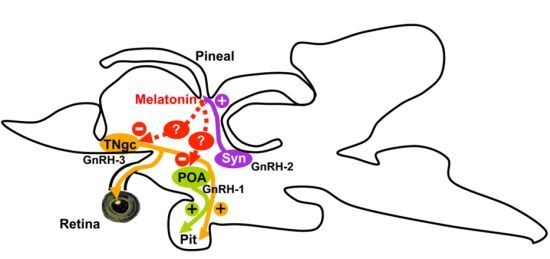
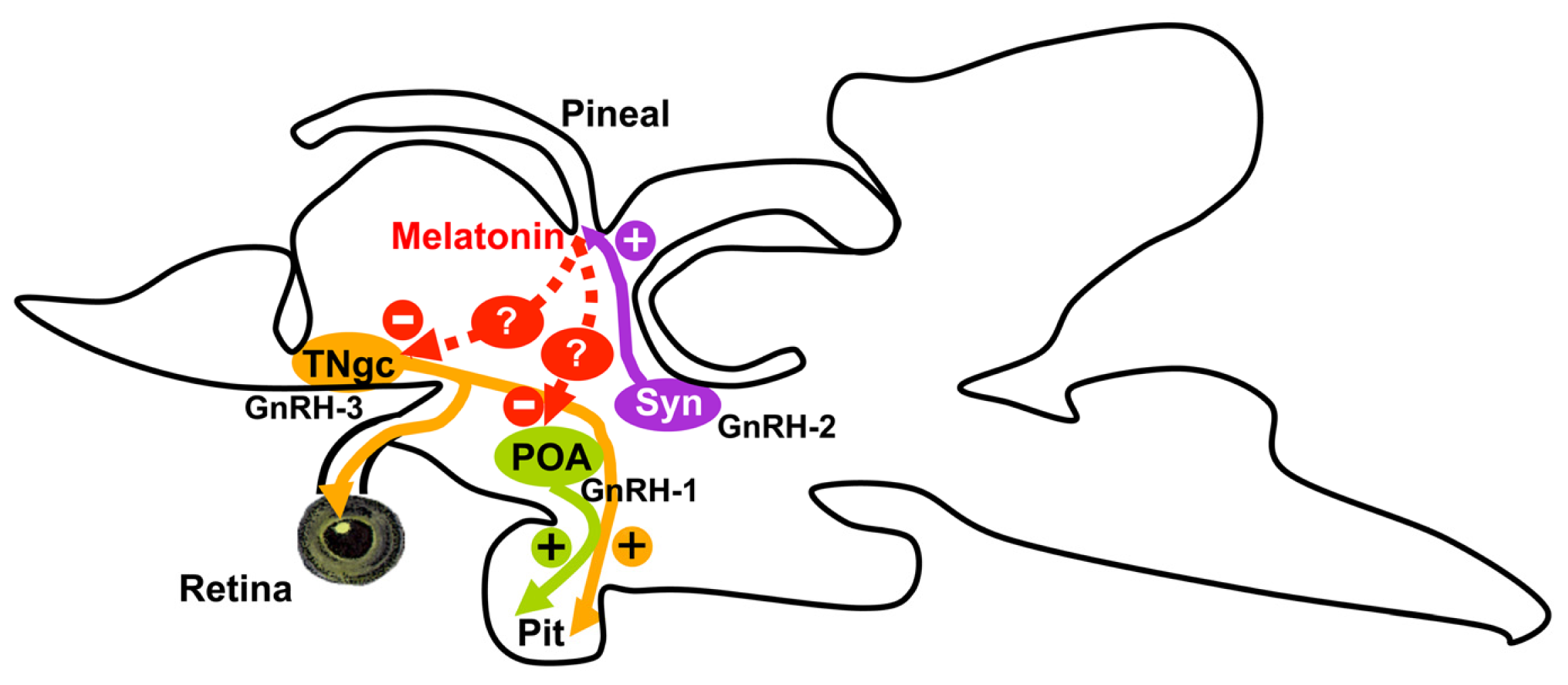
Melatonin Inhibits GnRH-1, GnRH-3 and GnRH Receptor Expression in the Brain of the European Sea Bass, Dicentrarchus labrax
Abstract
:1. Introduction
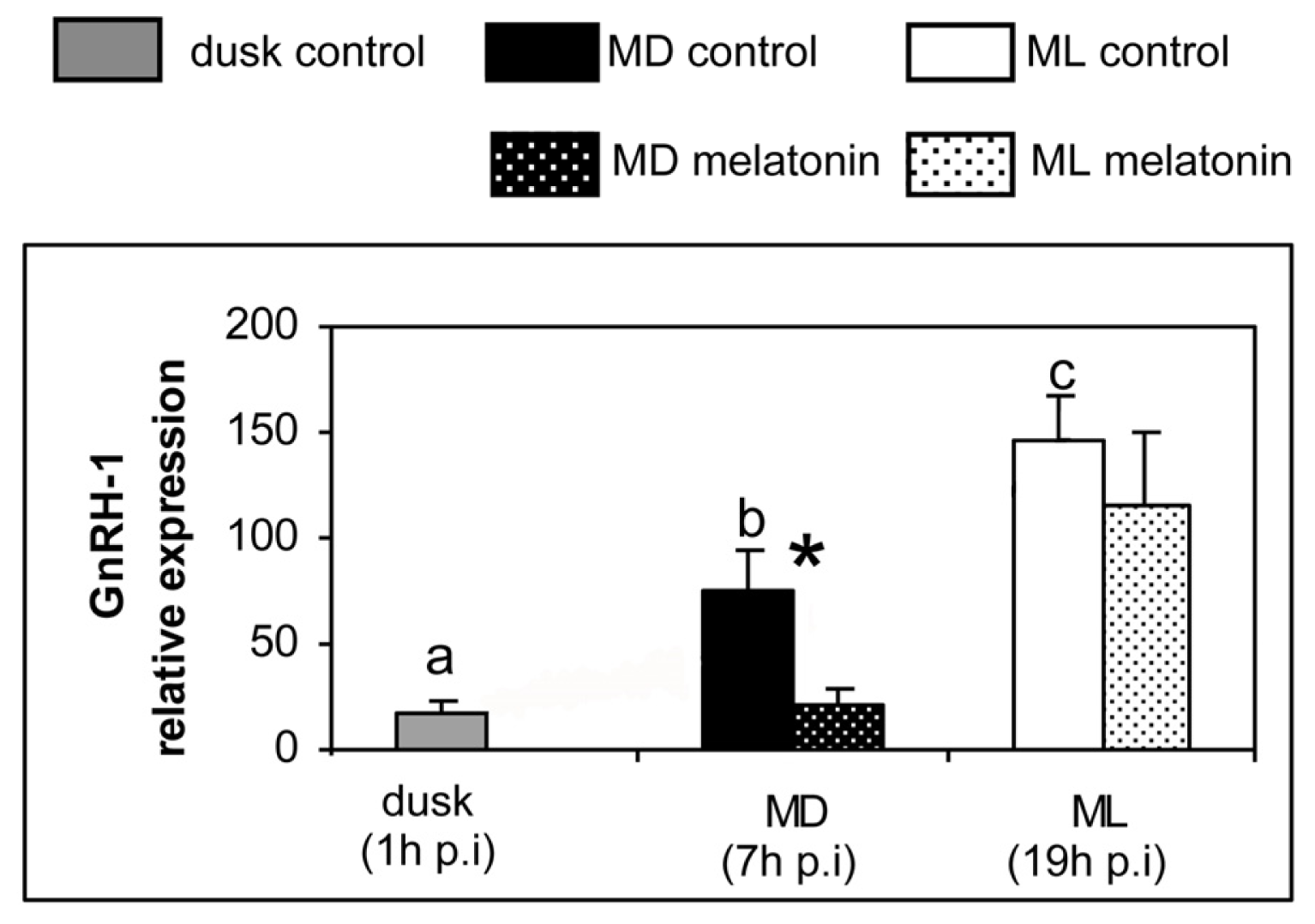
2. Results
3. Discussion
4. Material and Methods
4.1. Animals
4.2. Experimental Procedure
4.3. Quantitative Real Time PCR Assays in Sea Bass Brain
4.4. Data Analysis
5. Conclusions
Acknowledgements
Conflict of Interest
References
- Cassone, V.M. Melatonin’s role in vertebrate circadian rhythms. Chronobiol. Int 1998, 15, 457–473. [Google Scholar]
- Falcón, J.; Migaud, H.; Muñoz-Cueto, J.A.; Carrillo, M. Current knowledge on the melatonin system in teleost fish. Gen. Comp. Endocrinol 2010, 165, 469–482. [Google Scholar] [Green Version]
- Malpaux, B.; Migaud, M.; Tricoire, H.; Chemineau, P. Biology of mammalian photoperiodism and the critical role of the pineal gland and melatonin. J. Biol. Rhythms 2001, 16, 336–347. [Google Scholar]
- Zachmann, A.; Ali, M.A.; Falcón, J. Melatonin and its effects in fishes: An overview. In Rhythms in Fishes; Ali, M.A., Ed.; Plenum Press: New York, NY, USA, 1992; pp. 149–165. [Google Scholar]
