Alteration of Podocyte Protein Expression and Localization in the Early Stage of Various Hemodynamic Conditions
Abstract
:1. Introduction
2. Results and Discussion
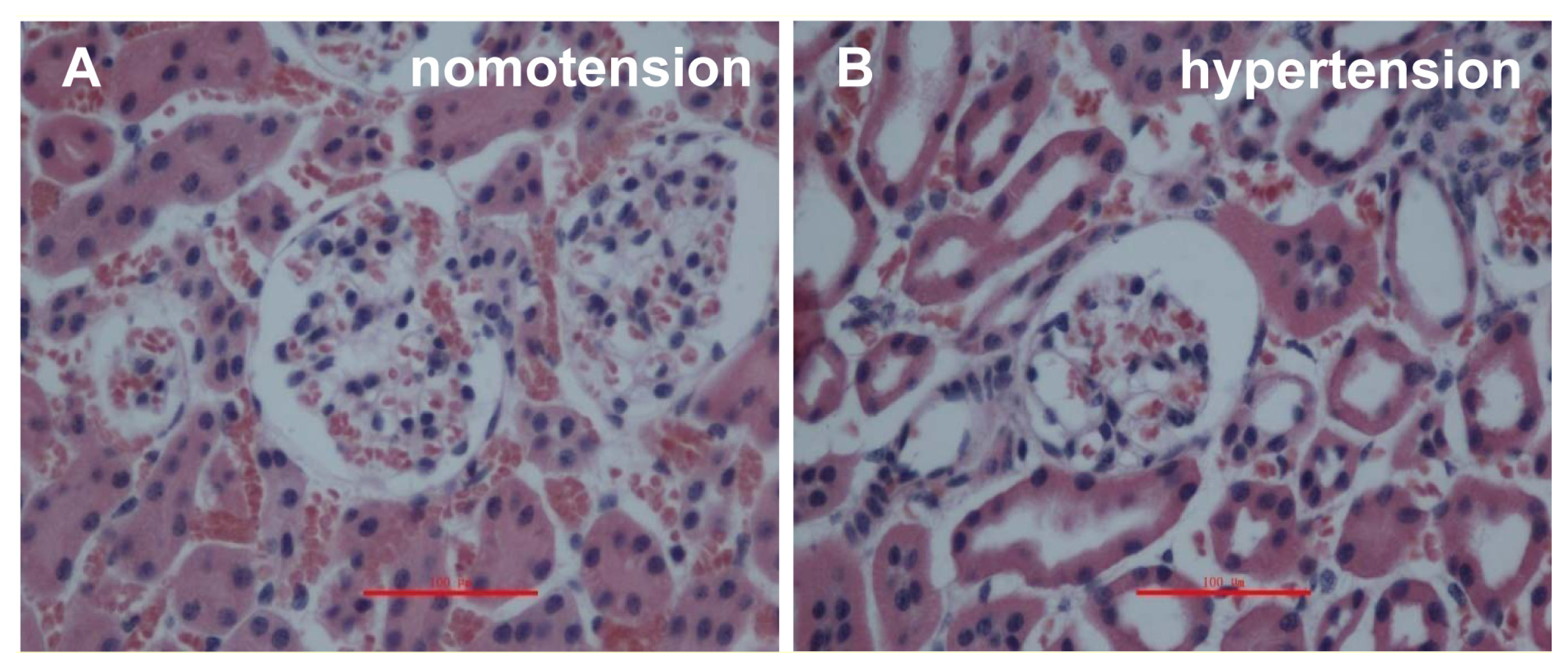
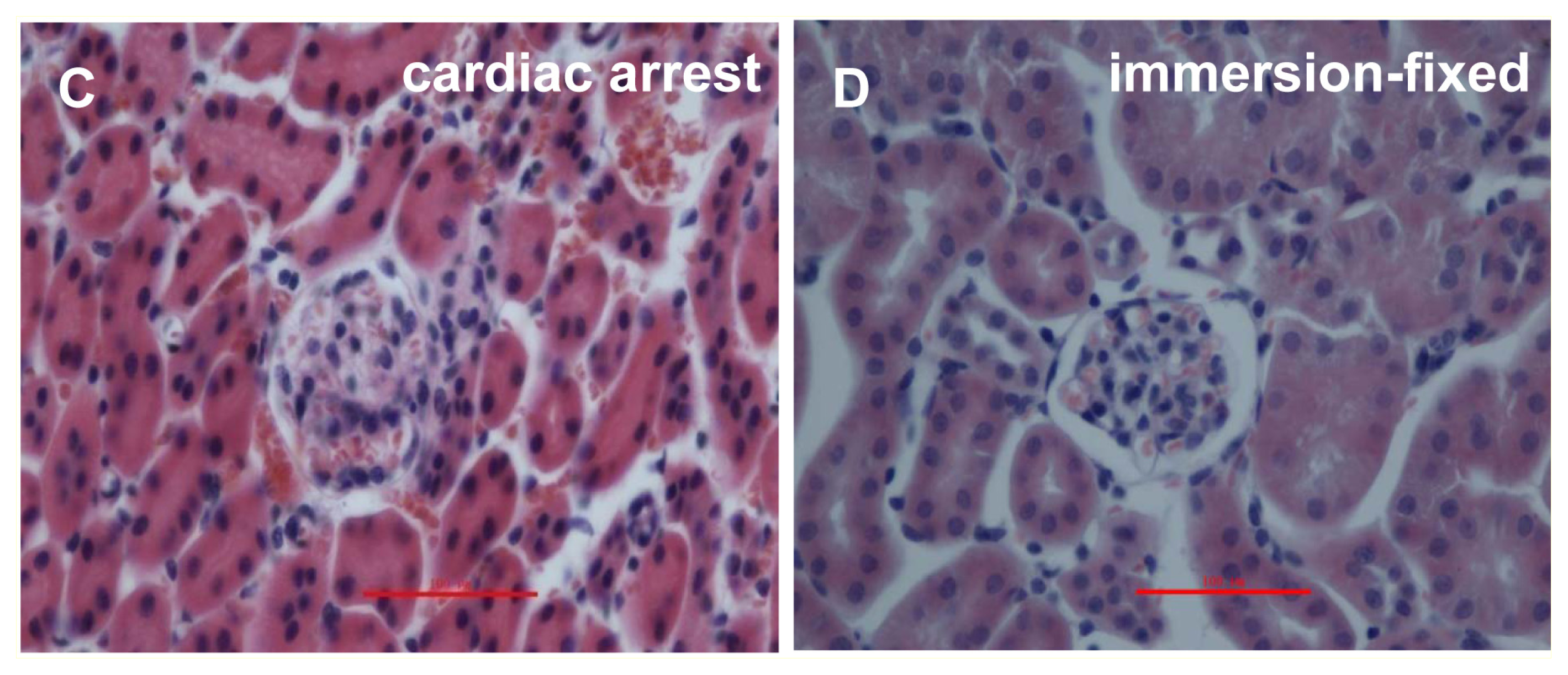
2.1. IVCT Exhibits a Clearer Morphological Alteration of the Glomeruli under Different Hemodynamic Conditions
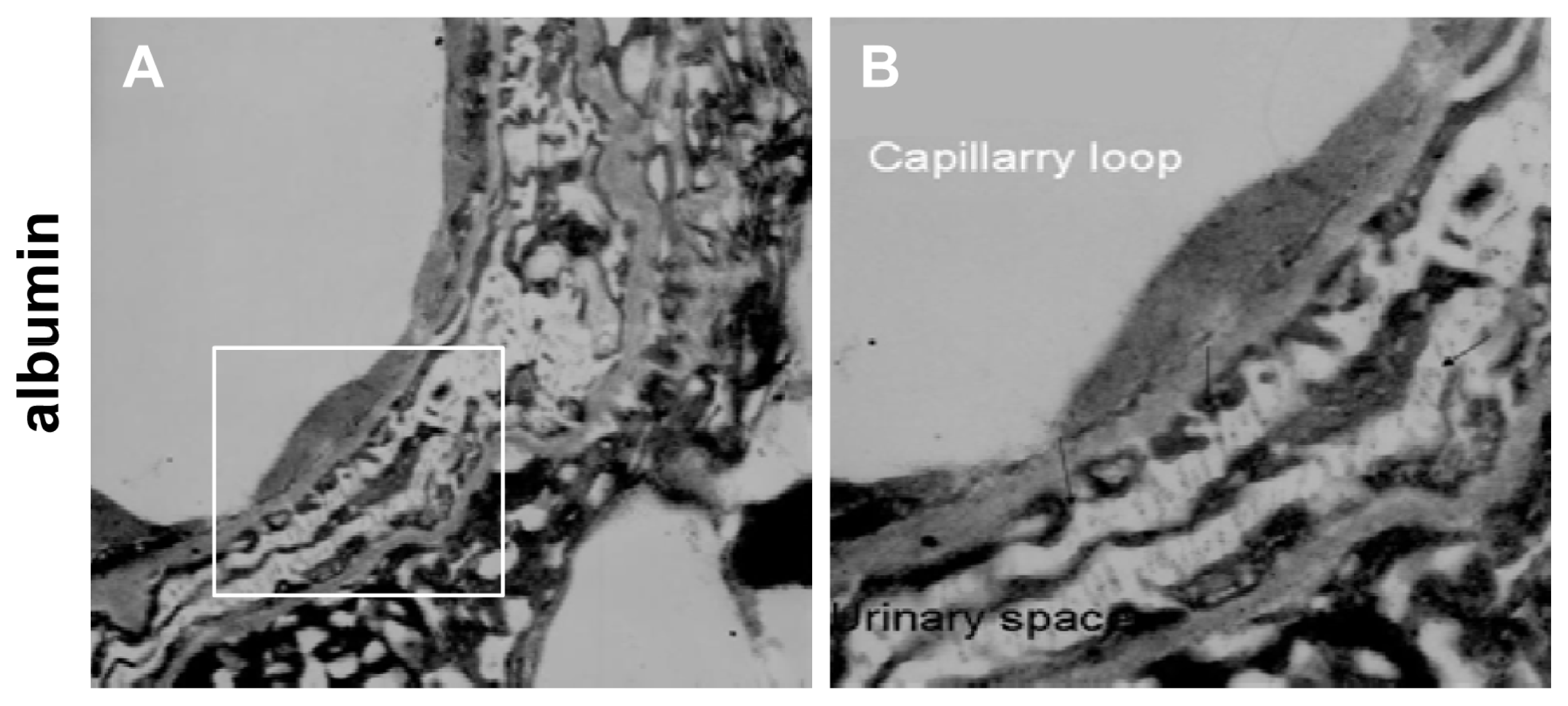
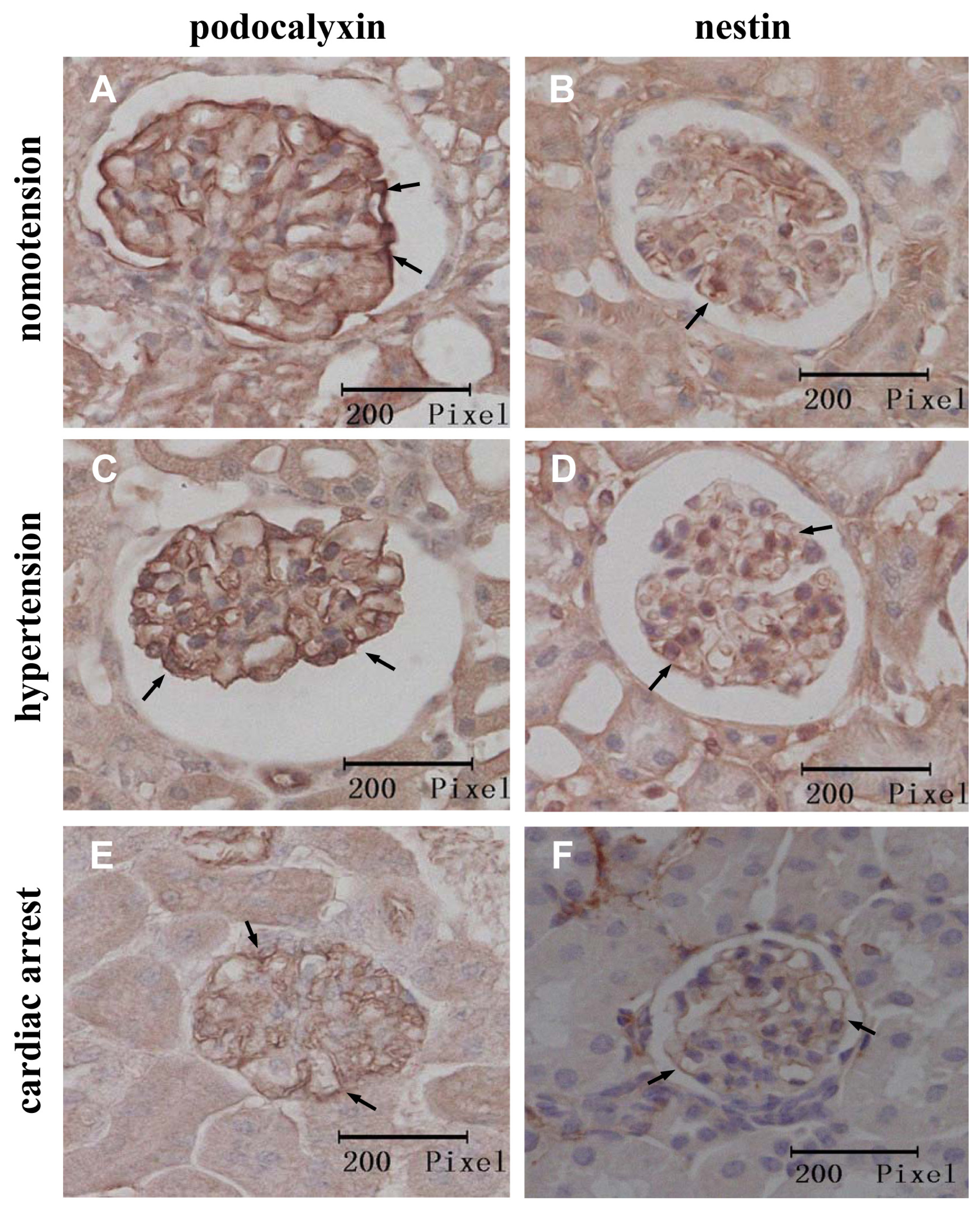
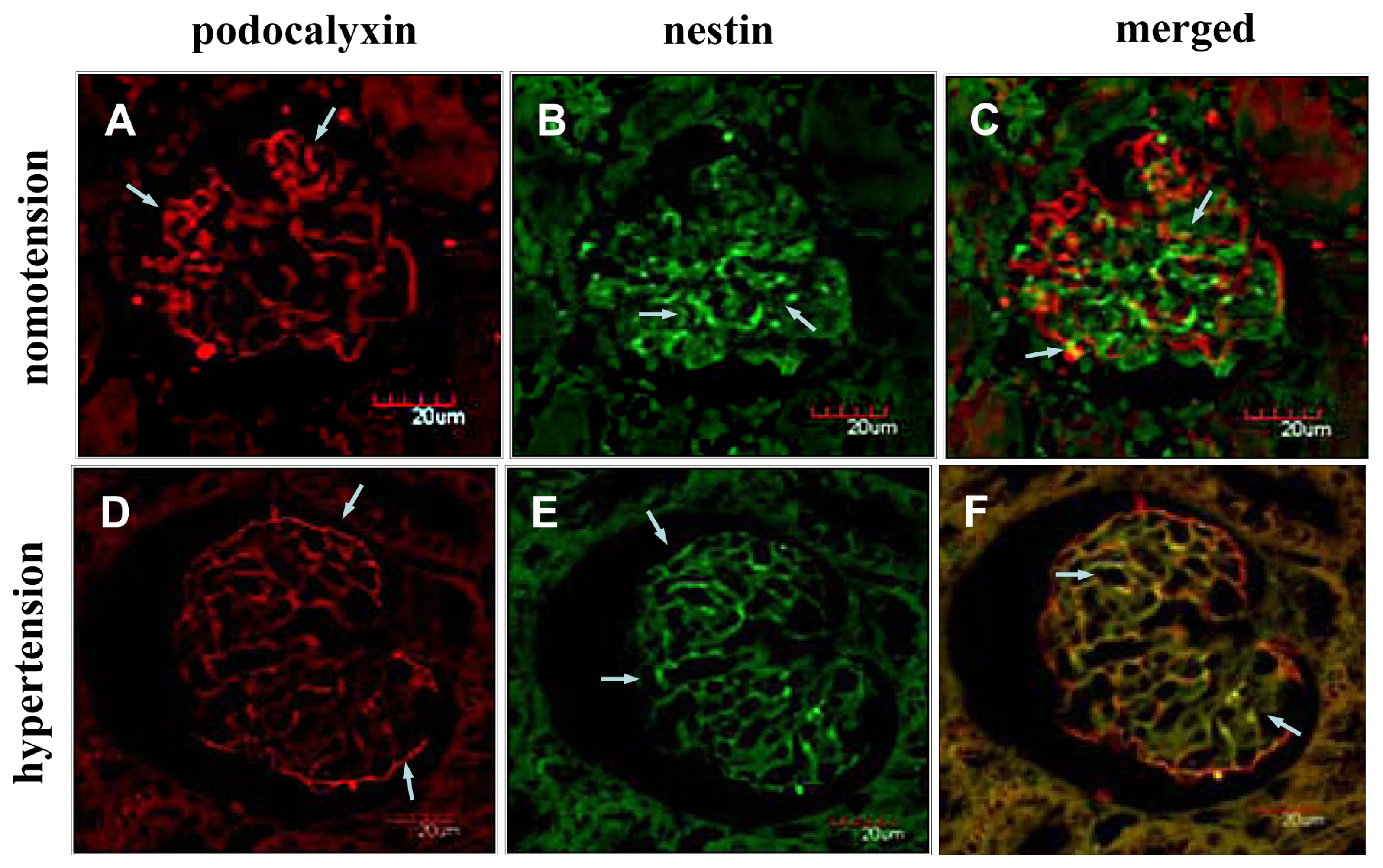
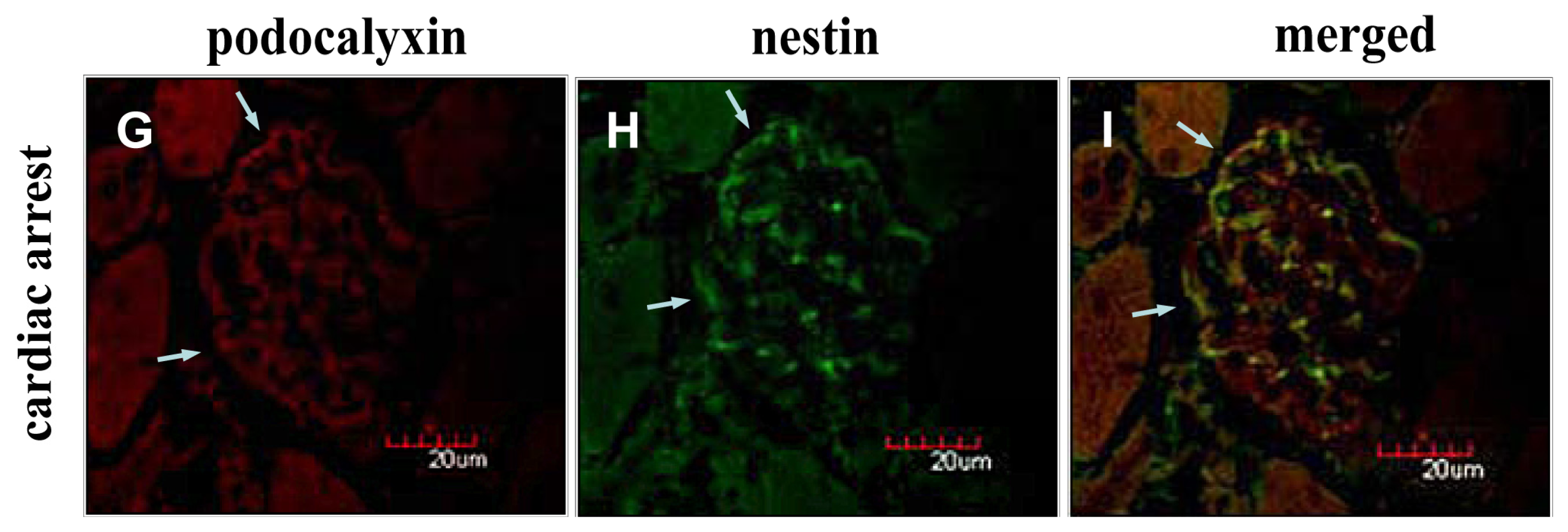
2.2. Instantaneous Changes in the Distribution and Expression of PCX and Nestin under Various Hemodynamic Conditions Are Examined by Immunohistochemistry and Immunofluorescence Analysis
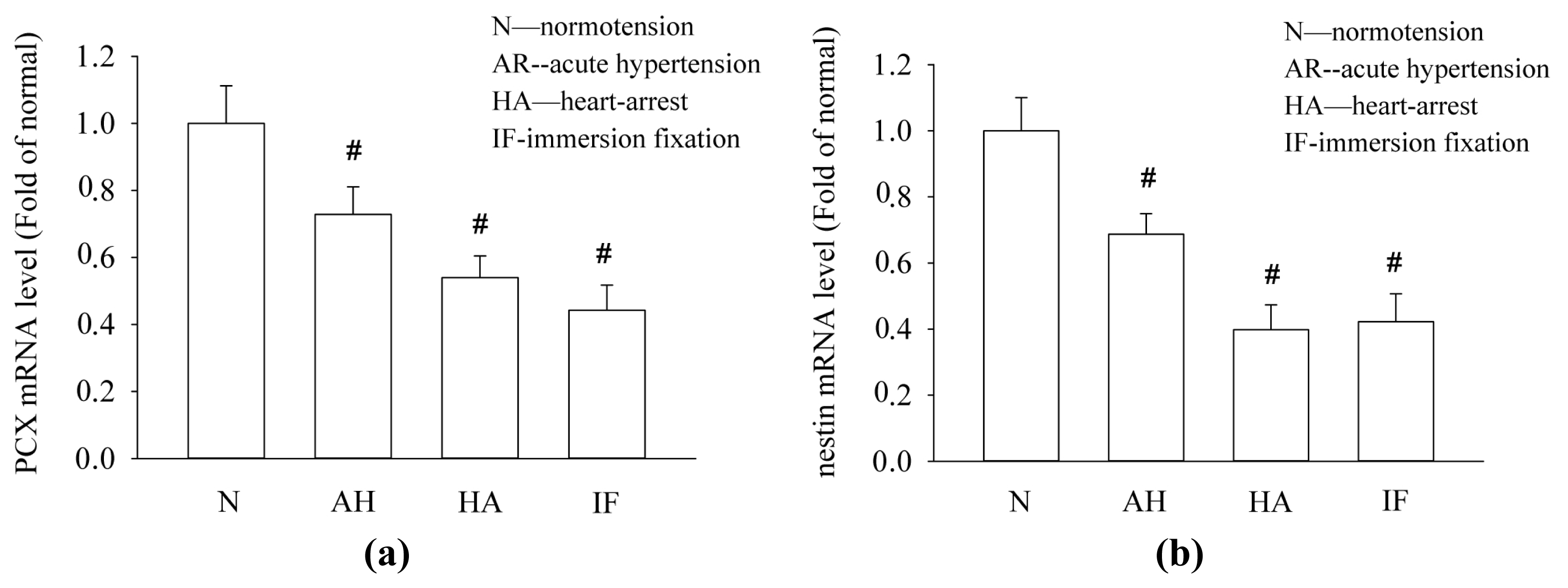
2.3. Acute Hypertensive and Cardiac Arrest Conditions Decrease the mRNA Levels of PCX and Nestin in Kidney Tissues
2.4. Discussion
3. Experimental Section
3.1. Antibodies
3.2. Animals
3.3. Preparation of Kidney Tissues
3.3.1. IVCT for Mouse Kidneys, Freeze-Substitution Fixation and Paraffin-Embedding
3.3.2. Immerse-Fixation of Resected Kidney Tissues
3.4. Immunostaining on Deparaffinized Sections
3.5. Immunoelectron Microscopy
