Wnt Secretion and Gradient Formation
Abstract
:1. Introduction
2. Wnt Proteins and Signaling
3. Wnt Secretion and Gradient Formation
4. Reggie/Flotillin Proteins
5. Wnt, Reggies and Breast Cancer
Acknowledgements
Conflict of Interest
References
- Logan, C.Y.; Nusse, R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol 2004, 20, 781–810. [Google Scholar]
- Clevers, H.; Nusse, R. Wnt/beta-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar]
- Herr, P.; Hausmann, G.; Basler, K. WNT secretion and signalling in human disease. Trends Mol. Med 2012, 18, 483–493. [Google Scholar]
- Polakis, P. Wnt signaling in cancer. Cold Spring Harbor Perspect. Biol. 2012. [Google Scholar] [CrossRef]
- Miller, J.R. The Wnts. Genome Biol. 2002, 3, reviews3001–reviews3001.15. [Google Scholar]
- Buechling, T.; Boutros, M. Wnt signaling signaling at and above the receptor level. Curr. Topics Dev. Biol 2011, 97, 21–53. [Google Scholar]
- Koval, A.; Purvanov, V.; Egger-Adam, D.; Katanaev, V.L. Yellow submarine of the Wnt/Frizzled signaling: Submerging from the G protein harbor to the targets. Biochem. Pharmacol 2011, 82, 1311–1319. [Google Scholar]
- Mikels, A.J.; Nusse, R. Purified Wnt5a protein activates or inhibits β-catenin-TCF signaling depending on receptor context. PLoS Biol 2006, 4, e115–e115. [Google Scholar]
- Harterink, M.; Korswagen, H.C. Dissecting the Wnt secretion pathway: Key questions on the modification and intracellular trafficking of Wnt proteins. Acta Physiol 2012, 204, 8–16. [Google Scholar]
- Port, F.; Basler, K. Wnt trafficking: New insights into Wnt maturation, secretion and spreading. Traffic 2010, 11, 1265–1271. [Google Scholar]
- Dubois, L.; Lecourtois, M.; Alexandre, C.; Hirst, E.; Vincent, J.P. Regulated endocytic routing modulates wingless signaling in Drosophila embryos. Cell 2001, 105, 613–624. [Google Scholar]
- Marois, E.; Mahmoud, A.; Eaton, S. The endocytic pathway and formation of the Wingless morphogen gradient. Development 2006, 133, 307–317. [Google Scholar]
- Hausmann, G.; Banziger, C.; Basler, K. Helping Wingless take flight: How WNT proteins are secreted. Nat. Rev. Mol. Cell Biol 2007, 8, 331–336. [Google Scholar]
- Bartscherer, K.; Pelte, N.; Ingelfinger, D.; Boutros, M. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell 2006, 125, 523–533. [Google Scholar]