- Molina-Borja, M.; Falcón, J.; Urquiola, E.; Oaknin, S. Characterization of 2–[125I]-iodomelatonin binding sites in the brain, intestine and gonad of the gilthead sea bream (Sparus aurata). Pflügers Arch 1994, 427, R5. [Google Scholar]
- Roy, D.; Angelini, N.L.; Fujieda, H.; Brown, G.M.; Belsham, D.D. Cyclical regulation of GnRH gene expression in GT1–7 GnRH-secreting neurons by melatonin. Endocrinology 2001, 142, 4711–4720. [Google Scholar]
- Sánchez-Vázquez, F.J.; Iigo, M.; Madrid, J.A.; Zamora, S.; Tabata, M. Daily cycle in plasma and ocular melatonin in demand fed sea bass, Dicentrarchus labrax L. J. Comp. Physiol. B 1997, 167, 409–415. [Google Scholar]
- Sébert, M.E.; Legros, C.; Weltzien, F.A.; Malpaux, B.; Chemineau, P.; Dufour, S. Melatonin activates brain dopaminergic systems in the eel with an inhibitory impact on reproductive function. J. Neuroendocrinol 2008, 20, 917–929. [Google Scholar]
- Khan, I.A.; Thomas, P. Melatonin influences gonadotropin II secretion in the Atlantic croaker (Micropogonias undulatus). Gen. Comp. Endocrinol 1996, 104, 231–242. [Google Scholar]
