Effect of Different Phospholipids on α-Secretase Activity in the Non-Amyloidogenic Pathway of Alzheimer’s Disease
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of FA Carbon Chain Length on α-Secretase Activity
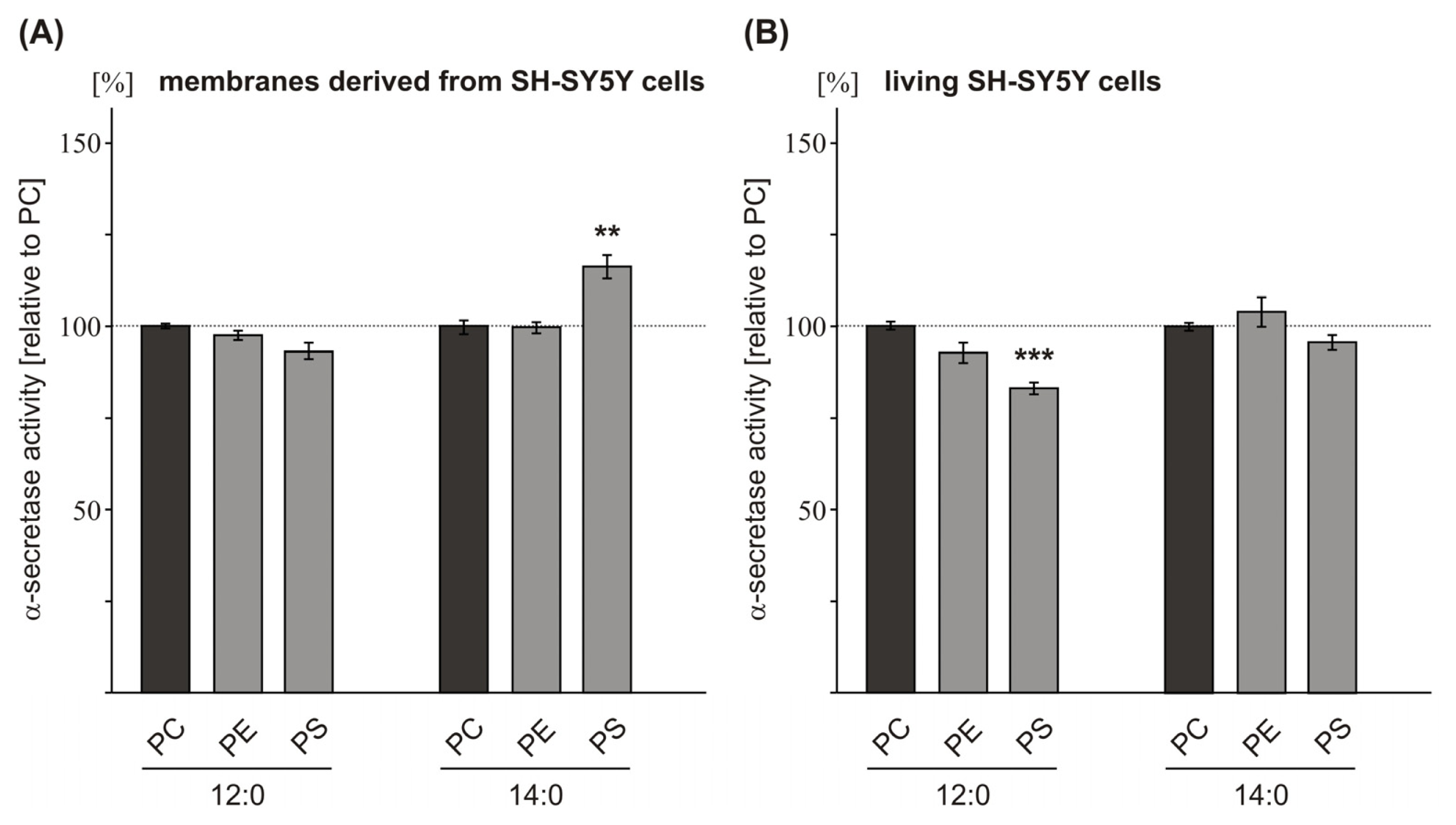
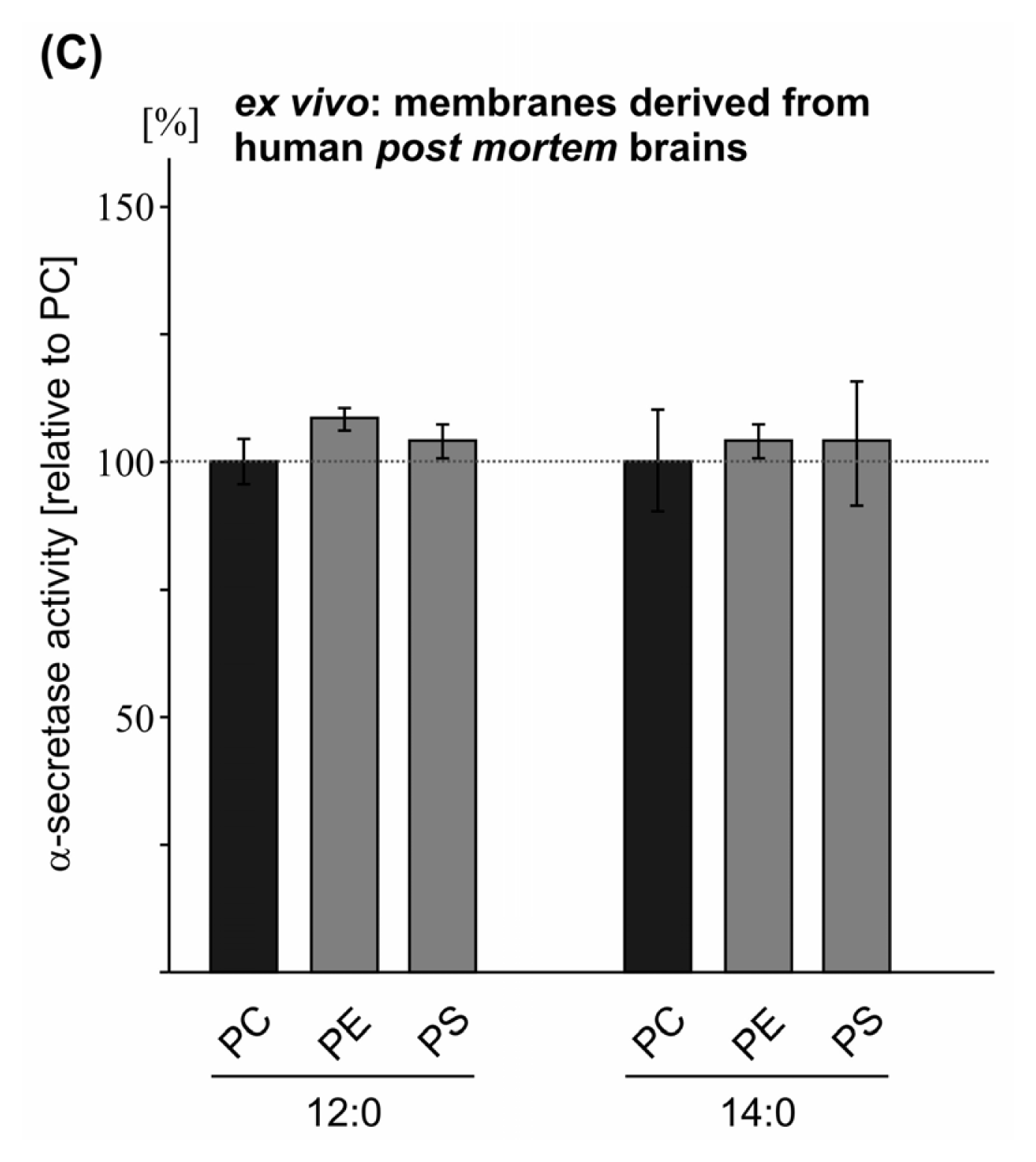
2.2. Variations in the Phospholipid Headgroup on α-Secretase Activity
2.3. Effect of FA Saturation on α-Secretase Activity
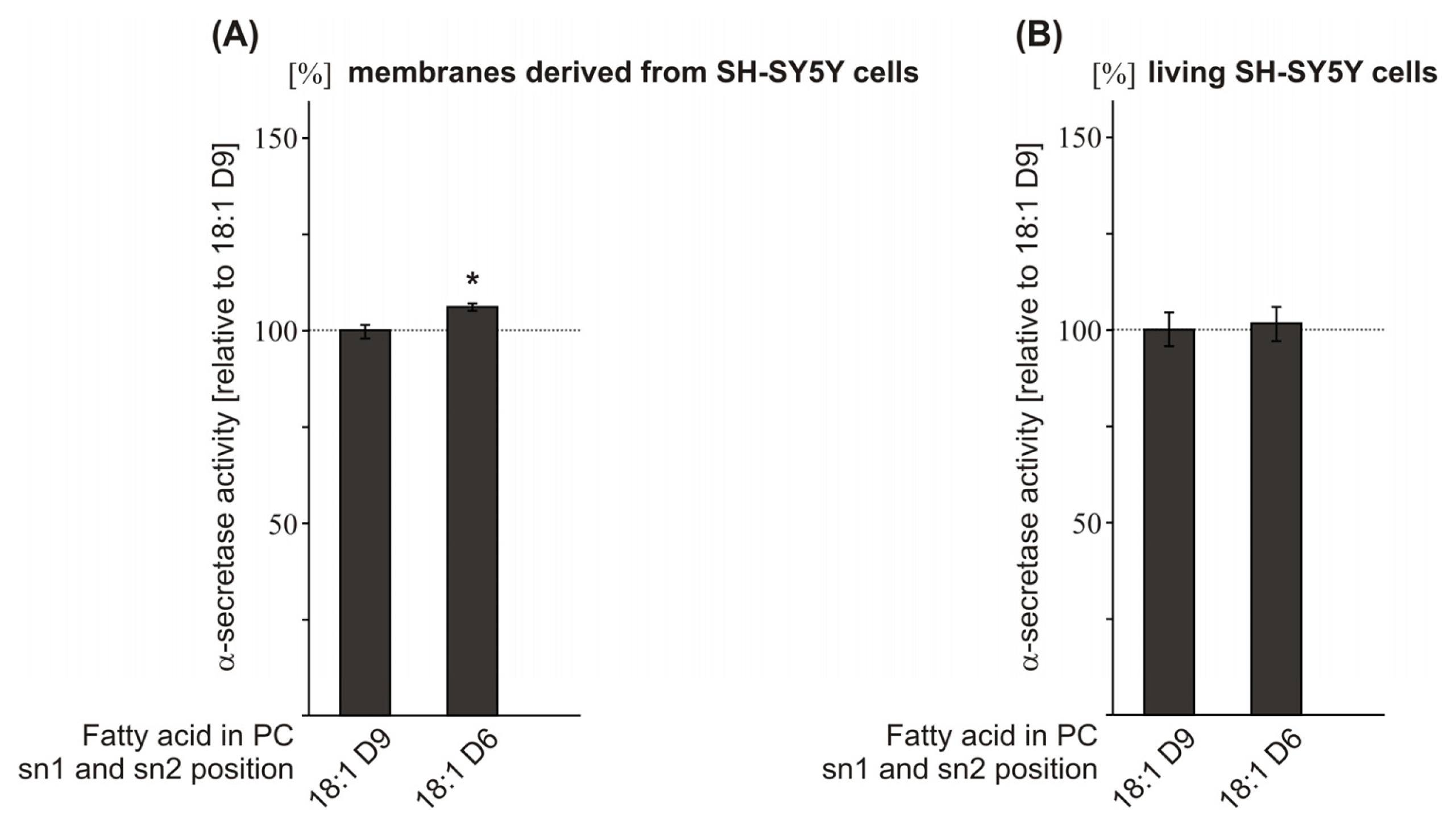
2.4. Effect of the Double-Bond Position on α-Secretase Activity
3. Experimental Section
3.1. Chemicals and Reagents
3.2. Cell Culture and Incubation with Phospholipids
3.3. Detection of α-Secretase Activity in Vivo
3.4. Human Post Mortem Brains
3.5. Preparation of Purified Membranes
3.6. Preparation of Human Brain Lipid Extract
3.7. Determination of α-Secretase Activity and in Vitro Incubation
3.8. Determination of ADAM10 Purified Enzyme Activity
3.9. Detection of Phospholipid Species in SH-SY5Ywt Cells and Human Post Mortem Brains
3.10. Mass Spectrometry Analysis
3.11. Statistical Analysis
4. Conclusions
Supplementary Information
ijms-14-05879-s001.pdfAcknowledgments
Conflict of Interest
References
- Masters, C.L.; Simms, G.; Weinman, N.A.; Multhaup, G.; McDonald, B.L.; Beyreuther, K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl. Acad. Sci. USA 1985, 82, 4245–4249. [Google Scholar]
- Selkoe, D.J. Alzheimer’s disease: Genes, proteins, and therapy. Physiol. Rev 2001, 81, 741–766. [Google Scholar]
- Dyrks, T.; Weidemann, A.; Multhaup, G.; Salbaum, J.M.; Lemaire, H.G.; Kang, J.; Muller-Hill, B.; Masters, C.L.; Beyreuther, K. Identification, transmembrane orientation and biogenesis of the amyloid A4 precursor of Alzheimer’s disease. EMBO J 1988, 7, 949–957. [Google Scholar]
- Vassar, R.; Bennett, B.D.; Babu-Khan, S.; Kahn, S.; Mendiaz, E.A.; Denis, P.; Teplow, D.B.; Ross, S.; Amarante, P.; Loeloff, R.; et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 1999, 286, 735–741. [Google Scholar]
- Sinha, S.; Anderson, J.P.; Barbour, R.; Basi, G.S.; Caccavello, R.; Davis, D.; Doan, M.; Dovey, H.F.; Frigon, N.; Hong, J.; et al. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature 1999, 402, 537–540. [Google Scholar]
- Haass, C.; Schlossmacher, M.G.; Hung, A.Y.; Vigo-Pelfrey, C.; Mellon, A.; Ostaszewski, B.L.; Lieberburg, I.; Koo, E.H.; Schenk, D.; Teplow, D.B.; et al. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature 1992, 359, 322–325. [Google Scholar]
- Shoji, M.; Golde, T.E.; Ghiso, J.; Cheung, T.T.; Estus, S.; Shaffer, L.M.; Cai, X.D.; McKay, D.M.; Tintner, R.; Frangione, B.; et al. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science 1992, 258, 126–129. [Google Scholar]