- Goodman, R.M.; Thombre, S.; Firtina, Z.; Gray, D.; Betts, D.; Roebuck, J.; Spana, E.P.; Selva, E.M. Sprinter: A novel transmembrane protein required for Wg secretion and signaling. Development 2006, 133, 4901–4911. [Google Scholar]
- Herr, P.; Basler, K. Porcupine-mediated lipidation is required for Wnt recognition by Wls. Dev. Biol 2012, 361, 392–402. [Google Scholar] [Green Version]
- Eaton, S. Retromer retrieves wntless. Dev. Cell 2008, 14, 4–6. [Google Scholar]
- Pfeiffer, S.; Ricardo, S.; Manneville, J.B.; Alexandre, C.; Vincent, J.P. Producing cells retain and recycle Wingless in Drosophila embryos. Curr. Biol 2002, 12, 957–962. [Google Scholar]
- Kicheva, A.; Bollenbach, T.; Wartlick, O.; Julicher, F.; Gonzalez-Gaitan, M. Investigating the principles of morphogen gradient formation: From tissues to cells. Curr. Opin. Genet. Dev 2012, 22, 527–532. [Google Scholar]
- Simmonds, A.J.; dosSantos, G.; Livne-Bar, I.; Krause, H.M. Apical localization of wingless transcripts is required for wingless signaling. Cell 2001, 105, 197–207. [Google Scholar]
- Strigini, M.; Cohen, S.M. Wingless gradient formation in the Drosophila wing. Curr. Biol 2000, 10, 293–300. [Google Scholar]
- Purvanov, V.; Koval, A.; Katanaev, V.L. A direct and functional interaction between Go and Rab5 during G protein-coupled receptor signaling. Sci. Signal. 2010, 3, ra65. [Google Scholar]
- Jaiswal, J.K.; Rivera, V.M.; Simon, S.M. Exocytosis of post-Golgi vesicles is regulated by components of the endocytic machinery. Cell 2009, 137, 1308–1319. [Google Scholar]
- Lin, X. Functions of heparan sulfate proteoglycans in cell signaling during development. Development 2004, 131, 6009–6021. [Google Scholar]
- Gallet, A.; Staccini-Lavenant, L.; Therond, P.P. Cellular trafficking of the glypican Dally-like is required for full-strength Hedgehog signaling and wingless transcytosis. Dev. Cell 2008, 14, 712–725. [Google Scholar]
- Han, C.; Yan, D.; Belenkaya, T.Y.; Lin, X. Drosophila glypicans Dally and Dally-like shape the extracellular Wingless morphogen gradient in the wing disc. Development 2005, 132, 667–679. [Google Scholar]
- Cadigan, K.M.; Peifer, M. Wnt signaling from development to disease: Insights from model systems. Cold Spring Harbor Perspect. Biol 2009, 1, a002881. [Google Scholar]
- Panakova, D.; Sprong, H.; Marois, E.; Thiele, C.; Eaton, S. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature 2005, 435, 58–65. [Google Scholar]