- Falcón, J.; Besseau, L.; Fazzari, D.; Attia, J.; Gaildrat, P.; Beauchaud, M.; Boeuf, G. Melatonin modulates secretion of growth hormone and prolactin by trout pituitary glands and cells in culture. Endocrinology 2003, 144, 4648–4658. [Google Scholar]
- Gaildrat, P.; Falcón, J. Expression of melatonin receptors and 2-[125I]-iodomelatonin binding sites in the pituitary of a teleost fish. Adv. Exp. Med. Biol 1999, 460, 61–72. [Google Scholar]
- Gaildrat, P.; Falcón, J. Melatonin receptors in the pituitary of a teleost fish: mRNA expression, 2-[(125)I]iodomelatonin binding and cyclic AMP response. Neuroendocrinology 2000, 72, 57–66. [Google Scholar]
- Sauzet, S.; Besseau, L.; Herrera Perez, P.; Covès, D.; Chatain, B.; Peyric, E.; Boeuf, G.; Muñoz-Cueto, J.A.; Falcón, J. Cloning and retinal expression of melatonin receptors in the European sea bass, Dicentrarchus labrax. Gen. Comp. Endocrinol 2008, 157, 186–195. [Google Scholar]
- Chattoraj, A.; Seth, M.; Maitra, S.K. Localization and dynamics of Mel(1a) melatonin receptor in the ovary of carp Catla catla in relation to serum melatonin levels. Comp. Biochem. Physiol. A Mol. Integr. Physiol 2009, 152, 327–333. [Google Scholar]
- Herrera-Pérez, P.; Munoz-Cueto, J.A. University of Cadiz: Puerto Real, Spain, Unpublished work; 2009.