3.6. Real-Time Quantitative PCR
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Miner, J.H. Glomerular basement membrane composition and the filtration barrier. Pediatr. Nephrol 2011, 26, 1413–1417. [Google Scholar]
- Guerrot, D.; Dussaule, J.C.; Mael-Ainin, M.; Xu-Dubois, Y.C.; Rondeau, E. Identification of periostin as a critical marker of progression/reversal of hypertensive nephropathy. PLoS One 2012, 7, e31974. [Google Scholar]
- Kretzler, M.; Koeppen-Hagemann, I.; Kriz, W. Podocyte damage is a critical step in the development of glomerulosclerosis in the uninephrectomised-desoxycorticosterone hypertensive rat. Virchows Arch 1994, 425, 181–193. [Google Scholar]
- Wang, G.; Lai, F.M.; Kwan, B.C.; Lai, K.B.; Chow, K.M. Podocyte loss in human hypertensive nephrosclerosis. Am. J. Hypertens 2009, 22, 300–306. [Google Scholar]
- Perysinaki, G.S.; Moysiadis, D.K.; Bertsias, G.; Giannopoulou, I.; Kyriacou, K. Podocyte main slit diaphragm proteins, nephrin and podocin, are affected at early stages of lupus nephritis and correlate with disease histology. Lupus 2011, 20, 781–791. [Google Scholar]