- Neumann, S.; Coudreuse, D.Y.; van der Westhuyzen, D.R.; Eckhardt, E.R.; Korswagen, H.C.; Schmitz, G.; Sprong, H. Mammalian Wnt3a is released on lipoprotein particles. Traffic 2009, 10, 334–343. [Google Scholar]
- Korkut, C.; Ataman, B.; Ramachandran, P.; Ashley, J.; Barria, R.; Gherbesi, N.; Budnik, V. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell 2009, 139, 393–404. [Google Scholar]
- Koles, K.; Nunnari, J.; Korkut, C.; Barria, R.; Brewer, C.; Li, Y.; Leszyk, J.; Zhang, B.; Budnik, V. Mechanism of evenness interrupted (Evi)-exosome release at synaptic boutons. J. Biol. Chem 2012, 287, 16820–16834. [Google Scholar]
- Greco, V.; Hannus, M.; Eaton, S. Argosomes: A potential vehicle for the spread of morphogens through epithelia. Cell 2001, 106, 633–645. [Google Scholar]
- Gross, J.C.; Chaudhary, V.; Bartscherer, K.; Boutros, M. Active Wnt proteins are secreted on exosomes. Nature Cell Biol 2012, 14, 1036–1045. [Google Scholar]
- Luga, V.; Zhang, L.; Viloria-Petit, A.M.; Ogunjimi, A.A.; Inanlou, M.R.; Chiu, E.; Buchanan, M.; Hosein, A.N.; Basik, M.; Wrana, J.L. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 2012, 151, 1542–1556. [Google Scholar]
- Simons, M.; Raposo, G. Exosomes—Vesicular carriers for intercellular communication. Curr. Opin. Cell Biol 2009, 21, 575–581. [Google Scholar]
- Mulligan, K.A.; Fuerer, C.; Ching, W.; Fish, M.; Willert, K.; Nusse, R. Secreted Wingless-interacting molecule (Swim) promotes long-range signaling by maintaining Wingless solubility. Proc. Natl. Acad. Sci. USA 2012, 109, 370–377. [Google Scholar]
- Leyns, L.; Bouwmeester, T.; Kim, S.H.; Piccolo, S.; de Robertis, E.M. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell 1997, 88, 747–756. [Google Scholar]
- Esteve, P.; Sandonis, A.; Ibanez, C.; Shimono, A.; Guerrero, I.; Bovolenta, P. Secreted frizzled-related proteins are required for Wnt/beta-catenin signalling activation in the vertebrate optic cup. Development 2011, 138, 4179–4184. [Google Scholar]
- Mii, Y.; Taira, M. Secreted Frizzled-related proteins enhance the diffusion of Wnt ligands and expand their signalling range. Development 2009, 136, 4083–4088. [Google Scholar]
- Katanaev, V.L.; Solis, G.P.; Hausmann, G.; Buestorf, S.; Katanayeva, N.; Schrock, Y.; Stuermer, C.A.; Basler, K. Reggie-1/flotillin-2 promotes secretion of the long-range signalling forms of Wingless and Hedgehog in Drosophila. EMBO J 2008, 27, 509–521. [Google Scholar]
