Melatonin May Curtail the Metabolic Syndrome: Studies on Initial and Fully Established Fructose-Induced Metabolic Syndrome in Rats
Abstract
:1. Introduction
2. Results and Discussion
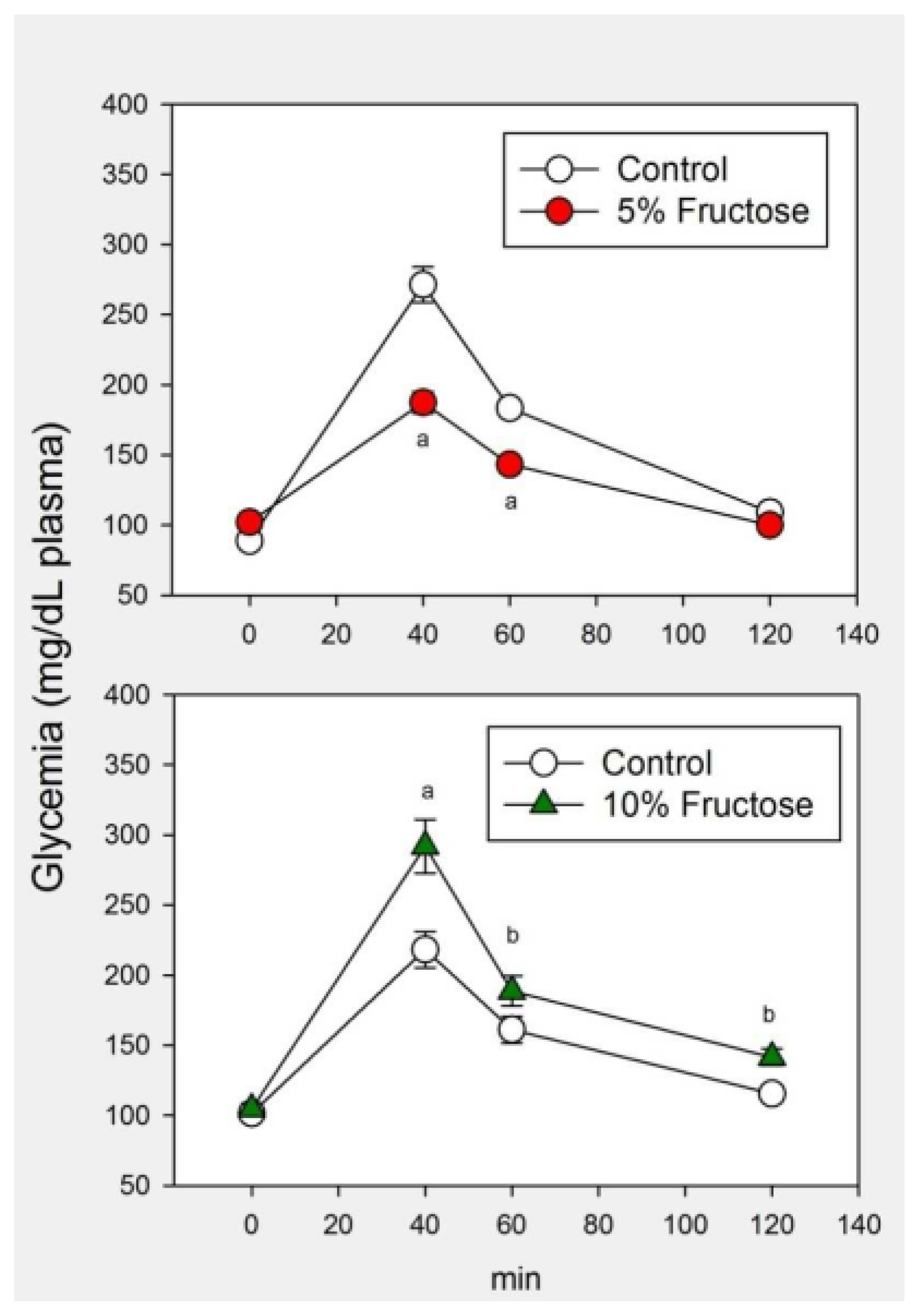
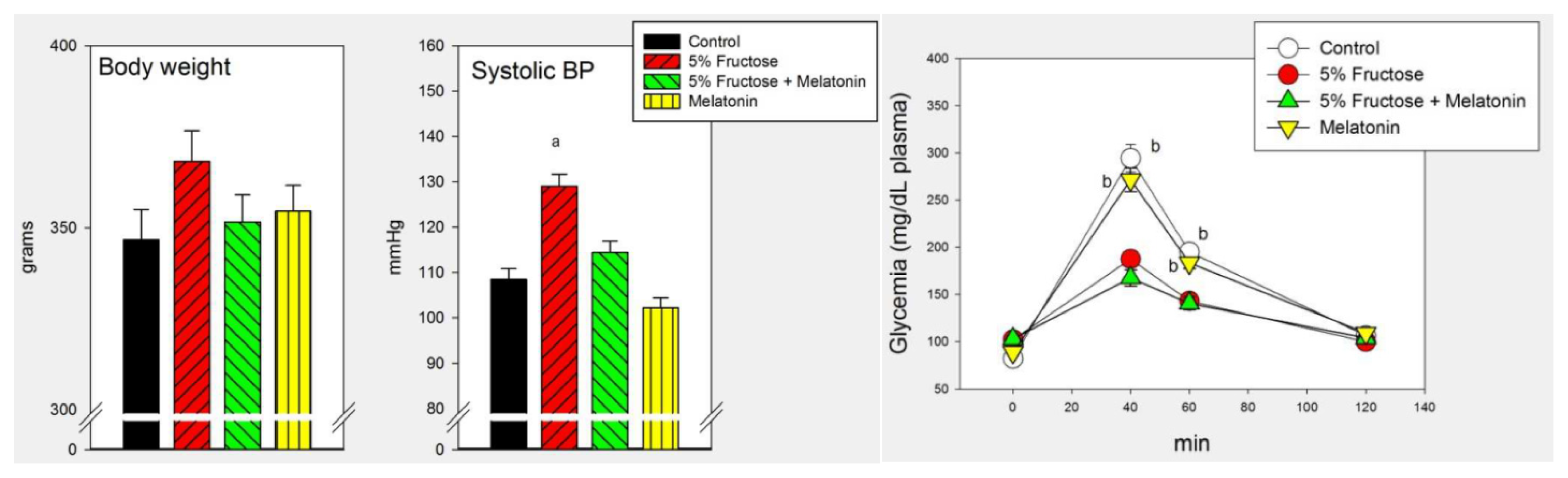
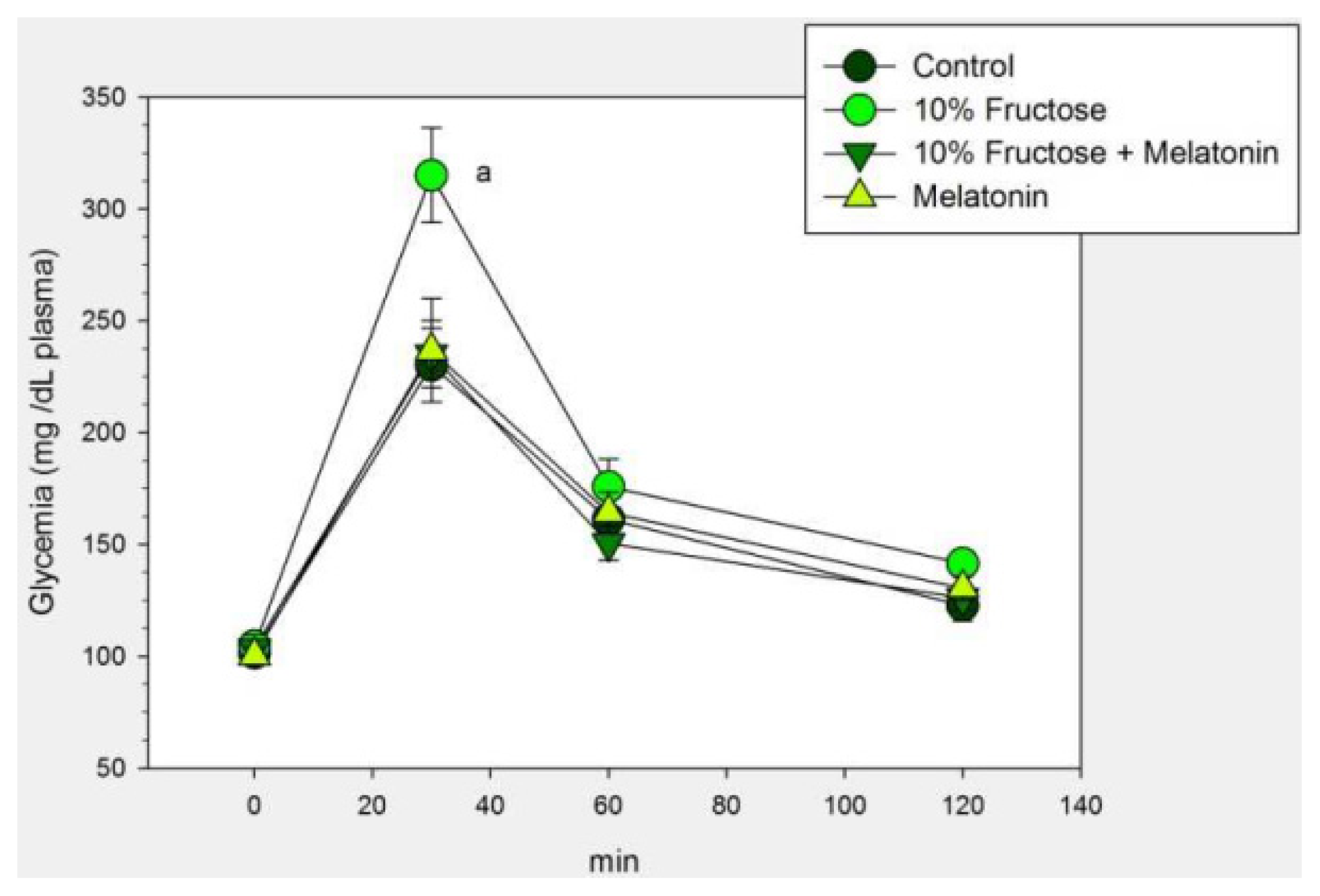
2.1. Results
2.2. Discussion
3. Experimental Section
3.1. Animals and Experimental Design
3.2. BP Measurement
3.3. Biochemical Assays
3.4. Statistical Analysis
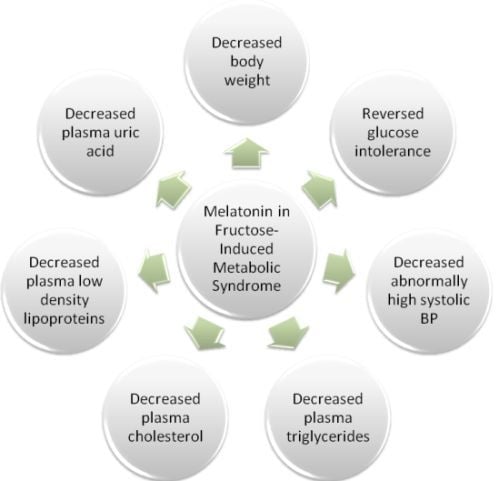
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Brown, T.; Avenell, A.; Edmunds, L.D.; Moore, H.; Whittaker, V.; Avery, L.; Summerbell, C. Systematic review of long-term lifestyle interventions to prevent weight gain and morbidity in adults. Obes. Rev 2009, 10, 627–638. [Google Scholar]
- Garaulet, M.; Madrid, J.A. Chronobiology, genetics and metabolic syndrome. Curr. Opin. Lipidol 2009, 20, 127–134. [Google Scholar]
- Maury, E.; Ramsey, K.M.; Bass, J. Circadian rhythms and metabolic syndrome: From experimental genetics to human disease. Circ. Res 2010, 106, 447–462. [Google Scholar]
- Tappy, L.; Le, K.A.; Tran, C.; Paquot, N. Fructose and metabolic diseases: New findings, new questions. Nutrition 2010, 26, 1044–1049. [Google Scholar]
- Tran, L.T.; Yuen, V.G.; McNeill, J.H. The fructose-fed rat: A review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol. Cell. Biochem 2009, 332, 145–159. [Google Scholar]
- Bremer, A.A.; Stanhope, K.L.; Graham, J.L.; Cummings, B.P.; Wang, W.; Saville, B.R.; Havel, P.J. Fructose-fed rhesus monkeys: A nonhuman primate model of insulin resistance, metabolic syndrome, and type 2 diabetes. Clin. Transl. Sci 2011, 4, 243–252. [Google Scholar]
- Shimamoto, K.; Ura, N. Mechanisms of insulin resistance in hypertensive rats. Clin. Exp. Hypertens 2006, 28, 543–552. [Google Scholar]