- Yasuno, K.; Ishihara, S.; Saito, R.; Ishikawa, M.; Kato, T. Early-onset podocyte injury and glomerular sclerosis in osborne-mendel rats. J. Vet. Med. Sci 2010, 72, 1319–1327. [Google Scholar]
- Le Hir, M.; Keller, C.; Eschmann, V.; Hahnel, B.; Hosser, H. Podocyte bridges between the tuft and Bowman’s capsule: An early event in experimental crescentic glomerulonephritis. J. Am. Soc. Nephrol 2001, 12, 2060–2071. [Google Scholar]
- Kerjaschki, D.; Sharkey, D.J.; Farquhar, M.G. Identification and characterization of podocalyxin—The major sialoprotein of the renal glomerular epithelial cell. J. Cell. Biol 1984, 98, 1591–1596. [Google Scholar]
- Doyonnas, R.; Kershaw, D.B.; Duhme, C.; Merkens, H.; Chelliah, S. Anuria, omphalocele, and perinatal lethality in mice lacking the CD34-related protein podocalyxin. J. Exp. Med 2001, 194, 13–27. [Google Scholar]
- Orlando, R.A.; Takeda, T.; Zak, B.; Schmieder, S.; Benoit, V.M. The glomerular epithelial cell anti-adhesin podocalyxin associates with the actin cytoskeleton through interactions with ezrin. J. Am. Soc. Nephrol 2001, 12, 1589–1598. [Google Scholar]
- Su, W.; Fang, C.; Yang, H.C.; Gu, Y.; Hao, C.M. Expression of nestin in human kidney and its clinical significance [in Chinese]. Zhonghua Bing Li Xue Za Zhi 2008, 37, 309–312. [Google Scholar]