- Babuke, T.; Tikkanen, R. Dissecting the molecular function of reggie/flotillin proteins. Eur. J. Cell Biol 2007, 86, 525–532. [Google Scholar]
- Langhorst, M.F.; Reuter, A.; Stuermer, C.A. Scaffolding microdomains and beyond: The function of reggie/flotillin proteins. Cell. Mol. Life Sci 2005, 62, 2228–2240. [Google Scholar]
- Otto, G.P.; Nichols, B.J. The roles of flotillin microdomains—Endocytosis and beyond. J. Cell Sci 2011, 124, 3933–3940. [Google Scholar]
- Neumann-Giesen, C.; Falkenbach, B.; Beicht, P.; Claasen, S.; Luers, G.; Stuermer, C.A.; Herzog, V.; Tikkanen, R. Membrane and raft association of reggie-1/flotillin-2: Role of myristoylation, palmitoylation and oligomerization and induction of filopodia by overexpression. Biochem. J 2004, 378, 509–518. [Google Scholar]
- Solis, G.P.; Hoegg, M.; Munderloh, C.; Schrock, Y.; Malaga-Trillo, E.; Rivera-Milla, E.; Stuermer, C.A. Reggie/flotillin proteins are organized into stable tetramers in membrane microdomains. Biochem. J 2007, 403, 313–322. [Google Scholar]
- Ludwig, A.; Otto, G.P.; Riento, K.; Hams, E.; Fallon, P.G.; Nichols, B.J. Flotillin microdomains interact with the cortical cytoskeleton to control uropod formation and neutrophil recruitment. J. Cell Biol 2010, 191, 771–781. [Google Scholar]
- Schulte, T.; Paschke, K.A.; Laessing, U.; Lottspeich, F.; Stuermer, C.A. Reggie-1 and reggie-2, two cell surface proteins expressed by retinal ganglion cells during axon regeneration. Development 1997, 124, 577–587. [Google Scholar]
- Lang, D.M.; Lommel, S.; Jung, M.; Ankerhold, R.; Petrausch, B.; Laessing, U.; Wiechers, M.F.; Plattner, H.; Stuermer, C.A. Identification of reggie-1 and reggie-2 as plasmamembrane-associated proteins which cocluster with activated GPI-anchored cell adhesion molecules in non-caveolar micropatches in neurons. J. Neurobiol 1998, 37, 502–523. [Google Scholar]
- Bodrikov, V.; Solis, G.P.; Stuermer, C.A. Prion protein promotes growth cone development through reggie/flotillin-dependent N-cadherin trafficking. J. Neurosci 2011, 31, 18013–18025. [Google Scholar]
- Koch, J.C.; Solis, G.P.; Bodrikov, V.; Michel, U.; Haralampieva, D.; Shypitsyna, A.; Tonges, L.; Bahr, M.; Lingor, P.; Stuermer, C.A. Upregulation of reggie-1/flotillin-2 promotes axon regeneration in the rat optic nerve in vivo and neurite growth in vitro. Neurobiol. Dis 2012, 51, 168–176. [Google Scholar]