- She, M.; Deng, X.; Guo, Z.; Laudon, M.; Hu, Z.; Liao, D.; Hu, X.; Luo, Y.; Shen, Q.; Su, Z.; Yin, W. NEU-P11, a novel melatonin agonist, inhibits weight gain and improves insulin sensitivity in high-fat/high-sucrose-fed rats. Pharmacol. Res 2009, 59, 248–253. [Google Scholar]
- Leibowitz, A.; Peleg, E.; Sharabi, Y.; Shabtai, Z.; Shamiss, A.; Grossman, E. The role of melatonin in the pathogenesis of hypertension in rats with metabolic syndrome. Am. J. Hypertens 2008, 21, 348–351. [Google Scholar]
- Kitagawa, A.; Ohta, Y.; Ohashi, K. Melatonin improves metabolic syndrome induced by high fructose intake in rats. J. Pineal Res 2012, 52, 403–413. [Google Scholar]
- Roglans, N.; Sanguino, E.; Peris, C.; Alegret, M.; Vazquez, M.; Adzet, T.; Diaz, C.; Hernandez, G.; Laguna, J.C.; Sanchez, R.M. Atorvastatin treatment induced peroxisome proliferator-activated receptor alpha expression and decreased plasma nonesterified fatty acids and liver triglyceride in fructose-fed rats. J. Pharmacol. Exp. Ther 2002, 302, 232–239. [Google Scholar]
- Park, J.; Lemieux, S.; Lewis, G.F.; Kuksis, A.; Steiner, G. Chronic exogenous insulin and chronic carbohydrate supplementation increase de novo VLDL triglyceride fatty acid production in rats. J. Lipid Res 1997, 38, 2529–2536. [Google Scholar]
- Dai, S.; McNeill, J.H. Fructose-induced hypertension in rats is concentration- and duration-dependent. J. Pharmacol. Toxicol. Methods 1995, 33, 101–107. [Google Scholar]
- Peschke, E.; Stumpf, I.; Bazwinsky, I.; Litvak, L.; Dralle, H.; Muhlbauer, E. Melatonin and type 2 diabetes—A possible link? J. Pineal Res 2007, 42, 350–358. [Google Scholar]
- Nduhirabandi, F.; Du Toit, E.F.; Lochner, A. Melatonin and the metabolic syndrome: A tool for effective therapy in obesity-associated abnormalities? Acta Physiol 2012, 205, 209–223. [Google Scholar]
- Kanter, M.; Uysal, H.; Karaca, T.; Sagmanligil, H.O. Depression of glucose levels and partial restoration of pancreatic beta-cell damage by melatonin in streptozotocin-induced diabetic rats. Arch. Toxicol 2006, 80, 362–369. [Google Scholar]