- Wagner, N.; Wagner, K.D.; Scholz, H.; Kirschner, K.M.; Schedl, A. Intermediate filament protein nestin is expressed in developing kidney and heart and might be regulated by the Wilms’ tumor suppressor Wt1. Am. J. Physiol. Regul. Integr. Comp. Physiol 2006, 291, R779–R787. [Google Scholar]
- Klein, T.; Ling, Z.; Heimberg, H.; Madsen, O.D.; Heller, R.S.; Serup, P. Nestin in expressed in vascular endothelial cells in the adult human pancreas. J. Histochem. Cytochem 2003, 51, 697–706. [Google Scholar]
- Perry, J.; Ho, M.; Viero, S.; Zheng, K.; Jacobs, R. The intermediate filament nestin is highly expressed in normal human podocytes and podocytes in glomerular disease. Pediatr. Dev. Pathol 2007, 10, 369–382. [Google Scholar]
- Zou, J.; Yaoita, E.; Watanabe, Y.; Yoshida, Y.; Nameta, M. Upregulation of nestin, vimentin, and desmin in rat podocytes in response to injury. Virchows Arch 2006, 448, 485–492. [Google Scholar]
- Li, Z.; Li, K.; Wang, J.; Zhai, X.; Wang, L.; Ohno, N.; Ohno, S. Application of novel “in vivo cryotechnique” in living animal kidneys. Microsc. Res. Tech 2012, 76, 113–120. [Google Scholar]