- Munderloh, C.; Solis, G.P.; Bodrikov, V.; Jaeger, F.A.; Wiechers, M.; Malaga-Trillo, E.; Stuermer, C.A. Reggies/flotillins regulate retinal axon regeneration in the zebrafish optic nerve and differentiation of hippocampal and N2a neurons. J. Neurosci 2009, 29, 6607–6615. [Google Scholar]
- Bickel, P.E.; Scherer, P.E.; Schnitzer, J.E.; Oh, P.; Lisanti, M.P.; Lodish, H.F. Flotillin and epidermal surface antigen define a new family of caveolae-associated integral membrane proteins. J. Biol. Chem 1997, 272, 13793–13802. [Google Scholar]
- Solis, G.P.; Schrock, Y.; Hulsbusch, N.; Wiechers, M.; Plattner, H.; Stuermer, C.A. Reggies/flotillins regulate E-cadherin-mediated cell contact formation by affecting EGFR trafficking. Mol. Biol. Cell 2012, 23, 1812–1825. [Google Scholar]
- Amaddii, M.; Meister, M.; Banning, A.; Tomasovic, A.; Mooz, J.; Rajalingam, K.; Tikkanen, R. Flotillin-1/reggie-2 protein plays dual role in activation of receptor-tyrosine kinase/mitogen-activated protein kinase signaling. J. Biol. Chem 2012, 287, 7265–7278. [Google Scholar]
- Malaga-Trillo, E.; Solis, G.P.; Schrock, Y.; Geiss, C.; Luncz, L.; Thomanetz, V.; Stuermer, C.A. Regulation of embryonic cell adhesion by the prion protein. PLoS Biol 2009, 7, e55. [Google Scholar]
- Schrock, Y.; Solis, G.P.; Stuermer, C.A. Regulation of focal adhesion formation and filopodia extension by the cellular prion protein (PrPC). FEBS Lett 2009, 583, 389–393. [Google Scholar]
- Solis, G.P.; Malaga-Trillo, E.; Plattner, H.; Stuermer, C.A. Cellular roles of the prion protein in association with reggie/flotillin microdomains. Front. Biosci 2010, 15, 1075–1085. [Google Scholar]
- Schneider, A.; Rajendran, L.; Honsho, M.; Gralle, M.; Donnert, G.; Wouters, F.; Hell, S.W.; Simons, M. Flotillin-dependent clustering of the amyloid precursor protein regulates its endocytosis and amyloidogenic processing in neurons. J. Neurosci 2008, 28, 2874–2882. [Google Scholar]
- Baumann, C.A.; Ribon, V.; Kanzaki, M.; Thurmond, D.C.; Mora, S.; Shigematsu, S.; Bickel, P.E.; Pessin, J.E.; Saltiel, A.R. CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature 2000, 407, 202–207. [Google Scholar]