- Nishida, S.; Sato, R.; Murai, I.; Nakagawa, S. Effect of pinealectomy on plasma levels of insulin and leptin and on hepatic lipids in type 2 diabetic rats. J. Pineal Res 2003, 35, 251–256. [Google Scholar]
- Nishida, S.; Segawa, T.; Murai, I.; Nakagawa, S. Long-term melatonin administration reduces hyperinsulinemia and improves the altered fatty-acid compositions in type 2 diabetic rats via the restoration of Delta-5 desaturase activity. J. Pineal Res 2002, 32, 26–33. [Google Scholar]
- Prunet-Marcassus, B.; Desbazeille, M.; Bros, A.; Louche, K.; Delagrange, P.; Renard, P.; Casteilla, L.; Penicaud, L. Melatonin reduces body weight gain in Sprague Dawley rats with diet-induced obesity. Endocrinology 2003, 144, 5347–5352. [Google Scholar]
- Puchalski, S.S.; Green, J.N.; Rasmussen, D.D. Melatonin effect on rat body weight regulation in response to high-fat diet at middle age. Endocrine 2003, 21, 163–167. [Google Scholar]
- Sartori, C.; Dessen, P.; Mathieu, C.; Monney, A.; Bloch, J.; Nicod, P.; Scherrer, U.; Duplain, H. Melatonin improves glucose homeostasis and endothelial vascular function in high-fat diet-fed insulin-resistant mice. Endocrinology 2009, 150, 5311–5317. [Google Scholar]
- Rios-Lugo, M.J.; Cano, P.; Jimenez-Ortega, V.; Fernandez-Mateos, M.P.; Scacchi, P.A.; Cardinali, D.P.; Esquifino, A.I. Melatonin effect on plasma adiponectin, leptin, insulin, glucose, triglycerides and cholesterol in normal and high fat-fed rats. J. Pineal Res 2010, 49, 342–348. [Google Scholar]
- Ladizesky, M.G.; Boggio, V.; Albornoz, L.E.; Castrillón, P.; Mautalen, C.A.; Cardinali, D.P. Melatonin increases oestradiol-induced bone formation in ovariectomized rats. J. Pineal Res 2003, 34, 143–151. [Google Scholar]
- Sanchez-Mateos, S.; Alonso-Gonzalez, C.; Gonzalez, A.; Martinez-Campa, C.M.; Mediavilla, M.D.; Cos, S.; Sanchez-Barcelo, E.J. Melatonin and estradiol effects on food intake, body weight, and leptin in ovariectomized rats. Maturitas 2007, 58, 91–101. [Google Scholar]