- Li, Z.; Ohno, N.; Terada, N.; Ohno, S. Immunolocalization of serum proteins in living mouse glomeruli under various hemodynamic conditions by “in vivo cryotechnique”. Histochem. Cell. Biol 2006, 126, 399–406. [Google Scholar]
- Ohno, S.; Terada, N.; Ohno, N.; Saitoh, S.; Saitoh, Y. Significance of “in vivo cryotechnique” for morphofunctional analyses of living animal organs. J. Electron. Microsc. Tokyo 2010, 59, 395–408. [Google Scholar]
- Shi, L.; Terada, N.; Saitoh, Y.; Saitoh, S.; Ohno, S. Immunohistochemical distribution of serum proteins in living mouse heart with In vivo cryotechnique. Acta Histochem. Cytochem 2011, 44, 61–72. [Google Scholar]
- Li, Z.; Terada, N.; Ohno, N.; Ohno, S. Immunohistochemical analyses on albumin and immunoglobulin in acute hypertensive mouse kidneys by “in vivo cryotechnique”. Histol. Histopathol 2005, 20, 807–816. [Google Scholar]
- Zhou, D.; Ohno, N.; Terada, N.; Li, Z.; Morita, H. Immunohistochemical analyses on serum proteins in nephrons of protein-overload mice by “in vivo cryotechnique”. Histol. Histopathol 2007, 22, 137–145. [Google Scholar]