- Ge, L.; Qi, W.; Wang, L.J.; Miao, H.H.; Qu, Y.X.; Li, B.L.; Song, B.L. Flotillins play an essential role in Niemann-Pick C1-like 1-mediated cholesterol uptake. Proc. Natl. Acad. Sci. USA 2011, 108, 551–556. [Google Scholar]
- Cremona, M.L.; Matthies, H.J.; Pau, K.; Bowton, E.; Speed, N.; Lute, B.J.; Anderson, M.; Sen, N.; Robertson, S.D.; Vaughan, R.A.; et al. Flotillin-1 is essential for PKC-triggered endocytosis and membrane microdomain localization of DAT. Nat. Neurosci 2011, 14, 469–477. [Google Scholar]
- Glebov, O.O.; Bright, N.A.; Nichols, B.J. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat. Cell Biol 2006, 8, 46–54. [Google Scholar]
- Stuermer, C.A. The reggie/flotillin connection to growth. Trends Cell Biol 2010, 20, 6–13. [Google Scholar]
- Langhorst, M.F.; Reuter, A.; Jaeger, F.A.; Wippich, F.M.; Luxenhofer, G.; Plattner, H.; Stuermer, C.A. Trafficking of the microdomain scaffolding protein reggie-1/flotillin-2. Eur. J. Cell Biol 2008, 87, 211–226. [Google Scholar]
- Zhai, L.; Chaturvedi, D.; Cumberledge, S. Drosophila wnt-1 undergoes a hydrophobic modification and is targeted to lipid rafts, a process that requires porcupine. J. Biol. Chem 2004, 279, 33220–33227. [Google Scholar]
- Simons, K.; Gerl, M.J. Revitalizing membrane rafts: New tools and insights. Nat. Rev. Mol. Cell Biol 2010, 11, 688–699. [Google Scholar]
- Ait-Slimane, T.; Galmes, R.; Trugnan, G.; Maurice, M. Basolateral internalization of GPI-anchored proteins occurs via a clathrin-independent flotillin-dependent pathway in polarized hepatic cells. Mol. Biol. Cell 2009, 20, 3792–3800. [Google Scholar]
- Polishchuk, R.; di Pentima, A.; Lippincott-Schwartz, J. Delivery of raft-associated, GPI-anchored proteins to the apical surface of polarized MDCK cells by a transcytotic pathway. Nat. Cell Biol 2004, 6, 297–307. [Google Scholar]
- Callejo, A.; Culi, J.; Guerrero, I. Patched, the receptor of Hedgehog, is a lipoprotein receptor. Proc. Natl. Acad. Sci. USA 2008, 105, 912–917. [Google Scholar]
- Packard, M.; Koo, E.S.; Gorczyca, M.; Sharpe, J.; Cumberledge, S.; Budnik, V. The Drosophila Wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell 2002, 111, 319–330. [Google Scholar]
- Mahr, A.; Aberle, H. The expression pattern of the Drosophila vesicular glutamate transporter: A marker protein for motoneurons and glutamatergic centers in the brain. Gene Expr. Patterns 2006, 6, 299–309. [Google Scholar]