- Hussein, M.R.; Ahmed, O.G.; Hassan, A.F.; Ahmed, M.A. Intake of melatonin is associated with amelioration of physiological changes, both metabolic and morphological pathologies associated with obesity: an animal model. Int. J. Exp. Pathol 2007, 88, 19–29. [Google Scholar]
- Raskind, M.A.; Burke, B.L.; Crites, N.J.; Tapp, A.M.; Rasmussen, D.D. Olanzapine-induced weight gain and increased visceral adiposity is blocked by melatonin replacement therapy in rats. Neuropsychopharmacology 2007, 32, 284–288. [Google Scholar]
- Bartness, T.J.; Demas, G.E.; Song, C.K. Seasonal changes in adiposity: The roles of the photoperiod, melatonin and other hormones, and sympathetic nervous system. Exp. Biol. Med 2002, 227, 363–376. [Google Scholar]
- Tan, D.X.; Manchester, L.C.; Fuentes-Broto, L.; Paredes, S.D.; Reiter, R.J. Significance and application of melatonin in the regulation of brown adipose tissue metabolism: Relation to human obesity. Obes. Rev 2011, 12, 167–188. [Google Scholar]
- Scheer, F.A.; van Montfrans, G.A.; van Someren, E.J.; Mairuhu, G.; Buijs, R.M. Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension 2004, 43, 192–197. [Google Scholar]
- Cagnacci, A.; Cannoletta, M.; Renzi, A.; Baldassari, F.; Arangino, S.; Volpe, A. Prolonged melatonin administration decreases nocturnal blood pressure in women. Am. J. Hypertens 2005, 18, 1614–1618. [Google Scholar]
- Grossman, E.; Laudon, M.; Yalcin, R.; Zengil, H.; Peleg, E.; Sharabi, Y.; Kamari, Y.; Shen-Orr, Z.; Zisapel, N. Melatonin reduces night blood pressure in patients with nocturnal hypertension. Am. J. Med 2006, 119, 898–902. [Google Scholar]
- Cavallo, A.; Daniels, S.R.; Dolan, L.M.; Bean, J.A.; Khoury, J.C. Blood pressure-lowering effect of melatonin in type 1 diabetes. J. Pineal Res 2004, 36, 262–266. [Google Scholar]
- Nakagawa, T.; Hu, H.; Zharikov, S.; Tuttle, K.R.; Short, R.A.; Glushakova, O.; Ouyang, X.; Feig, D.I.; Block, E.R.; Herrera-Acosta, J.; et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am. J. Physiol. Renal Physiol 2006, 290, F625–F631. [Google Scholar]