- Ohno, S.; Kato, Y.; Xiang, T.; Terada, N.; Takayama, I. Ultrastructural study of mouse renal glomeruli under various hemodynamic conditions by an “in vivo cryotechnique”. Ital. J. Anat. Embryol 2001, 106, 431–438. [Google Scholar]
- Economou, C.G.; Kitsiou, P.V.; Tzinia, A.K.; Panagopoulou, E.; Marinos, E. Enhanced podocalyxin expression alters the structure of podocyte basal surface. J. Cell. Sci 2004, 117, 3281–3294. [Google Scholar]
- Kershaw, D.B.; Thomas, P.E.; Wharram, B.L.; Goyal, M.; Wiggins, J.E. Molecular cloning, expression, and characterization of podocalyxin-like protein 1 from rabbit as a transmembrane protein of glomerular podocytes and vascular endothelium. J. Biol. Chem 1995, 270, 29439–29446. [Google Scholar]
- Wegner, B.; Al-Momany, A.; Kulak, S.C.; Kozlowski, K.; Obeidat, M. CLIC5A, a component of the ezrin-podocalyxin complex in glomeruli, is a determinant of podocyte integrity. Am. J. Physiol. Renal. Physiol 2010, 298, F1492–F1503. [Google Scholar]
- Qi, J.; Xiao, Y.F.; Zhang, D.J.; Yang, G.R.; Huang, H.C. High glucose downregulates the expression of podocalyxin protein in glomerular podocytes of mice [in Chinese]. Beijing Da Xue Xue Bao 2007, 39, 167–170. [Google Scholar]