- Herreman, A.; Serneels, L.; Annaert, W.; Collen, D.; Schoonjans, L.; de Strooper, B. Total inactivation of gamma-secretase activity in presenilin-deficient embryonic stem cells. Nat. Cell. Biol 2000, 2, 461–462. [Google Scholar]
- Takasugi, N.; Tomita, T.; Hayashi, I.; Tsuruoka, M.; Niimura, M.; Takahashi, Y.; Thinakaran, G.; Iwatsubo, T. The role of presenilin cofactors in the gamma-secretase complex. Nature 2003, 422, 438–441. [Google Scholar]
- Kimberly, W.T.; LaVoie, M.J.; Ostaszewski, B.L.; Ye, W.; Wolfe, M.S.; Selkoe, D.J. Gamma-secretase is a membrane protein complex comprised of presenilin, nicastrin, Aph-1, and Pen-2. Proc. Natl. Acad. Sci. USA 2003, 100, 6382–6387. [Google Scholar]
- Duering, M.; Grimm, M.O.; Grimm, H.S.; Schroder, J.; Hartmann, T. Mean age of onset in familial Alzheimer’s disease is determined by amyloid beta 42. Neurobiol. Aging 2005, 26, 785–788. [Google Scholar]
- Haass, C.; Hung, A.Y.; Schlossmacher, M.G.; Teplow, D.B.; Selkoe, D.J. Beta-Amyloid peptide and a 3-kDa fragment are derived by distinct cellular mechanisms. J. Biol. Chem 1993, 268, 3021–3024. [Google Scholar]
- Lichtenthaler, S.F. Alpha-secretase in Alzheimer’s disease: Molecular identity, regulation and therapeutic potential. J. Neurochem 2011, 116, 10–21. [Google Scholar]
- Buxbaum, J.D.; Liu, K.N.; Luo, Y.; Slack, J.L.; Stocking, K.L.; Peschon, J.J.; Johnson, R.S.; Castner, B.J.; Cerretti, D.P.; Black, R.A. Evidence that tumor necrosis factor alpha converting enzyme is involved in regulated alpha-secretase cleavage of the Alzheimer amyloid protein precursor. J. Biol. Chem 1998, 273, 27765–27767. [Google Scholar]
- Lammich, S.; Kojro, E.; Postina, R.; Gilbert, S.; Pfeiffer, R.; Jasionowski, M.; Haass, C.; Fahrenholz, F. Constitutive and regulated alpha-secretase cleavage of Alzheimer’s amyloid precursor protein by a disintegrin metalloprotease. Proc. Natl. Acad. Sci. USA 1999, 96, 3922–3927. [Google Scholar]
- Koike, H.; Tomioka, S.; Sorimachi, H.; Saido, T.C.; Maruyama, K.; Okuyama, A.; Fujisawa-Sehara, A.; Ohno, S.; Suzuki, K.; Ishiura, S. Membrane-anchored metalloprotease MDC9 has an alpha-secretase activity responsible for processing the amyloid precursor protein. Biochem. J 1999, 343, 371–375. [Google Scholar]
- Allinson, T.M.; Parkin, E.T.; Turner, A.J.; Hooper, N.M. ADAMs family members as amyloid precursor protein alpha-secretases. J. Neurosci. Res 2003, 74, 342–352. [Google Scholar]
- Hartmann, T.; Kuchenbecker, J.; Grimm, M.O. Alzheimer’s disease: The lipid connection. J. Neurochem 2007, 103, 159–170. [Google Scholar]
- Osenkowski, P.; Ye, W.; Wang, R.; Wolfe, M.S.; Selkoe, D.J. Direct and potent regulation of gamma-secretase by its lipid microenvironment. J. Biol. Chem 2008, 283, 22529–22540. [Google Scholar]