- Scheer, F.A.; Hilton, M.F.; Mantzoros, C.S.; Shea, S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458. [Google Scholar]
- Wilson, S.J.; Nutt, D.J.; Alford, C.; Argyropoulos, S.V.; Baldwin, D.S.; Bateson, A.N.; Britton, T.C.; Crowe, C.; Dijk, D.J.; Espie, C.A.; et al. British association for psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders. J. Psychopharmacol 2010, 24, 1577–1601. [Google Scholar]
- Oxenkrug, G.F.; Summergrad, P. Ramelteon attenuates age-associated hypertension and weight gain in spontaneously hypertensive rats. Ann. N. Y. Acad. Sci 2010, 1199, 114–120. [Google Scholar]
- Guardiola-Lemaitre, B. Melatoninergic receptor agonists and antagonists: Therapeutic perspectives. J. Soc. Biol 2007, 201, 105–113. [Google Scholar]
- Cardinali, D.P.; Cano, P.; Jimenez-Ortega, V.; Esquifino, A.I. Melatonin and the metabolic syndrome: Physiopathologic and therapeutical implications. Neuroendocrinology 2011, 93, 133–142. [Google Scholar]
- Hardeland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin—A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol 2011, 93, 350–384. [Google Scholar]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J 2007, 22, 659–661. [Google Scholar]

| Control | 5% Fructose | 10% Fructose | F | p | |
|---|---|---|---|---|---|
| Initial body weight (g) | 273 ± 15 | 267 ± 9 | 269 ± 11 | 0.07 | NS |
| Final body weight (g) | 354 ± 13 | 339 ± 11 | 409 ± 19 | 6.26 | 0.007 |
| Systolic BP (mmHg) | 108 ± 4 | 124 ± 5 * | 129 ± 5 * | 5.47 | 0.012 |
| LDL-c (mg/dL) | 37 ± 5 | 45 ± 4 | 54 ± 4 * | 3.81 | 0.039 |
| HDL-c (mg/dL) | 53 ± 2 | 52 ± 1 | 54 ± 4 | 0.14 | NS |
| Cholesterol (mg/dL) | 68 ± 4 | 84 ± 4 * | 88 ± 6 * | 4.94 | 0.017 |
| Triglycerides (mg/dL) | 95 ± 6 | 112 ± 11 | 233 ± 19 * | 32.8 | <0.001 |
| Creatinine (mg/dL) | 1.1 ± 0.1 | 1.2 ± 0.2 | 1.1 ± 0.1 | 1.11 | NS |
| Urea (mg/dL) | 44 ± 5 | 49 ± 6 | 39 ± 3 | 0.35 | NS |