- Zhang, J.; Li, P.H.; Yang, L.; Du, Q.S.; Guo, T.T. Connective tissue growth factor mediates high glucose-induced down-regulation of podocalyxin expression in mouse podocytes [in Chinese]. Nan Fang Yi Ke Da Xue Xue Bao 2011, 31, 839–843. [Google Scholar]
- Chen, J.; Boyle, S.; Zhao, M.; Su, W.; Takahashi, K. Differential expression of the intermediate filament protein nestin during renal development and its localization in adult podocytes. J. Am. Soc. Nephrol 2006, 17, 1283–1291. [Google Scholar]
- Wen, D.; You, L.; Zhang, Q.; Zhang, L.; Gu, Y. Upregulation of nestin protects podocytes from apoptosis induced by puromycin aminonucleoside. Am. J. Nephrol 2011, 34, 423–434. [Google Scholar]
- Ritz, E.; Fliser, D.; Siebels, M. Pathophysiology of hypertensive renal damage. Am. J. Hypertens 1993, 6, S241–S244. [Google Scholar]
- Advani, A.; Kelly, D.J.; Advani, S.L.; Cox, A.J.; Thai, K. Role of VEGF in maintaining renal structure and function under normotensive and hypertensive conditions. Proc. Natl. Acad. Sci. USA 2007, 104, 14448–14453. [Google Scholar]
- Deen, W.M. What determines glomerular capillary permeability? J. Clin. Invest 2004, 114, 1412–1414. [Google Scholar]