- Grimm, M.O.; Rothhaar, T.L.; Hartmann, T. The role of APP proteolytic processing in lipid metabolism. Exp. Brain Res 2012, 217, 365–375. [Google Scholar]
- Lemkul, J.A.; Bevan, D.R. Lipid composition influences the release of Alzheimer’s amyloid beta-peptide from membranes. Protein Sci 2011, 20, 1530–1545. [Google Scholar]
- Grimm, M.O.; Rothhaar, T.L.; Grösgen, S.; Burg, V.K.; Hundsdorfer, B.; Haupenthal, V.J.; Friess, P.; Kins, S.; Grimm, H.S.; Hartmann, T. Trans fatty acids enhance amyloidogenic processing of the Alzheimer amyloid precursor protein (APP). J. Nutr. Biochem 2012, 23, 1214–1223. [Google Scholar]
- Rothhaar, T.L.; Grosgen, S.; Haupenthal, V.J.; Burg, V.K.; Hundsdorfer, B.; Mett, J.; Riemenschneider, M.; Grimm, H.S.; Hartmann, T.; Grimm, M.O. Plasmalogens inhibit APP processing by directly affecting gamma-secretase activity in Alzheimer’s disease. Sci. World J 2012, 2012, 141240. [Google Scholar]
- Marenchino, M.; Williamson, P.T.; Murri, S.; Zandomeneghi, G.; Wunderli-Allenspach, H.; Meier, B.H.; Kramer, S.D. Dynamics and Cleavability at the alpha-cleavage site of APP(684–726) in different lipid environments. Biophys. J 2008, 95, 1460–1473. [Google Scholar]
- Barrett, P.J.; Song, Y.; van Horn, W.D.; Hustedt, E.J.; Schafer, J.M.; Hadziselimovic, A.; Beel, A.J.; Sanders, C.R. The amyloid precursor protein has a flexible transmembrane domain and binds cholesterol. Science 2012, 336, 1168–1171. [Google Scholar]
- Simons, M.; Keller, P.; de Strooper, B.; Beyreuther, K.; Dotti, C.G.; Simons, K. Cholesterol depletion inhibits the generation of beta-amyloid in hippocampal neurons. Proc. Natl. Acad. Sci. USA 1998, 95, 6460–6464. [Google Scholar]
- Grösgen, S.; Grimm, M.O.; Friess, P.; Hartmann, T. Role of amyloid beta in lipid homeostasis. Biochim. Biophys. Acta 2010, 1801, 966–974. [Google Scholar]
- Grimm, M.O.; Grimm, H.S.; Tomic, I.; Beyreuther, K.; Hartmann, T.; Bergmann, C. Independent inhibition of Alzheimer disease beta- and gamma-secretase cleavage by lowered cholesterol levels. J. Biol. Chem 2008, 283, 11302–11311. [Google Scholar]
- Grimm, M.O.; Grimm, H.S.; Patzold, A.J.; Zinser, E.G.; Halonen, R.; Duering, M.; Tschape, J.A.; De Strooper, B.; Muller, U.; Shen, J.; et al. Regulation of cholesterol and sphingomyelin metabolism by amyloid-beta and presenilin. Nat. Cell. Biol. 2005, 7, 1118–1123. [Google Scholar]
- Zha, Q.; Ruan, Y.; Hartmann, T.; Beyreuther, K.; Zhang, D. GM1 ganglioside regulates the proteolysis of amyloid precursor protein. Mol. Psychiatry 2004, 9, 946–952. [Google Scholar]