- Zito, K.; Parnas, D.; Fetter, R.D.; Isacoff, E.Y.; Goodman, C.S. Watching a synapse grow: Noninvasive confocal imaging of synaptic growth in Drosophila. Neuron 1999, 22, 719–729. [Google Scholar]
- Wu, L.; Gonias, S.L. The low-density lipoprotein receptor-related protein-1 associates transiently with lipid rafts. J. Cell. Biochem 2005, 96, 1021–1033. [Google Scholar]
- Zhang, H.; Links, P.H.; Ngsee, J.K.; Tran, K.; Cui, Z.; Ko, K.W.; Yao, Z. Localization of low density lipoprotein receptor-related protein 1 to caveolae in 3T3-L1 adipocytes in response to insulin treatment. J. Biol. Chem 2004, 279, 2221–2230. [Google Scholar]
- Taylor, D.R.; Hooper, N.M. The low-density lipoprotein receptor-related protein 1 (LRP1) mediates the endocytosis of the cellular prion protein. Biochem. J 2007, 402, 17–23. [Google Scholar]
- Yoon, I.S.; Chen, E.; Busse, T.; Repetto, E.; Lakshmana, M.K.; Koo, E.H.; Kang, D.E. Low-density lipoprotein receptor-related protein promotes amyloid precursor protein trafficking to lipid rafts in the endocytic pathway. FASEB J 2007, 21, 2742–2752. [Google Scholar]
- Chen, M.H.; Li, Y.J.; Kawakami, T.; Xu, S.M.; Chuang, P.T. Palmitoylation is required for the production of a soluble multimeric Hedgehog protein complex and long-range signaling in vertebrates. Genes Dev 2004, 18, 641–659. [Google Scholar]
- Nusse, R.; Varmus, H.E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 1982, 31, 99–109. [Google Scholar]
- Turashvili, G.; Bouchal, J.; Burkadze, G.; Kolar, Z. Wnt signaling pathway in mammary gland development and carcinogenesis. Pathobiology 2006, 73, 213–223. [Google Scholar]
- Benhaj, K.; Akcali, K.C.; Ozturk, M. Redundant expression of canonical Wnt ligands in human breast cancer cell lines. Oncol. Rep 2006, 15, 701–707. [Google Scholar]
- King, T.D.; Suto, M.J.; Li, Y. The Wnt/beta-catenin signaling pathway: A potential therapeutic target in the treatment of triple negative breast cancer. J. Cell. Biochem 2012, 113, 13–18. [Google Scholar]
- Jessen, J.R. Noncanonical Wnt signaling in tumor progression and metastasis. Zebrafish 2009, 6, 21–28. [Google Scholar]
- Klemm, F.; Bleckmann, A.; Siam, L.; Chuang, H.N.; Rietkotter, E.; Behme, D.; Schulz, M.; Schaffrinski, M.; Schindler, S.; Trumper, L.; et al. beta-Catenin-independent WNT signaling in basal-like breast cancer and brain metastasis. Carcinogenesis 2011, 32, 434–442. [Google Scholar]
