Out of the Lab and into the Bathroom: Evening Short-Term Exposure to Conventional Light Suppresses Melatonin and Increases Alertness Perception
Abstract
:1. Introduction
2. Results and Discussion
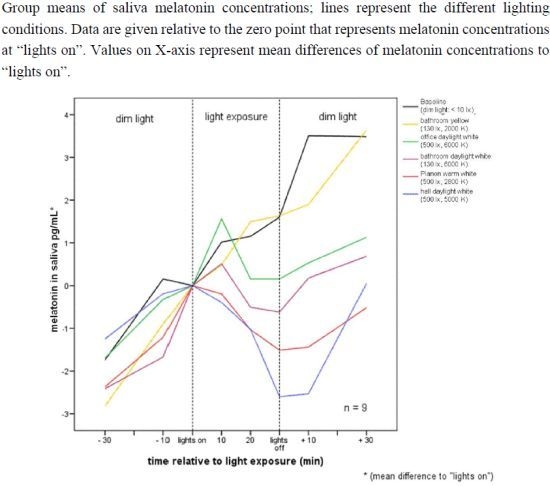
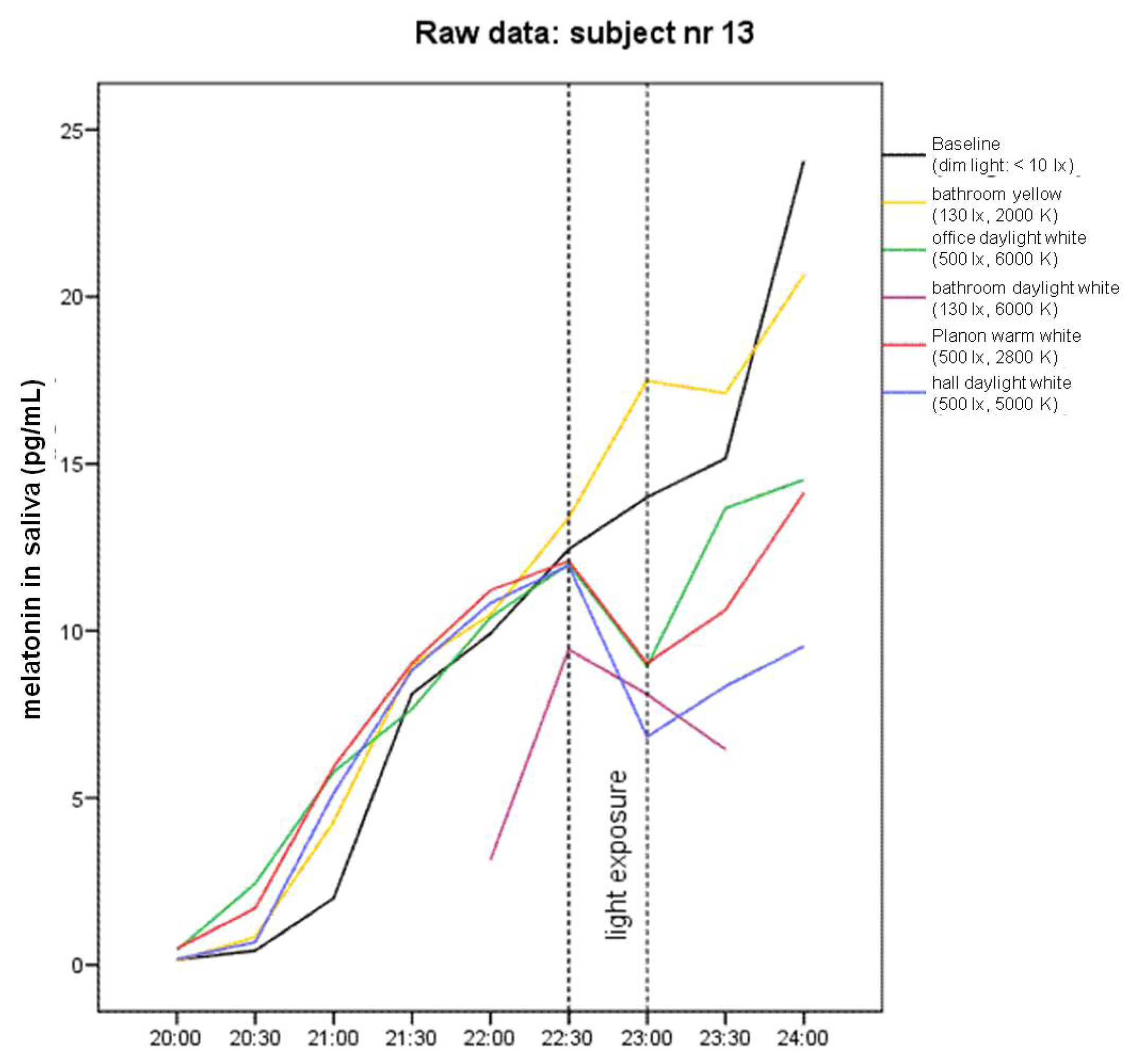
2.1. Raw Data and Descriptive Statistics
2.2. Data Analysis
2.2.1. Melatonin Levels in Different Lighting Regimes
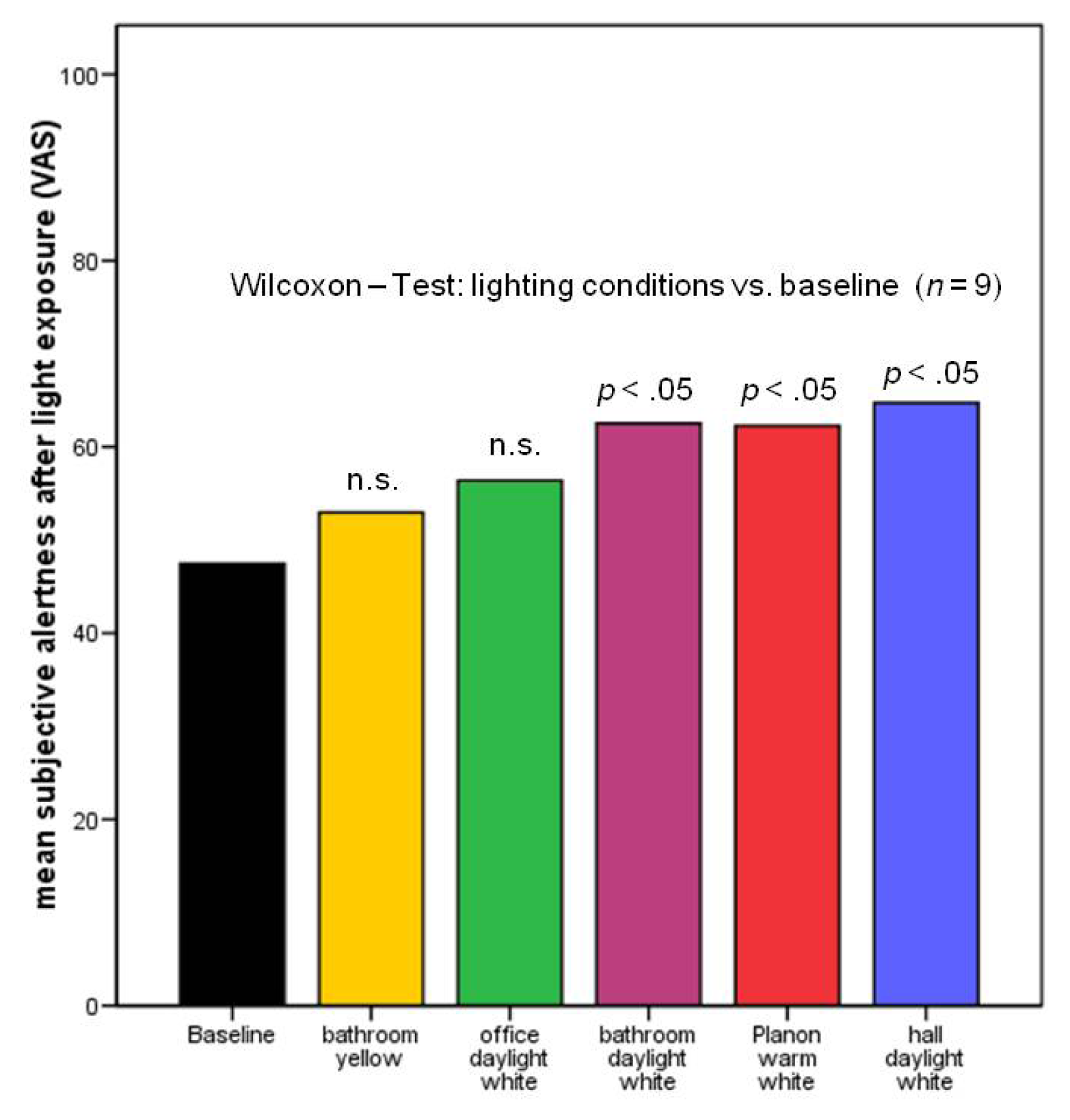
2.2.2. Subjective Alertness, Calmness and Contentedness in Different Lighting Regimes
2.3. Discussion
3. Experimental Section
Statistical Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Ohayon, M.M.; Reynolds, C.F., III. Epidemiological and clinical relevance of insomnia diagnosis algorithms according to the DSM-IV and the International Classification of Sleep Disorders (ICSD). Sleep Med. 2009, 10, 952–960. [Google Scholar]
- Olesen, J.; Gustavsson, A.; Svensson, M.; Wittchen, H.U.; Jonsson, B. The economic cost of brain disorders in Europe. Eur. J. Neurol 2012, 19, 155–162. [Google Scholar]
- Hickie, I.B.; Rogers, N.L. Novel melatonin-based therapies: Potential advances in the treatment of major depression. Lancet 2011, 378, 621–631. [Google Scholar]
- Pittendrigh, C.S. Temporal organization: Reflections of a Darwinian clock-watcher. Annu. Rev. Physiol 1993, 55, 16–54. [Google Scholar]
- Boivin, D.B.; Czeisler, C.A.; Dijk, D.J.; Duffy, J.F.; Folkard, S.; Minors, D.S.; Totterdell, P.; Waterhouse, J.M. Complex interaction of the sleep-wake cycle and circadian phase modulates mood in healthy subjects. Arch. Gen. Psychiatry 1997, 54, 145–152. [Google Scholar]