- Grimm, M.O.; Zinser, E.G.; Grösgen, S.; Hundsdorfer, B.; Rothhaar, T.L.; Burg, V.K.; Kaestner, L.; Bayer, T.A.; Lipp, P.; Muller, U.; et al. Amyloid precursor protein (APP) mediated regulation of ganglioside homeostasis linking Alzheimer’s disease pathology with ganglioside metabolism. PLoS One 2012, 7, e34095. [Google Scholar]
- Svennerholm, L.; Gottfries, C.G. Membrane lipids, selectively diminished in Alzheimer brains, suggest synapse loss as a primary event in early-onset form (type I) and demyelination in late-onset form (type II). J. Neurochem 1994, 62, 1039–1047. [Google Scholar]
- Wells, K.; Farooqui, A.A.; Liss, L.; Horrocks, L.A. Neural membrane phospholipids in Alzheimer disease. Neurochem. Res 1995, 20, 1329–1333. [Google Scholar]
- Prasad, M.R.; Lovell, M.A.; Yatin, M.; Dhillon, H.; Markesbery, W.R. Regional membrane phospholipid alterations in Alzheimer’s disease. Neurochem. Res 1998, 23, 81–88. [Google Scholar]
- Holmes, O.; Paturi, S.; Ye, W.; Wolfe, M.S.; Selkoe, D.J. Effects of membrane lipids on the activity and processivity of purified gamma-secretase. Biochemistry 2012, 51, 3565–3575. [Google Scholar]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar]
- Vetrivel, K.S.; Thinakaran, G. Membrane rafts in Alzheimer’s disease beta-amyloid production. Biochim. Biophys. Acta 2010, 1801, 860–867. [Google Scholar]
- Riddell, D.R.; Christie, G.; Hussain, I.; Dingwall, C. Compartmentalization of beta-secretase (Asp2) into low-buoyant density, noncaveolar lipid rafts. Curr. Biol 2001, 11, 1288–1293. [Google Scholar]
- Vetrivel, K.S.; Cheng, H.; Lin, W.; Sakurai, T.; Li, T.; Nukina, N.; Wong, P.C.; Xu, H.; Thinakaran, G. Association of gamma-secretase with lipid rafts in post-Golgi and endosome membranes. J. Biol. Chem 2004, 279, 44945–44954. [Google Scholar]
- Vetrivel, K.S.; Cheng, H.; Kim, S.H.; Chen, Y.; Barnes, N.Y.; Parent, A.T.; Sisodia, S.S.; Thinakaran, G. Spatial segregation of gamma-secretase and substrates in distinct membrane domains. J. Biol. Chem 2005, 280, 25892–25900. [Google Scholar]
- Koumanov, K.S.; Tessier, C.; Momchilova, A.B.; Rainteau, D.; Wolf, C.; Quinn, P.J. Comparative lipid analysis and structure of detergent-resistant membrane raft fractions isolated from human and ruminant erythrocytes. Arch. Biochem. Biophys 2005, 434, 150–158. [Google Scholar]
- Kojro, E.; Gimpl, G.; Lammich, S.; Marz, W.; Fahrenholz, F. Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the α-secretase ADAM 10. Proc. Natl. Acad. Sci. USA 2001, 98, 5815–5820. [Google Scholar]
- Ehehalt, R.; Keller, P.; Haass, C.; Thiele, C.; Simons, K. Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J. Cell Biol 2003, 160, 113–123. [Google Scholar]
- Parr-Sturgess, C.A.; Rushton, D.J.; Parkin, E.T. Ectodomain shedding of the Notch ligand Jagged1 is mediated by ADAM17, but is not a lipid-raft-associated event. Biochem. J 2010, 432, 283–294. [Google Scholar]