- Clemens, J.W.; Jarzynka, M.J.; Witt-Enderby, P.A. Down-regulation of mt1 melatonin receptors in rat ovary following estrogen exposure. Life Sci 2001, 69, 27–35. [Google Scholar]
- Lee, C.J.; Do, B.R.; Lee, Y.H.; Park, J.H.; Kim, S.J.; Kim, J.K.; Roh, S.I.; Yoon, Y.D.; Yoon, H.S. Ovarian expression of melatonin Mel(1a) receptor mRNA during mouse development. Mol. Reprod. Dev 2001, 59, 126–132. [Google Scholar]
- Soares, J.M., Jr; Masana, M.I.; Ersahin, C.; Dubocovich, M.L. Functional melatonin receptors in rat ovaries at various stages of the estrous cycle. J. Pharmacol. Exp. Ther. 2003, 306, 694–702. [Google Scholar]
- Amano, M.; Iigo, M.; Ikuta, K.; Kitamura, S.; Yamada, H.; Yamamori, K. Roles of melatonin in gonadal maturation of underyearling precocious male masu salmon. Gen. Comp. Endocrinol 2000, 120, 190–197. [Google Scholar]
- Carrillo, M.; Zanuy, S.; Prat, F.; Cerda, J.; Ramos, J.; Mananos, E.; Bromage, N. Sea Bass. In Broodstock Management and Egg and Larval Quality; Bromage, N., Roberts, F.J., Eds.; Blackwell: Oxford, UK, 1995; pp. 138–168. [Google Scholar]
- Amano, M.; Iigo, M.; Ikuta, K.; Kitamura, S.; Okuzawa, K.; Yamada, H.; Yamamori, K. Disturbance of plasma melatonin profile by high dose melatonin administration inhibits testicular maturation of precocious male masu salmon. Zoolog. Sci 2004, 21, 79–85. [Google Scholar]
- Ghosh, J.; Nath, P. Seasonal effects of melatonin on ovary and plasma gonadotropin and vitellogenin levels in intact and pinealectomized catfish, Clarias batrachus (Linn). Indian J. Exp. Biol 2005, 43, 224–232. [Google Scholar]
- González-Martínez, D.; Madigou, T.; Zmora, N.; Anglade, I.; Zanuy, S.; Zohar, Y.; Elizur, A.; Muñoz-Cueto, J.A.; Kah, O. Differential expression of three different prepro-GnRH (gonadotrophin-releasing hormone) messengers in the brain of the european sea bass (Dicentrarchus labrax). J. Comp. Neurol 2001, 429, 144–155. [Google Scholar]
- González-Martínez, D.; Zmora, N.; Mañanos, E.; Saligaut, D.; Zanuy, S.; Zohar, Y.; Elizur, A.; Kah, O.; Muñoz-Cueto, J.A. Immunohistochemical localization of three different prepro-GnRHs in the brain and pituitary of the European sea bass (Dicentrarchus labrax) using antibodies to the corresponding GnRH-associated peptides. J. Comp. Neurol 2002, 446, 95–113. [Google Scholar]
- González-Martínez, D.; Zmora, N.; Zanuy, S.; Sarasquete, C.; Elizur, A.; Kah, O.; Muñoz-Cueto, J.A. Developmental expression of three different prepro-GnRH (gonadotrophin-releasing hormone) messengers in the brain of the European sea bass (Dicentrarchus labrax). J. Chem. Neuroanat 2002, 23, 255–267. [Google Scholar]
- Servili, A.; Lethimonier, C.; Lareyre, J.J.; López-Olmeda, J.F.; Sánchez-Vázquez, F.J.; Kah, O.; Muñoz-Cueto, J.A. The highly conserved gonadotropin-releasing hormone-2 form acts as a melatonin-releasing factor in the pineal of a teleost fish, the European sea bass Dicentrarchus labrax. Endocrinology 2010, 151, 2265–2275. [Google Scholar]
