; Stirnberg, P.; Turnbull, C.; Srinivasan, M.; Goddard, P.; Leyser, O. MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Dev. Cell 2005, 8, 443–449. [Google Scholar]
- Marzec, M.; Muszynska, A. Strigolactones—New candidates for plant hormones. Post. Biol. Kom 2012, 39, 63–86. [Google Scholar]
- Beveridge, C.A.; Kyozuka, J. New genes in the strigolactone-related shoot branching pathway. Curr. Opin. Plant Biol 2010, 13, 34–39. [Google Scholar]
- Stirnberg, P.; Furner, I.J.; Leyser, O. MAX2 participates in an SCF complex which acts locally at the node to suppress shoot branching. Plant J 2007, 50, 80–94. [Google Scholar]
- Hamiaux, C.; Drummond, R.S.; Janssen, B.J.; Ledger, S.E.; Cooney, J.M.; Newcomb, R.D.; Snowden, K.C. DAD2 is an α/β hydrolase likely to be involved in the perception of the plant branching hormone, strigolactone. Curr. Biol 2012, 22, 2032–2036. [Google Scholar]
- Shen, H.; Zhu, L.; Bu, Q.Y.; Huq, E. MAX2 affects multiple hormones to promote photomorphogenesis. Mol. Plant 2012, 5, 750–762. [Google Scholar]
- Arite, T.; Umehara, M.; Ishikawa, S.; Hanada, A.; Maekawa, M.; Yamaguchi, S.; Kyozuka, J. d14, a strigolactone-insensitive mutant of rice, shows an accelerated outgrowth of tillers. Plant Cell Physiol 2009, 50, 1416–1424. [Google Scholar]
- Gao, Z.; Qian, Q.; Liu, X.; Yan, M.; Feng, Q.; Dong, G.; Liu, J.; Han, B. Dwarf 88, a novel putative esterase gene affecting architecture of rice plant. Plant Mol. Biol 2009, 71, 265–276. [Google Scholar]
- Liu, W.; Wu, C.; Fu, Y.; Hu, G.; Si, H.; Zhu, L.; Luan, W.; He, Z.; Sun, Z. Identification and characterization of HTD2: A novel gene negatively regulating tiller bud outgrowth in rice. Planta 2009, 230, 649–658. [Google Scholar]
- Waters, M.T.; Nelson, D.C.; Scaffidi, A.; Flematti, G.R.; Sun, Y.K.; Dixon, K.W.; Smith, S.M. Specialisation within the DWARF14 protein family confers distinct responses to karrikins and strigolactones in Arabidopsis. Development 2012, 139, 1285–1295. [Google Scholar]
- Zhao, L.H.; Zhou, X.E.; Wu, Z.S.; Yi, W.; Xu, Y.; Li, S.; Xu, T.H.; Liu, Y.; Chen, R.Z.; Kovach, A.; et al. Crystal structures of two phytohormone signal-transducing α/β hydrolases: Karrikin-signaling KAI2 and strigolactone signaling DWARF14. Cell Res 2013, 23, 436–439. [Google Scholar]
- Smith, S.M.; Waters, M.T. Strigolactones: Destruction-dependent perception? Curr. Biol 2012, 22, 924–927. [Google Scholar]
- Kretzschmar, T.; Kohlen, W.; Sasse, J.; Borghi, L.; Schlegel, M.; Bachelier, J.B.; Reinhardt, D.; Bours, R.; Bouwmeester, H.J.; Martinoia, E. A petunia ABC protein controls strigolactone-dependent symbiotic signalling and branching. Nature 2012, 7389, 341–344. [Google Scholar]
- Kohlen, W.; Charnikhova, T.; Liu, Q.; Bours, R.; Domagalska, M.A.; Beguerie, S.; Verstappen, F.; Leyser, O.; Bouwmeester, H.; Ruyter-Spira, C. Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in nonarbuscular mycorrhizal host Arabidopsis. Plant Physiol 2011, 155, 974–987. [Google Scholar]