- Bruguerole, B. Chronopharmacology. In Biologic Rhythms in Clinical and Laboratory Medicine; Touitou, Y., Haus, E., Eds.; Springer Verlag: Paris, France, 1992; pp. 114–137. [Google Scholar]
- Shearman, L.P.; Sriram, S.; Weaver, D.R.; Maywood, E.S.; Chaves, I.; Zheng, B.; Kume, K.; Lee, C.C.; van der Horst, G.T.; Hastings, M.H.; et al. Interacting molecular loops in the mammalian circadian clock. Science 2000, 288, 1013–1019. [Google Scholar]
- Van Cauter, E.; Plat, L.; Leproult, R.; Copinschi, G. Alterations of circadian rhythmicity and sleep in aging: Endocrine consequences. Horm. Res 1998, 49, 147–152. [Google Scholar]
- Wirz-Justice, A. Circadian rhythms in mammalian neurotransmitter receptors. Prog. Neurobiol 1987, 29, 219–259. [Google Scholar]
- Wyatt, J.K.; Ritz-De Cecco, A.; Czeisler, C.A.; Dijk, D.J. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am. J. Physiol 1999, 277, R1152–R1163. [Google Scholar]
- Davidson, A.J.; Sellix, M.T.; Daniel, J.; Yamazaki, S.; Menaker, M.; Block, G.D. Chronic jet-lag increases mortality in aged mice. Curr. Biol 2006, 16, R914–R916. [Google Scholar]
- Spiegel, K.; Leproult, R.; van Cauter, E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999, 354, 1435–1439. [Google Scholar]
- Stevens, R.G.; Blask, D.E.; Brainard, G.C.; Hansen, J.; Lockley, S.W.; Provencio, I.; Rea, M.S.; Reinlib, L. Meeting report: The role of environmental lighting and circadian disruption in cancer and other diseases. Environ. Health Perspect 2007, 115, 1357–1362. [Google Scholar]