- Kuhn, P.H.; Wang, H.; Dislich, B.; Colombo, A.; Zeitschel, U.; Ellwart, J.W.; Kremmer, E.; Rossner, S.; Lichtenthaler, S.F. ADAM10 is the physiologically relevant, constitutive alpha-secretase of the amyloid precursor protein in primary neurons. EMBO J 2010, 29, 3020–3032. [Google Scholar]
- Stokes, C.E.; Hawthorne, J.N. Reduced phosphoinositide concentrations in anterior temporal cortex of Alzheimer-diseased brains. J. Neurochem 1987, 48, 1018–1021. [Google Scholar]
- Nitsch, R.M.; Blusztajn, J.K.; Pittas, A.G.; Slack, B.E.; Growdon, J.H.; Wurtman, R.J. Evidence for a membrane defect in Alzheimer disease brain. Proc. Natl. Acad. Sci. USA 1992, 89, 1671–1675. [Google Scholar]
- Nesic, I.; Guix, F.X.; Vennekens, K.; Michaki, V.; van Veldhoven, P.P.; Feiguin, F.; de Strooper, B.; Dotti, C.G.; Wahle, T. Alterations in phosphatidylethanolamine levels affect the generation of Abeta. Aging Cell 2012, 11, 63–72. [Google Scholar]
- Escriba, P.V.; Gonzalez-Ros, J.M.; Goni, F.M.; Kinnunen, P.K.; Vigh, L.; Sanchez-Magraner, L.; Fernandez, A.M.; Busquets, X.; Horvath, I.; Barcelo-Coblijn, G. Membranes: A meeting point for lipids, proteins and therapies. J. Cell. Mol. Med 2008, 12, 829–875. [Google Scholar]
- Bazan, N.G.; Scott, B.L. Dietary omega-3 fatty acids and accumulation of docosahexaenoic acid in rod photoreceptor cells of the retina and at synapses. Ups. J. Med. Sci. Suppl 1990, 48, 97–107. [Google Scholar]
- Ansari, K.A.; Shoeman, D.W. Arachidonic and docosahexanoic acid content of bovine brain myelin: Implications for the pathogenesis of multiple sclerosis. Neurochem. Res 1990, 15, 7–11. [Google Scholar]
- Horrocks, L.A.; Farooqui, A.A. Docosahexaenoic acid in the diet: Its importance in maintenance and restoration of neural membrane function. Prostaglandins Leukot Essent Fatty Acids 2004, 70, 361–372. [Google Scholar]
- Yang, X.; Sheng, W.; Sun, G.Y.; Lee, J.C. Effects of fatty acid unsaturation numbers on membrane fluidity and alpha-secretase-dependent amyloid precursor protein processing. Neurochem. Int 2011, 58, 321–329. [Google Scholar]
- Eckert, G.P.; Chang, S.; Eckmann, J.; Copanaki, E.; Hagl, S.; Hener, U.; Muller, W.E.; Kogel, D. Liposome-incorporated DHA increases neuronal survival by enhancing non-amyloidogenic APP processing. Biochim. Biophys. Acta 2011, 1808, 236–243. [Google Scholar]
- Soderberg, M.; Edlund, C.; Kristensson, K.; Dallner, G. Fatty acid composition of brain phospholipids in aging and in Alzheimer’s disease. Lipids 1991, 26, 421–425. [Google Scholar]
- Tully, A.M.; Roche, H.M.; Doyle, R.; Fallon, C.; Bruce, I.; Lawlor, B.; Coakley, D.; Gibney, M.J. Low serum cholesteryl ester-docosahexaenoic acid levels in Alzheimer’s disease: A case-control study. Br. J. Nutr 2003, 89, 483–489. [Google Scholar]