- Amtmann, A.; Armengaud, P. Effects of N, P, K and S on metabolism: New knowledge gained from multi-level analysis. Curr. Opin. Plant Biol 2009, 12, 275–283. [Google Scholar]
- Beatty, P.H.; Good, A.G. Plant science. Future prospects for cereals that fix nitrogen. Nature 2011, 6041, 416–417. [Google Scholar]
- Hodge, A. Plastic plants and patchy soils. J. Exp. Bot 2006, 57, 401–411. [Google Scholar]
- Yoneyama, K.; Yoneyama, K.; Takeuchi, Y.; Sekimoto, H. Phosphorus deficiency in red clover promotes exudation of orobanchol, the signal for mycorrhizal symbionts and germination stimulant for root parasites. Planta 2007, 225, 1031–1038. [Google Scholar]
- Yoneyama, K.; Xie, X.; Kusumoto, D.; Sekimoto, H.; Sugimoto, Y.; Takeuchi, Y.; Yoneyama, K. Nitrogen deficiency as well as phosphorus deficiency in sorghum promotes the production and exudation of 5-deoxystrigol, the host recognition signal for arbuscular mycorrhizal fungi and root parasites. Planta 2007, 227, 125–132. [Google Scholar]
- Yoneyama, K.; Xie, X.; Kim, H.I.; Kisugi, T.; Nomura, T.; Sekimoto, H.; Yokota, T.; Yoneyama, K. How do nitrogen and phosphorus deficiencies affect strigolactone production and exudation? Planta 2012, 235, 1197–1207. [Google Scholar]
- Foo, E.; Yoneyama, K.; Hugill, C.J.; Quittenden, L.J.; Reid, J.B. Strigolactones and the regulation of pea symbioses in response to nitrate and phosphate deficiency. Mol. Plant 2013, 6, 76–87. [Google Scholar]
- Umehara, M.; Hanada, A.; Magome, H.; Takeda-Kamiya, N.; Yamaguchi, S. Contribution of strigolactones to the inhibition of tiller bud outgrowth under phosphate deficiency in rice. Plant Cell Physiol 2010, 51, 1118–1126. [Google Scholar]
- Jamil, M.; Charnikhova, T.; Cardoso, C.; Jamil, T.; Ueno, K.; Verstappen, F.; Asami, T.; Bouwmeester, H.J. Quantification of the relationship between strigolactones and Striga hermonthica infection in rice under varying levels of nitrogen and phosphorus. Weed Res 2011, 51, 373–385. [Google Scholar]
- López-Ráez, J.A.; Charnikhova, T.; Gómez-Roldán, V.; Matusova, R.; Kohlen, W.; de Vos, R.; Verstappen, F.; Puech-Pages, V.; Bécard, G.; Mulder, P.; et al. Tomato strigolactones are derived from carotenoids and their biosynthesis is promoted by phosphate starvation. New Phytol 2008, 178, 863–874. [Google Scholar]
- Luquet, D.; Zhang, B.G.; Dingkuhn, M.; Dexet, A.; Clement-Vidal, A. Phenotypic plasticity of rice seedlings: Case of phosphorus deficiency. Plant Prod. Sci 2005, 8, 145–151. [Google Scholar]
- Umehara, M. Strigolactone, a key regulator of nutrient allocation in plants. Plant Biotechnol 2011, 28, 429–437. [Google Scholar]
- López-Bucio, J.; Cruz-Ramírez, A.; Herrera-Estrella, L. The role of nutrient availability in regulating root architecture. Curr. Opin. Plant Biol 2003, 6, 280–287. [Google Scholar]
- Péret, B.; Clément, M.; Nussaume, L.; Desnos, T. Root developmental adaptation to phosphate starvation: Better safe than sorry. Trends Plant Sci 2011, 16, 442–450. [Google Scholar]