- Hu, K.; Van Someren, E.J.; Shea, S.A.; Scheer, F.A. Reduction of scale invariance of activity fluctuations with aging and Alzheimer’s disease: Involvement of the circadian pacemaker. Proc. Natl. Acad. Sci. USA 2009, 106, 2490–2494. [Google Scholar]
- Hansen, J. Risk of breast cancer after night- and shift work: Current evidence and ongoing studies in Denmark. Cancer Causes Control 2006, 17, 531–537. [Google Scholar]
- Knutsson, A.; Akerstedt, T.; Jonsson, B.G.; Orth-Gomer, K. Increased risk of ischaemic heart disease in shift workers. Lancet 1986, 2, 89–92. [Google Scholar]
- Schernhammer, E.S.; Schulmeister, K. Melatonin and cancer risk: Does light at night compromise physiologic cancer protection by lowering serum melatonin levels? Br. J. Cancer 2004, 90, 941–943. [Google Scholar]
- Czeisler, C.A.; Shanahan, T.L.; Klerman, E.B.; Martens, H.; Brotman, D.J.; Emens, J.S.; Klein, T.; Rizzo, J.F., III. Suppression of melatonin secretion in some blind patients by exposure to bright light. N. Engl. J. Med. 1995, 332, 6–11. [Google Scholar]
- Provencio, I.; Rodriguez, I.R.; Jiang, G.; Hayes, W.P.; Moreira, E.F.; Rollag, M.D. A novel human opsin in the inner retina. J. Neurosci 2000, 20, 600–605. [Google Scholar]
- Foster, R.G. Neurobiology: Bright blue times. Nature 2005, 433, 698–699. [Google Scholar]
- Hanifin, J.P.; Brainard, G.C. Photoreception for circadian, neuroendocrine, and neurobehavioral regulation. J. Physiol. Anthropol 2007, 26, 87–94. [Google Scholar]
- Brainard, G.C.; Hanifin, J.P.; Greeson, J.M.; Byrne, B.; Glickman, G.; Gerner, E.; Rollag, M.D. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. J. Neurosci 2001, 21, 6405–6412. [Google Scholar]