- Cunnane, S.C.; Schneider, J.A.; Tangney, C.; Tremblay-Mercier, J.; Fortier, M.; Bennett, D.A.; Morris, M.C. Plasma and brain fatty acid profiles in mild cognitive impairment and Alzheimer’s disease. J. Alzheimers Dis 2012, 29, 691–697. [Google Scholar]
- Barberger-Gateau, P.; Letenneur, L.; Deschamps, V.; Peres, K.; Dartigues, J.F.; Renaud, S. Fish, meat, and risk of dementia: Cohort study. Br. Med. J 2002, 325, 932–933. [Google Scholar]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Wilson, R.S.; Aggarwal, N.; Schneider, J. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch. Neurol 2003, 60, 940–946. [Google Scholar]
- Schaefer, E.J.; Bongard, V.; Beiser, A.S.; Lamon-Fava, S.; Robins, S.J.; Au, R.; Tucker, K.L.; Kyle, D.J.; Wilson, P.W.; Wolf, P.A. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: The Framingham Heart Study. Arch. Neurol 2006, 63, 1545–1550. [Google Scholar]
- Van Gelder, B.M.; Tijhuis, M.; Kalmijn, S.; Kromhout, D. Fish consumption, n-3 fatty acids, and subsequent 5-y cognitive decline in elderly men: The Zutphen Elderly Study. Am. J. Clin. Nutr 2007, 85, 1142–1147. [Google Scholar]
- Lukiw, W.J.; Cui, J.G.; Marcheselli, V.L.; Bodker, M.; Botkjaer, A.; Gotlinger, K.; Serhan, C.N.; Bazan, N.G. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J. Clin. Invest 2005, 115, 2774–2783. [Google Scholar]
- Perez, S.E.; Berg, B.M.; Moore, K.A.; He, B.; Counts, S.E.; Fritz, J.J.; Hu, Y.S.; Lazarov, O.; Lah, J.J.; Mufson, E.J. DHA diet reduces AD pathology in young APPswe/PS1 Delta E9 transgenic mice: Possible gender effects. J. Neurosci. Res 2010, 88, 1026–1040. [Google Scholar]
- Oksman, M.; Iivonen, H.; Hogyes, E.; Amtul, Z.; Penke, B.; Leenders, I.; Broersen, L.; Lutjohann, D.; Hartmann, T.; Tanila, H. Impact of different saturated fatty acid, polyunsaturated fatty acid and cholesterol containing diets on beta-amyloid accumulation in APP/PS1 transgenic mice. Neurobiol. Dis 2006, 23, 563–572. [Google Scholar]
- Calon, F.; Lim, G.P.; Yang, F.; Morihara, T.; Teter, B.; Ubeda, O.; Rostaing, P.; Triller, A.; Salem, N., Jr.; Ashe, K.H.; et al. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer’s disease mouse model. Neuron 2004, 43, 633–645. [Google Scholar]
- Hooijmans, C.R.; Rutters, F.; Dederen, P.J.; Gambarota, G.; Veltien, A.; van Groen, T.; Broersen, L.M.; Lutjohann, D.; Heerschap, A.; Tanila, H.; et al. Changes in cerebral blood volume and amyloid pathology in aged Alzheimer APP/PS1 mice on a docosahexaenoic acid (DHA) diet or cholesterol enriched Typical Western Diet (TWD). Neurobiol. Dis. 2007, 28, 16–29. [Google Scholar]