- Ruyter-Spira, C.; Kohlen, W.; Charnikhova, T.; van Zeijl, A.; van Bezouwen, L.; de Ruijter, N.; Cardoso, C.; Lopez-Raez, J.A.; Matusova, R.; Bours, R.; et al. Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: Another belowground role for strigolactones? Plant Physiol 2011, 155, 721–734. [Google Scholar]
- Rasmussen, A.; Mason, M.G.; de Cuyper, C.; Brewer, P.B.; Herold, S.; Agusti, J.; Geelen, D.; Greb, T.; Goormachtig, S.; Beeckman, T.; et al. Strigolactones suppress adventitious rooting in Arabidopsis and pea. Plant Physiol 2012, 158, 1976–1987. [Google Scholar]
- Mayzlish-Gati, E.; de Cuyper, C.; Goormachtig, S.; Beeckman, T.; Vuylsteke, M.; Brewer, P.B.; Beveridge, C.A.; Yermiyahu, U.; Kaplan, Y.; Enzer, Y.; et al. Strigolactones are involved in root response to low phosphate conditions in Arabidopsis. Plant Physiol 2012, 160, 1329–1341. [Google Scholar]
- Koltai, H. Strigolactones activate different hormonal pathways for regulation of root development in response to phosphate growth conditions. Ann. Bot. 2012. [Google Scholar] [CrossRef]
- Kitahata, N.; Ito, S.; Kato, A.; Ueno, K.; Nakano, T.; Yoneyama, K.; Yoneyama, K.; Asami, T. Abamine as a basis for new designs of regulators of strigolactone production. J. Pestic. Sci 2011, 36, 53–57. [Google Scholar]
- Chiou, T.J.; Lin, S.I. Signaling network in sensing phosphate availability in plants. Annu. Rev. Plant. Biol 2011, 62, 185–206. [Google Scholar]
- Ramaiah, M.; Jain, A.; Baldwin, J.C.; Karthikeyan, A.S.; Raghothama, K.G. Characterization of the phosphate starvation-induced glycerol-3-phosphate permease gene family in Arabidopsis. Plant Physiol 2011, 157, 279–291. [Google Scholar]
- Shin, H.; Shin, H.S.; Dewbre, G.R.; Harrison, M.J. Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J 2004, 39, 629–642. [Google Scholar]
- Lei, M.; Liu, Y.; Zhang, B.; Zhao, Y.; Wang, X.; Zhou, Y.; Raghothama, K.G.; Liu, D. Genetic and genomic evidence that sucrose is a global regulator of plant responses to phosphate starvation in Arabidopsis. Plant Physiol 2011, 156, 1116–1130. [Google Scholar]
- Brown, R.; Greenwood, A.D.; Johnson, A.W.; Long, A.G. The stimulant involved in the germination of Orobanche minor Sm. I. Assay technique and bulk preparation of the stimulant. Biochem. J 1951, 48, 559–564. [Google Scholar]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed; Academic Press: San Diego, CA, USA, 2008. [Google Scholar]
- Govindarajulu, M.; Pfeffer, P.E.; Jin, H.; Abubaker, J.; Douds, D.D.; Allen, J.W.; Bücking, H.; Lammers, P.J.; Shachar-Hill, Y. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 2005, 7043, 819–823. [Google Scholar]
- Remy, W.; Taylor, T.N.; Hass, H.; Kerp, H. Four hundred million-year-old vesicular arbuscular mycorrhizae. Proc. Natl. Acad. Sci. USA 2004, 91, 11841–11843. [Google Scholar]
- Besserer, A.; Puech-Pagès, V.; Kiefer, P.; Gomez-Roldan, V.; Jauneau, A.; Roy, S.; Portais, J.C.; Roux, C.; Bécard, G.; Séjalon-Delmas, N. Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol 2006, 7, e226. [Google Scholar]