- Zmora, N.; González-Martínez, D.; Muñoz-Cueto, J.A.; Madigou, T.; Mañanos-Sánchez, E.; Doste, S.Z.; Zohar, Y.; Kah, O.; Elizur, A. The GnRH system in the European sea bass (Dicentrarchus labrax). J. Endocrinol 2002, 172, 105–116. [Google Scholar]
- González-Martínez, D.; Madigou, T.; Mañanos, E.; Cerdá-Reverter, J.M.; Zanuy, S.; Kah, O.; Muñoz-Cueto, J.A. Cloning and expression of gonadotropin-releasing hormone receptor in the brain and pituitary of the European sea bass: An in situ hybridization study. Biol. Reprod 2004, 70, 1380–1391. [Google Scholar]
- Lethimonier, C.; Madigou, T.; Muñoz-Cueto, J.A.; Lareyre, J.J.; Kah, O. Evolutionary aspects of GnRHs, GnRH neuronal systems and GnRH receptors in teleost fish. Gen. Comp. Endocrinol 2004, 135, 1–16. [Google Scholar]
- Moncaut, N.; Somoza, G.; Power, D.M.; Canario, A.V. Five gonadotrophin-releasing hormone receptors in a teleost fish: Isolation, tissue distribution and phylogenetic relationships. J. Mol. Endocrinol 2005, 34, 767–779. [Google Scholar]
- Zohar, Y.; Muñoz-Cueto, J.A.; Elizur, A.; Kah, O. Neuroendocrinology of reproduction in teleost fish. Gen. Comp. Endocrinol 2010, 165, 438–455. [Google Scholar]
- Garcia-Allegue, R.; Madrid, J.A.; Sanchez-Vazquez, F.J. Melatonin rhythms in European sea bass plasma and eye: Influence of seasonal photoperiod and water temperature. J. Pineal Res 2001, 31, 68–75. [Google Scholar]
- Iigo, M.; Aida, K. Effects of season, temperature, and photoperiod on plasma melatonin rhythms in the goldfish, Carassius auratus. J. Pineal. Res 1995, 18, 62–68. [Google Scholar]
- Bayarri, M.J.; Iigo, M.; Muñoz-Cueto, J.A.; Isorna, E.; Delgado, M.J.; Madrid, J.A.; Sánchez-Vázquez, F.J.; Alonso-Gómez, A.L. Binding characteristics and daily rhythms of melatonin receptors are distinct in the retina and the brain areas of the European sea bass retina (Dicentrarchus labrax). Brain Res 2004, 1029, 241–250. [Google Scholar]
- Bayarri, M.J.; Falcon, J.; Zanuy, S.; Carrillo, M. Continuous light and melatonin: Daily and seasonal variations of brain binding sites and plasma concentration during the first reproductive cycle of sea bass. Gen. Comp. Endocrinol 2010, 169, 58–64. [Google Scholar] [Green Version]
- Herrera-Pérez, P.; Rendón, M.C.; Besseau, L.; Sauzet, S.; Falcón, J.; Muñoz-Cueto, J.A. Melatonin receptors in the brain of the European sea bass: An in situ hybridization and autoradiographic study. J. Comp. Neurol 2010, 518, 3495–3511. [Google Scholar]
- Bayarri, M.J.; Rodríguez, L.; Zanuy, S.; Madrid, J.A.; Sánchez-Vázquez, F.J.; Kagawa, H.; Okuzawa, K.; Carrillo, M. Effect of photoperiod manipulation on the daily rhythms of melatonin and reproductive hormones in caged European sea bass (Dicentrarchus labrax). Gen. Comp. Endocrinol 2004, 136, 72–81. [Google Scholar]