- Hazarika, P.; McCarty, M.F.; Prieto, V.G.; George, S.; Babu, D.; Koul, D.; Bar-Eli, M.; Duvic, M. Up-regulation of Flotillin-2 is associated with melanoma progression and modulates expression of the thrombin receptor protease activated receptor 1. Cancer Res 2004, 64, 7361–7369. [Google Scholar]
- Song, L.; Gong, H.; Lin, C.; Wang, C.; Liu, L.; Wu, J.; Li, M.; Li, J. Flotillin-1 promotes tumor necrosis factor-alpha receptor signaling and activation of NF-κB in esophageal squamous cell carcinoma cells. Gastroenterology 2012, 143, 995–1005. [Google Scholar]
- Berger, T.; Ueda, T.; Arpaia, E.; Chio, II; Shirdel, E.A.; Jurisica, I.; Hamada, K.; You-Ten, A.; Haight, J.; Wakeham, A.; et al. Flotillin-2 deficiency leads to reduced lung metastases in a mouse breast cancer model. Oncogene 2012. [Google Scholar] [CrossRef]
- Lin, C.; Wu, Z.; Lin, X.; Yu, C.; Shi, T.; Zeng, Y.; Wang, X.; Li, J.; Song, L. Knockdown of FLOT1 impairs cell proliferation and tumorigenicity in breast cancer through upregulation of FOXO3a. Clin. Cancer Res 2011, 17, 3089–3099. [Google Scholar]
- Pust, S.; Klokk, T.I.; Musa, N.; Jenstad, M.; Risberg, B.; Erikstein, B.; Tcatchoff, L.; Liestol, K.; Danielsen, H.E.; van Deurs, B.; et al. Flotillins as regulators of ErbB2 levels in breast cancer. Oncogene 2012. [Google Scholar] [CrossRef]
- Boettner, B.; Govek, E.E.; Cross, J.; van Aelst, L. The junctional multidomain protein AF-6 is a binding partner of the Rap1A GTPase and associates with the actin cytoskeletal regulator profilin. Proc. Natl. Acad. Sci. USA 2000, 97, 9064–9069. [Google Scholar]
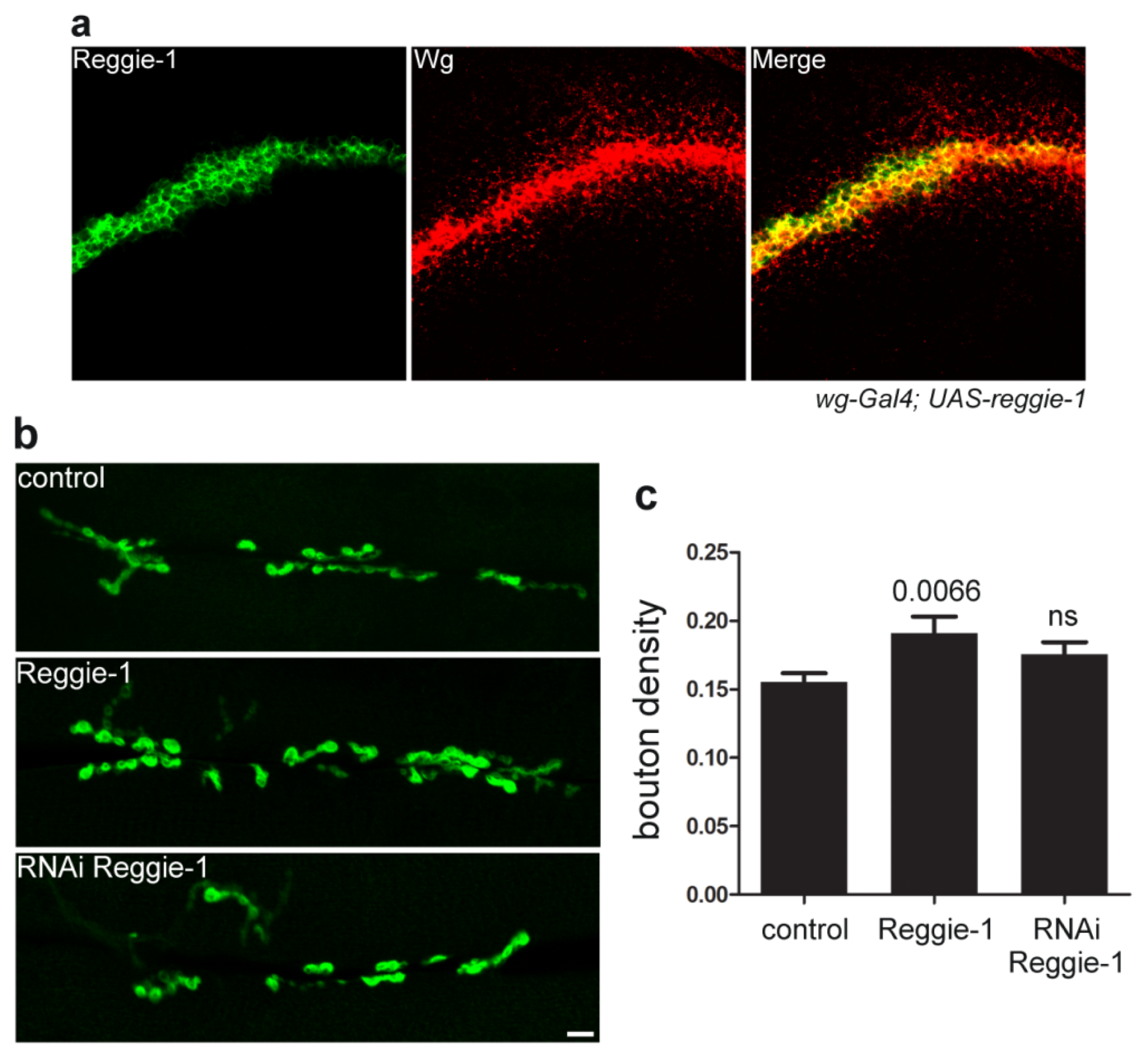

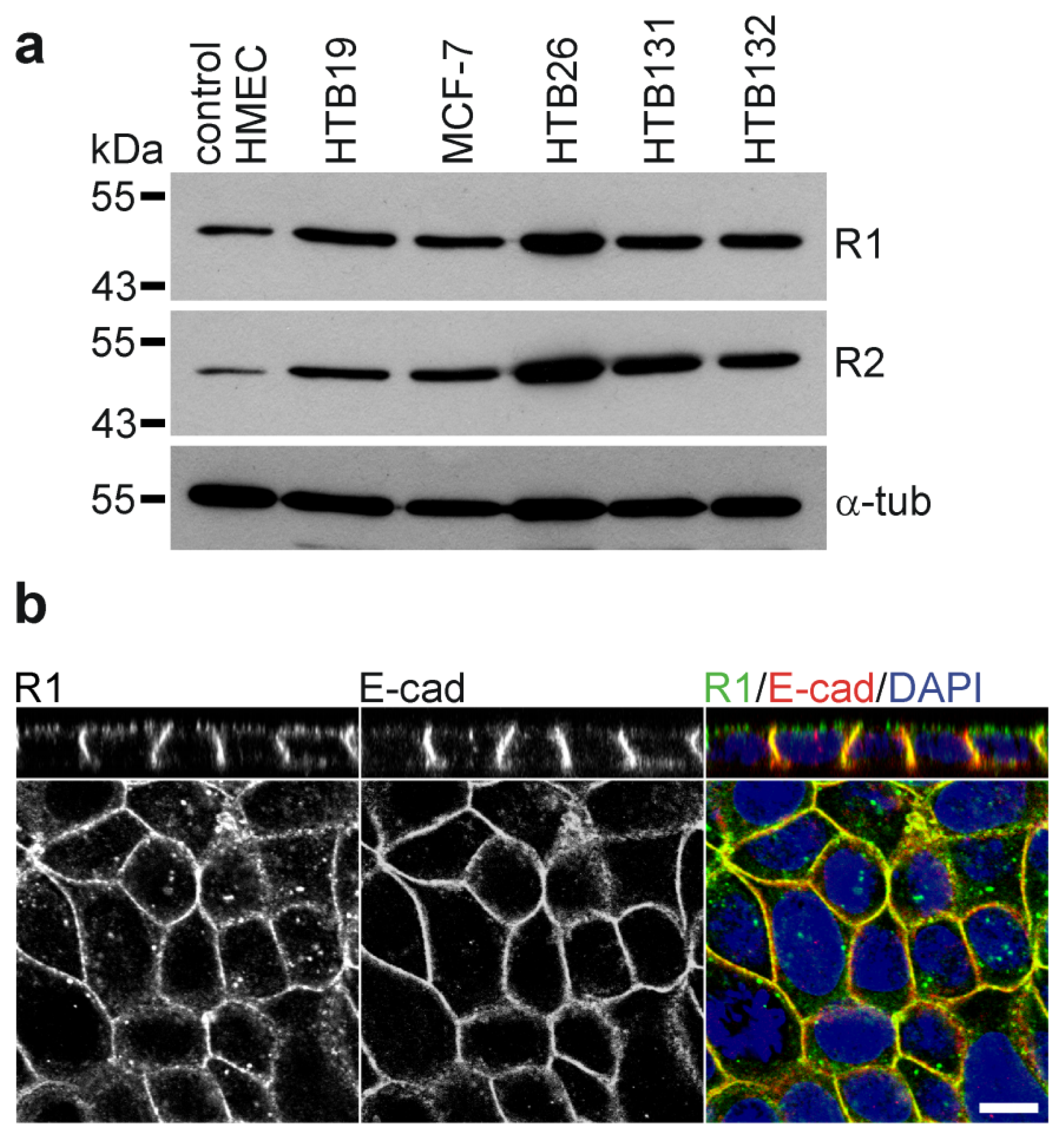
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Solis, G.P.; Lüchtenborg, A.-M.; Katanaev, V.L. Wnt Secretion and Gradient Formation. Int. J. Mol. Sci. 2013, 14, 5130-5145. https://doi.org/10.3390/ijms14035130
Solis GP, Lüchtenborg A-M, Katanaev VL. Wnt Secretion and Gradient Formation. International Journal of Molecular Sciences. 2013; 14(3):5130-5145. https://doi.org/10.3390/ijms14035130
Chicago/Turabian StyleSolis, Gonzalo P., Anne-Marie Lüchtenborg, and Vladimir L. Katanaev. 2013. "Wnt Secretion and Gradient Formation" International Journal of Molecular Sciences 14, no. 3: 5130-5145. https://doi.org/10.3390/ijms14035130