- Besserer, A.; Bécard, G.; Jauneau, A.; Roux, C.; Séjalon-Delmas, N. GR24, a synthetic analog of strigolactones, stimulates the mitosis and growth of the arbuscular mycorrhizal fungus Gigaspora rosea by boosting its energy metabolism. Plant Physiol 2008, 148, 402–413. [Google Scholar]
- Gutjahr, C.; Radovanovic, D.; Geoffroy, J.; Zhang, Q.; Siegler, H.; Chiapello, M.; Casieri, L.; An, K.; An, G.; Guiderdoni, E.; et al. The half-size ABC transporters STR1 and STR2 are indispensable for mycorrhizal arbuscule formation in rice. Plant J 2012, 69, 906–920. [Google Scholar]
- Kohlen, W.; Charnikhova, T.; Lammers, M.; Pollina, T.; Tóth, P.; Haider, I.; Pozo, M.J.; de Maagd, R.A.; Ruyter-Spira, C.; Bouwmeester, H.J.; et al. The tomato CAROTENOID CLEAVAGE DIOXYGENASE8 (SlCCD8) regulates rhizosphere signaling, plant architecture and affects reproductive development through strigolactone biosynthesis. New Phytol 2012, 196, 535–547. [Google Scholar]
- López-Ráez, J.A.; Charnikhova, T.; Fernández, I.; Bouwmeester, H.; Pozo, M.J. Arbuscular mycorrhizal symbiosis decreases strigolactone production in tomato. J. Plant Physiol 2011, 168, 294–297. [Google Scholar]
- Yoshida, S.; Kameoka, H.; Tempo, M.; Akiyama, K.; Umehara, M.; Yamaguchi, S.; Hayashi, H.; Kyozuka, J.; Shirasu, K. The D3 F-box protein is a key component in host strigolactone responses essential for arbuscular mycorrhizal symbiosis. New Phytol 2012, 196, 1208–1216. [Google Scholar]
- Balzergue, C.; Puech-Pagès, V.; Bécard, G.; Rochange, S.F. The regulation of arbuscular mycorrhizal symbiosis by phosphate in pea involves early and systemic signalling events. J. Exp. Bot 2011, 62, 1049–1060. [Google Scholar]
- Gu, M.; Xu, K.; Chen, A.; Zhu, Y.; Tang, G.; Xu, G. Expression analysis suggests potential roles of microRNAs for phosphate and arbuscular mycorrhizal signaling in Solanum lycopersicum. Physiol. Plant 2010, 138, 226–237. [Google Scholar]
- Breuillin, F.; Schramm, J.; Hajirezaei, M.; Ahkami, A.; Favre, P.; Druege, U.; Hause, B.; Bucher, M.; Kretzschmar, T.; Bossolini, E.; et al. Phosphate systemically inhibits development of arbuscular mycorrhiza in Petunia hybrida and represses genes involved in mycorrhizal functioning. Plant J 2010, 64, 1002–1017. [Google Scholar]
- Markmann, K.; Parniske, M. Evolution of root endosymbiosis with bacteria: How novel are nodules? Trends Plant Sci 2009, 14, 77–86. [Google Scholar]
- Ferguson, B.J.; Indrasumunar, A.; Hayashi, S.; Lin, Y.H.; Lin, M.H.; Reid, D.; Gresshoff, P.M. Molecular analysis of legume nodule development and autoregulation. J. Integr. Plant Biol 2010, 52, 61–76. [Google Scholar]
- Wang, D.; Griffitts, J.; Starker, C.; Fedorova, E.; Limpens, E.; Ivanov, S.; Bisseling, T.; Long, S. A nodule-specific protein secretory pathway required for nitrogen-fixing symbiosis. Science 2010, 5969, 1126–1129. [Google Scholar]