- Mañanós, E.L.; Zanuy, S.; Carrillo, M. Photoperiodic manipulations of the reproductive cycle of sea bass (Dicentrarchus labrax) and their effects on gonadal development, and plasma 17β-estradiol and vitellogenin levels. Fish Physiol. Biochem 1997, 16, 211–222. [Google Scholar]
- Moles, G.; Carrillo, M.; Mañanós, E.; Mylonas, C.C.; Zanuy, S. Temporal profile of brain and pituitary GnRHs, GnRH-R and gonadotropin mRNA expression and content during early development in European sea bass (Dicentrarchus labrax L.). Gen. Comp. Endocrinol 2007, 150, 75–86. [Google Scholar]
- Prat, F.; Zanuy, S.; Carrillo, M.; de Mones, A.; Fostier, A. Seasonal changes in plasma levels of gonadal steroids of sea bass, Dicentrarchus labrax L. Gen. Comp. Endocrinol 1990, 78, 361–373. [Google Scholar]
- Rodríguez, L.; Carrillo, M.; Sorbera, L.A.; Soubrier, M.A.; Mañanós, E.; Holland, M.C.; Zohar, Y.; Zanuy, S. Pituitary levels of three forms of GnRH in the male European sea bass (Dicentrarchus labrax, L.) during sex differentiation and first spawning season. Gen. Comp. Endocrinol 2000, 120, 67–74. [Google Scholar]
- Bayarri, M.J.; Zanuy, S.; Yilmaz, O.; Carrillo, M. Effects of continuous light on the reproductive system of European sea bass gauged by alterations of circadian variations during their first reproductive cycle. Chronobiol. Int 2009, 26, 184–199. [Google Scholar]
- Confente, F.; Munoz-Cueto, J.A. University of Cadiz: Puerto Real, Spain, Unpublished work; 2009.
- Gothilf, Y.; Meiri, I.; Elizur, A.; Zohar, Y. Preovulatory changes in the levels of three gonadotropin-releasing hormone-encoding messenger ribonucleic acids (mRNAs), gonadotropin beta-subunit mRNAs, plasma gonadotropin, and steroids in the female gilthead seabream, Sparus aurata. Biol. Reprod 1997, 57, 1145–1154. [Google Scholar]
- Schirman-Hildesheim, T.D.; Ben-Aroya, N.; Koch, Y. Daily GnRH and GnRH-receptor mRNA expression in the ovariectomized and intact rat. Mol. Cell. Endocrinol 2006, 252, 120–125. [Google Scholar]
- Bauer-Dantoin, A.C.; Weiss, J.; Jameson, J.L. Roles of estrogen, progesterone, and gonadotropin-releasing hormone (GnRH) in the control of pituitary GnRH receptor gene expression at the time of the preovulatory gonadotropin surges. Endocrinology 1995, 136, 1014–1019. [Google Scholar]
- Kah, O.; Lethimonier, C.; Somoza, G.; Guilgur, L.G.; Vaillant, C.; Lareyre, J.J. GnRH and GnRH receptors in metazoa: A historical, comparative, and evolutive perspective. Gen. Comp. Endocrinol. 2007, 153, 346–364. [Google Scholar]
- Hernandez-Rauda, R.; Miguez, J.M.; Ruibal, C.; Aldegunde, M. Effects of melatonin on dopamine metabolism in the hypothalamus and the pituitary of the rainbow trout, Oncorhynchus mykiss. J. Exp. Zool 2000, 287, 440–444. [Google Scholar]
- Pinillos, M.L.; de Pedro, N.; Alonso-Gómez, A.L.; Alonso-Bedate, M.; Delgado, M.J. Food intake inhibition by melatonin in goldfish (Carassius auratus). Physiol. Behav 2001, 72, 629–634. [Google Scholar]