| Uric acid (mg/dL) | 1.8 ± 0.2 | 1.7 ± 0.2 | 1.6 ± 0.3 | 0.18 | NS |
| Control | 10% Fructose | 10% Fructose + Melatonin | Melatonin | F | p | |
|---|---|---|---|---|---|---|
| Initial body weight (g) | 265 ± 22 | 284 ± 19 | 273 ± 16 | 269 ± 26 | 0.15 | NS |
| Final body weight (g) | 351 ± 30 | 479 ± 36 a | 370 ± 32 | 371 ± 30 | 3.29 | 0.035 |
| Systolic BP (mmHg) | 102 ± 8 | 129 ± 6 a | 103 ± 4 | 100 ± 8 b | 4.18 | 0.014 |
| LDL-c (mg/dL) | 39 ± 4 | 59 ± 6 c | 19 ± 3 d | 22 ± 4 | 17.6 | <0.001 |
| HDL-c (mg/dL) | 54 ± 7 | 55 ± 8 | 62 ± 6 | 58 ± 5 | 0.29 | NS |
| Cholesterol (mg/dL) | 65 ± 6 | 88 ± 4 e | 67 ± 5 | 71 ± 4 | 4.71 | 0.009 |
| Triglycerides (mg/dL) | 175 ± 23 | 302 ± 26 f | 215 ± 19 g | 164 ± 13 | 9.04 | <0.001 |
| Creatinine (mg/dL) | 1.1 ± 0.1 | 1.2 ± 0.2 | 1.1 ± 0.1 | 1.3 ± 0.1 | 0.24 | NS |
| Urea (mg/dL) | 44 ± 5 | 40 ± 6 | 38 ± 3 | 42 ± 4 | 0.31 | NS |
| Uric acid (mg/dL) | 1.7 ± 0.1 | 1.9 ± 0.2 h | 1.2 ± 0.1 | 1.1 ± 0.1 d | 8.52 | <0.001 |
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cardinali, D.P.; Bernasconi, P.A.S.; Reynoso, R.; Toso, C.F.R.; Scacchi, P. Melatonin May Curtail the Metabolic Syndrome: Studies on Initial and Fully Established Fructose-Induced Metabolic Syndrome in Rats. Int. J. Mol. Sci. 2013, 14, 2502-2514. https://doi.org/10.3390/ijms14022502
Cardinali DP, Bernasconi PAS, Reynoso R, Toso CFR, Scacchi P. Melatonin May Curtail the Metabolic Syndrome: Studies on Initial and Fully Established Fructose-Induced Metabolic Syndrome in Rats. International Journal of Molecular Sciences. 2013; 14(2):2502-2514. https://doi.org/10.3390/ijms14022502
Chicago/Turabian StyleCardinali, Daniel P., Pablo A. Scacchi Bernasconi, Roxana Reynoso, Carlos F. Reyes Toso, and Pablo Scacchi. 2013. "Melatonin May Curtail the Metabolic Syndrome: Studies on Initial and Fully Established Fructose-Induced Metabolic Syndrome in Rats" International Journal of Molecular Sciences 14, no. 2: 2502-2514. https://doi.org/10.3390/ijms14022502