- Soto, M.J.; Fernandez-Aparicio, M.; Castellanos-Morales, V.; Garcia-Garrido, J.A.; Delgado, M.J.; Vierheilig, H. First indications for the involvement of strigolactones on nodule formation in alfalfa (Medicago sativa). Soil Biol. Biochem 2010, 42, 383–385. [Google Scholar]
- Aroca, R.; Ruiz-Lozano, J.M.; Zamarreño, A.M.; Paz, J.A.; García-Mina, J.M.; Pozo, M.J.; López-Ráez, J.A. Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J. Plant Physiol 2013, 170, 47–55. [Google Scholar]
- Bartoli, C.G.; Casalongué, C.A.; Simontacchi, M.; Marquez-Garcia, B.; Foyer, H.F. Interactions between hormone and redox signalling pathways in the control of growth and cross tolerance to stress. Environ. Exp. Bot. 2012. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Managing the cellular redox hub in photosynthetic organisms. Plant Cell Environ 2012, 35, 199–201. [Google Scholar]
- Woo, H.R.; Chung, K.M.; Park, J.H.; Oh, S.A.; Ahn, T.; Hong, S.H.; Jang, S.K.; Nam, H.G. ORE9, an F-box protein that regulates leaf senescence in Arabidopsis. Plant Cell 2001, 13, 1779–1790. [Google Scholar]
- Stirnberg, P.; van de Sande, K.; Leyser, O. MAX1 and MAX2 control shoot lateral branching in Arabidopsis. Development 2002, 129, 1131–1141. [Google Scholar]
- Woo, H.R.; Kim, J.H.; Nam, H.G.; Lim, P.O. The delayed leaf senescence mutants of Arabidopsis, ore1, ore3 and ore9 are tolerant to oxidative stress. Plant Cell Physiol 2004, 45, 923–932. [Google Scholar]
- Johnson, M.P.; Havaux, M.; Triantaphylidès, C.; Ksas, B.; Pascal, A.A.; Robert, B.; Davison, P.A.; Ruban, A.V.; Horton, P. Elevated zeaxanthin bound to oligomeric LHCII enhances the resistance of Arabidopsis to photooxidative stress by a lipid-protective, antioxidant mechanism. J. Biol. Chem 2007, 282, 22605–22618. [Google Scholar]
- Triantaphylidès, C.; Havaux, M. Singlet oxygen in plants: Production, detoxification and signaling. Trends Plant Sci 2009, 14, 219–228. [Google Scholar]
- Ramel, F.; Mialoundama, A.S.; Havaux, M. Nonenzymic carotenoid oxidation and photooxidative stress signalling in plants. J. Exp. Bot 2013, 64, 799–805. [Google Scholar]
- Mayzlish-Gati, E.; LekKala, S.P.; Resnick, N.; Wininger, S.; Bhattacharya, C.; Lemcoff, J.H.; Kapulnik, Y.; Koltai, H. Strigolactones are positive regulators of light-harvesting genes in tomato. J. Exp. Bot 2010, 61, 3129–3136. [Google Scholar]
- Koltai, H.; Kapulnik, Y. Strigolactones as mediators of plant growth responses to environmental conditions. Plant Signal. Behav 2011, 6, 37–41. [Google Scholar]
- Brewer, P.B.; Koltai, H.; Beveridge, C.A. Diverse roles of strigolactones in plant development. Mol. Plant 2013, 6, 18–28. [Google Scholar]
- Snowden, K.C.; Simkin, A.J.; Janssen, B.J.; Templeton, K.R.; Loucas, H.M.; Simons, J.L.; Karunairetnam, S.; Gleave, A.P.; Clark, D.G.; Klee, H.J. The Decreased apical dominance1/Petunia hybrida CAROTENOID CLEAVAGE DIOXYGENASE8 gene affects branch production and plays a role in leaf senescence, root growth, and flower development. Plant Cell 2005, 17, 746–759. [Google Scholar]