- Escobar, S.; Felip, A.; Gueguen, M.M.; Zanuy, S.; Carrillo, M.; Kah, O.; Servili, A. Expression of kisspeptins in the brain and pituitary of the European sea bass (Dicentrarchus labrax). J. Comp. Neurol. 2013, 521, 933–948. [Google Scholar]
- Rodriguez, L.; Begtashi, I.; Zanuy, S.; Carrillo, M. Long-term exposure to continuous light inhibits precocity in European male sea bass (Dicentrarchus labrax, L.): Hormonal aspects. Gen. Comp. Endocrinol 2005, 140, 116–125. [Google Scholar]
- Servili, A.; Herrera-Pérez, P.; Kah, O.; Muñoz-Cueto, J.A. The retina is a target for GnRH-3 system in the European sea bass, Dicentrarchus labrax. Gen. Comp. Endocrinol 2012, 175, 398–406. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar]



| Gene | Forward primer sequence | Reverse primer sequence | Annealing temperature | GenBank accession no. |
|---|---|---|---|---|
| GnRH-1 | qPCR-GnRH-1-F: GGTCCTATGGACTGAGTCCAGG | qPCR-GnRH-1-R: TGATTCCTCTGCACAACCTAA | 60 °C | AF224279 |
| GnRH-2 | qPCR-GnRH-2-F: GTGTGAGGCAGGAGAATGCA | qPCR-GnRH-2-R: CTGGCTAAGGCATCCAGAATG | 60 °C | AF224281 |
| GnRH-3 | qPCR-GnRH-3-F: TGTGGGAGAGCTAGAGGCAAC | qPCR-GnRH-3-R: GTTTGGGCACTCGCCTCTT | 60 °C | AF224280 |
| GnRH-R II-1a | qPCR-1a-F: CTCTGGCTATCAATAAGGC | qPCR-1a-R: CTCGGGATGGATGATGGT | 59 °C | AJ419594 |
| GnRH-R II-1b | qPCR-1b-F: CTGCTGATGTTCTTGAAACTGG | qPCR-1b-R: GAAGTTCTCTGGCACTGTGATG | 64 °C | AJ606686 |
| GnRH-R II-1c | qPCR-1c-F: TGATGGTGGCGTGGACTA | qPCR-1c-R: GAGTAAAGTTTGCTGGATAAG | 59 °C | AJ606684 |
| GnRH-R II-2a | qPCR-2a-F: TGACGCTGTATGTCTTCCC | qPCR-2a-R: CATCCGGGCTTTGGGTAT | 59 °C | AJ606683 |
| GnRH-R II-2b | qPCR-2b-F: AGACTCTGAAGATGACGGTGGT | qPCR-2b-R: AGTGAAGCGTCTCTTCTCATCC | 64 °C | AJ606685 |
| 18S | qPCR-18S-F: GCATGGCCGTTCTTAGTTGGT | qPCR-18S-R: GCATGCCGGAGTCTCGTT | 48 °C | AY831388 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Servili, A.; Herrera-Pérez, P.; Del Carmen Rendón, M.; Muñoz-Cueto, J.A. Melatonin Inhibits GnRH-1, GnRH-3 and GnRH Receptor Expression in the Brain of the European Sea Bass, Dicentrarchus labrax. Int. J. Mol. Sci. 2013, 14, 7603-7616. https://doi.org/10.3390/ijms14047603
Servili A, Herrera-Pérez P, Del Carmen Rendón M, Muñoz-Cueto JA. Melatonin Inhibits GnRH-1, GnRH-3 and GnRH Receptor Expression in the Brain of the European Sea Bass, Dicentrarchus labrax. International Journal of Molecular Sciences. 2013; 14(4):7603-7616. https://doi.org/10.3390/ijms14047603
Chicago/Turabian StyleServili, Arianna, Patricia Herrera-Pérez, María Del Carmen Rendón, and José Antonio Muñoz-Cueto. 2013. "Melatonin Inhibits GnRH-1, GnRH-3 and GnRH Receptor Expression in the Brain of the European Sea Bass, Dicentrarchus labrax" International Journal of Molecular Sciences 14, no. 4: 7603-7616. https://doi.org/10.3390/ijms14047603