- Cole, G.M.; Ma, Q.L.; Frautschy, S.A. Omega-3 fatty acids and dementia. Prostaglandins Leukot Essent Fatty Acids 2009, 81, 213–221. [Google Scholar]
- Grimm, M.O.; Kuchenbecker, J.; Grösgen, S.; Burg, V.K.; Hundsdorfer, B.; Rothhaar, T.L.; Friess, P.; de Wilde, M.C.; Broersen, L.M.; Penke, B.; et al. Docosahexaenoic acid reduces amyloid beta production via multiple pleiotropic mechanisms. J. Biol. Chem. 2011, 286, 14028–14039. [Google Scholar]
- Grziwa, B.; Grimm, M.O.; Masters, C.L.; Beyreuther, K.; Hartmann, T.; Lichtenthaler, S.F. The transmembrane domain of the amyloid precursor protein in microsomal membranes is on both sides shorter than predicted. J. Biol. Chem 2003, 278, 6803–6808. [Google Scholar]
- Duyckaerts, C.; Hauw, J.J. Diagnosis and staging of Alzheimer disease. Neurobiol. Aging 1997, 18, S33–S42. [Google Scholar]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem 1985, 150, 76–85. [Google Scholar]
- Grimm, M.O.; Grösgen, S.; Rothhaar, T.L.; Burg, V.K.; Hundsdorfer, B.; Haupenthal, V.J.; Friess, P.; Muller, U.; Fassbender, K.; Riemenschneider, M.; et al. Intracellular APP domain regulates serine-palmitoyl-coa transferase expression and is affected in Alzheimer’s disease. Int. J. Alzheimers Dis. 2011, 2011, 695413. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol 1959, 37, 911–917. [Google Scholar]
- Grimm, M.O.; Kuchenbecker, J.; Rothhaar, T.L.; Grösgen, S.; Hundsdorfer, B.; Burg, V.K.; Friess, P.; Muller, U.; Grimm, H.S.; Riemenschneider, M.; et al. Plasmalogen synthesis is regulated via alkyl-dihydroxyacetonephosphate-synthase by amyloid precursor protein processing and is affected in Alzheimer’s disease. J. Neurochem. 2011, 116, 916–925. [Google Scholar]
- Ruiz, J.I.; Ochoa, B. Quantification in the subnanomolar range of phospholipids and neutral lipids by monodimensional thin-layer chromatography and image analysis. J. Lipid Res 1997, 38, 1482–1489. [Google Scholar]
- Grimm, M.O.; Grösgen, S.; Riemenschneider, M.; Tanila, H.; Grimm, H.S.; Hartmann, T. From brain to food: Analysis of phosphatidylcholins, lyso-phosphatidylcholins and phosphatidylcholin-plasmalogens derivates in Alzheimer’s disease human post mortem brains and mice model via mass spectrometry. J. Chromatogr. A 2011, 1218, 7713–7722. [Google Scholar]



| SH-SY5Y membranes | living SH-SY5Y cells | purified ADAM10 | human post mortem brain | |
|---|---|---|---|---|
| Mean % (SEM % +/− Sign.) | Mean % (SEM % +/− Sign.) | Mean % (SEM % +/− Sign.) | Mean % (SEM % +/− Sign.) | |
| Effect of chain length | ||||
| PC 10:0 | 127.7 (2.2 n.s.) | 124.8 (1.3 ***) | ||
| PC 12:0 | 144.0 (9.3 **) | 126.9 (1.3 ***) | 132.0 (9.6 *) | 141.9 (7.6 **) |
| PC 14:0 | 128.0 (11.9 n.s.) | 103.5 (1.2 n.s.) | ||
| PC 16:0 | 99.8 (5.9 n.s.) | 99.2 (1.3 n.s.) | ||