- Yan, H.; Saika, H.; Maekawa, M.; Takamure, I.; Tsutsumi, N.; Kyozuka, J.; Nakazono, M. Rice tillering dwarf mutant dwarf3 has increased leaf longevity during darkness-induced senescence or hydrogen peroxide-induced cell death. Genes Genet. Syst 2007, 82, 361–366. [Google Scholar]

| Protein | Gene | Process | |||
|---|---|---|---|---|---|
| Arabidopsis | Rice | Pea | Petunia | ||
| Iron-containing protein | AtD27 | D27 | Biosynthesis | ||
| CCD7 | MAX3 | HTD1/D17 | RMS5 | DAD3 | |
| CCD8 | MAX4 | D10 | RMS1 | DAD1 | |
| cytochrome P450 | MAX1 | ||||
| F-box protein | MAX2 | D3 | RMS4 | Signaling | |
| α/β hydrolase | AtD14 | D14/D88/ | HTD2 | DAD2 | |
| Species | Low level of P | Low level of N | Ref. | ||
|---|---|---|---|---|---|
| SL name | Change | SL name | Change | ||
| Non-legume plants | |||||
| Sorghum bicolor L. (cv. Hybrid) | 5-deoxystrigol | 30-fold | 5-deoxystrigol | 30-fold | [40] |
| Calendula officinalis L. (cv. Super) | Orobanchol | ++ | Orobanchol | + | [41] |
| Orobanchyl acetate | ++ | Orobanchyl acetate | + | ||
| Triticum aestivum L. (cv. Chinese Spring) | Orobanchol | ++ | Orobanchol | + | [41] |
| Lactuca sativa L. (cv. Chirimensha) | Orobanchol | ++ | Orobanchol | + | [41] |
| Orobanchyl acetate | ++ | Orobanchyl acetate | + | ||
| Solanum lycopersicum L. (cv. MoneyMaker) | Orobanchol | + | na | na | [45] |
| Solanacol | + | na | na | ||
| Solanum lycopersicum L. (cv. M82) | Orobanchol | 100-fold | Orobanchol | - | [41] |
| Oryza sativa (cv. IAC 165) | Orobanchol | ++ | Orobanchol | ++ | [44] |
| 2′-epi-5-deoxystrigol | ++ | 2′-epi-5-deoxystrigol | ++ | ||
| Oryza sativa (cv. TN 1) | Orobanchol | + | orobanchol | + | [44] |
| 2′-epi-5-deoxystrigol | + | 2′-epi-5-deoxystrigol | + | ||
| Legume plants | |||||
| Trifolium pretense | Orobanchol | 20-fold | Orobanchol | - | [39] |
| Medicago sativa L. (cv. BRS511) | Orobanchol | + | Orobanchol | - | [41] |
| Orobanchyl acetate | + | Orobanchyl acetate | - | ||
| Astragalus sinicus L. (cv. Pinkyfield) | Sorgomol | 14,000-fold | Sorgomol, | 1000-fold | [41] |
| 5-deoxystrigol | 1000-fold | 5-deoxystrigol | 20-fold | ||
| Pisum sativum L. | Fabacyl acetate | 10-fold | Fabacyl acetate, | 3-fold | [42] |
| Orobanchyl acetate | 10-fold | Orobanchyl acetate | 3-fold | ||
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Marzec, M.; Muszynska, A.; Gruszka, D. The Role of Strigolactones in Nutrient-Stress Responses in Plants. Int. J. Mol. Sci. 2013, 14, 9286-9304. https://doi.org/10.3390/ijms14059286
Marzec M, Muszynska A, Gruszka D. The Role of Strigolactones in Nutrient-Stress Responses in Plants. International Journal of Molecular Sciences. 2013; 14(5):9286-9304. https://doi.org/10.3390/ijms14059286
Chicago/Turabian StyleMarzec, Marek, Aleksandra Muszynska, and Damian Gruszka. 2013. "The Role of Strigolactones in Nutrient-Stress Responses in Plants" International Journal of Molecular Sciences 14, no. 5: 9286-9304. https://doi.org/10.3390/ijms14059286