- Karumanchi, S.A.; Epstein, F.H.; Stillman, I.E. Is loss of podocyte foot processes necessary for the induction of proteinuria? Am. J. Kidney Dis 2005, 45, 436. [Google Scholar]
- Stillman, I.E.; Karumanchi, S.A. The glomerular injury of preeclampsia. J. Am. Soc. Nephrol 2007, 18, 2281–2284. [Google Scholar]


© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, K.; Wang, J.; Yin, X.; Zhai, X.; Li, Z. Alteration of Podocyte Protein Expression and Localization in the Early Stage of Various Hemodynamic Conditions. Int. J. Mol. Sci. 2013, 14, 5998-6011. https://doi.org/10.3390/ijms14035998
Li K, Wang J, Yin X, Zhai X, Li Z. Alteration of Podocyte Protein Expression and Localization in the Early Stage of Various Hemodynamic Conditions. International Journal of Molecular Sciences. 2013; 14(3):5998-6011. https://doi.org/10.3390/ijms14035998
Chicago/Turabian StyleLi, Kai, Juan Wang, Xiaohui Yin, Xiaoyue Zhai, and Zilong Li. 2013. "Alteration of Podocyte Protein Expression and Localization in the Early Stage of Various Hemodynamic Conditions" International Journal of Molecular Sciences 14, no. 3: 5998-6011. https://doi.org/10.3390/ijms14035998