| PC 18:0 | 100.0 (3.2) | 100.0 (2.3 n.s.) | 100.0 (6.4) | 100.0 (5.7) |
| PC 20:0 | 102.3 (0.5 n.s.) | 98.8 (1.9 n.s.) | ||
| PC 22:0 | 98.8 (1.9 n.s.) | 100.1 (2.3 n.s.) | ||
| PC 24:0 | 101.7 (1.5 n.s.) | 107.8 (2.2 n.s.) | ||
| Mean % (SEM % +/− Sign.) | Mean % (SEM % +/− Sign.) | Mean % (SEM % +/− Sign.) | Mean % (SEM % +/− Sign.) | |
| Effect of headgroup | ||||
| PC 12:0 | 100.0 (0.7) | 100.0 (1.1) | 100.0 (4.5) | |
| PE 12:0 | 98.5 (1.3 n.s.) | 93.6 (2.8 n.s.) | 109.2 (3.1 n.s.) | |
| PS 12:0 | 93.4 (2.5 n.s.) | 83.8 (1.6 **) | 104.9 (3.7 n.s.) | |
| PC 14:0 | 100.0 (1.9) | 100.0 (1.1) | 100.0 (10.9) | |
| PE 14:0 | 101.0 (1.5 n.s.) | 105.0 (4.0 n.s.) | 105.7 (3.6 n.s.) | |
| PS 14:0 | 116.2 (3.1 ***) | 96.6 (2.0 n.s.) | 104.7 (12.2 n.s.) | |
| Mean % (SEM % +/− Sign.) | Mean % (SEM % +/− Sign.) | Mean % (SEM % +/− Sign.) | Mean % (SEM % +/− Sign.) | |
| Effect of saturation | ||||
| PC 18:0 | 100.0 (1.2) | 100.0 (2.3) | 100.0 (4.8) | 100.0 (3.1) |
| PC 18:1 | 102.0 (1.5 n.s.) | 110.3 (4.3 n.s.) | ||
| PC 18:2 | 97.1 (2.1 n.s.) | 115.0 (6.6 n.s.) | ||
| PC 18:3 | 98.2 (2.0 n.s.) | 117.4 (2.3 n.s.) | 148.2 (4.2 ***) | 106.3 (2.7 n.s.) |
| PC 20:4 | 104.2 (4.8 n.s.) | 124.7 (2.0 **) | ||
| PC 20:5 | 123.1 (7.6 **) | 140.1 (14.2 ***) | ||
| PC 22:6 | 108.6 (2.6 n.s.) | 136.8 (2.2 ***) | 229.7 (2.7 ***) | 152.0 (17.4 **) |
| Mean % (SEM % +/− Sign.) | Mean % (SEM % +/− Sign.) | Mean % (SEM % +/− Sign.) | Mean % (SEM % +/− Sign.) | |
| Effect of double-bond position | ||||
| PC 18:1D9 | 100.0 (1.8) | 100.0 (4.4) | ||
| PC 18:1D6 | 106.1 (1.0 *) | 101.7 (4.5 n.s.) | ||
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Grimm, M.O.W.; Haupenthal, V.J.; Rothhaar, T.L.; Zimmer, V.C.; Grösgen, S.; Hundsdörfer, B.; Lehmann, J.; Grimm, H.S.; Hartmann, T. Effect of Different Phospholipids on α-Secretase Activity in the Non-Amyloidogenic Pathway of Alzheimer’s Disease. Int. J. Mol. Sci. 2013, 14, 5879-5898. https://doi.org/10.3390/ijms14035879
Grimm MOW, Haupenthal VJ, Rothhaar TL, Zimmer VC, Grösgen S, Hundsdörfer B, Lehmann J, Grimm HS, Hartmann T. Effect of Different Phospholipids on α-Secretase Activity in the Non-Amyloidogenic Pathway of Alzheimer’s Disease. International Journal of Molecular Sciences. 2013; 14(3):5879-5898. https://doi.org/10.3390/ijms14035879
Chicago/Turabian StyleGrimm, Marcus O. W., Viola J. Haupenthal, Tatjana L. Rothhaar, Valerie C. Zimmer, Sven Grösgen, Benjamin Hundsdörfer, Johannes Lehmann, Heike S. Grimm, and Tobias Hartmann. 2013. "Effect of Different Phospholipids on α-Secretase Activity in the Non-Amyloidogenic Pathway of Alzheimer’s Disease" International Journal of Molecular Sciences 14, no. 3: 5879-5898. https://doi.org/10.3390/ijms14035879