- Thapan, K.; Arendt, J.; Skene, D.J. An action spectrum for melatonin suppression: Evidence for a novel non-rod, non-cone photoreceptor system in humans. J. Physiol 2001, 535, 261–267. [Google Scholar]
- Cajochen, C.; Munch, M.; Kobialka, S.; Krauchi, K.; Steiner, R.; Oelhafen, P.; Orgul, S.; Wirz-Justice, A. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J. Clin. Endocrinol. Metab 2005, 90, 1311–1316. [Google Scholar]
- Lockley, S.W.; Evans, E.E.; Scheer, F.A.; Brainard, G.C.; Czeisler, C.A.; Aeschbach, D. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep 2006, 29, 161–168. [Google Scholar]
- Munch, M.; Kobialka, S.; Steiner, R.; Oelhafen, P.; Wirz-Justice, A.; Cajochen, C. Wavelength-dependent effects of evening light exposure on sleep architecture and sleep EEG power density in men. Am. J. Physiol. Regul. Integr. Comp. Physiol 2006, 290, R1421–R1428. [Google Scholar]
- Cajochen, C.; Jud, C.; Munch, M.; Kobialka, S.; Wirz-Justice, A.; Albrecht, U. Evening exposure to blue light stimulates the expression of the clock gene PER2 in humans. Eur. J. Neurosci 2006, 23, 1082–1086. [Google Scholar]
- Enezi, J.; Revell, V.; Brown, T.; Wynne, J.; Schlangen, L.; Lucas, R. A “melanopic” spectral efficiency function predicts the sensitivity of melanopsin photoreceptors to polychromatic lights. J. Biol. Rhythms 2011, 26, 314–323. [Google Scholar]
- Gooley, J.J.; Chamberlain, K.; Smith, K.A.; Khalsa, S.B.; Rajaratnam, S.M.; van Reen, E.; Zeitzer, J.M.; Czeisler, C.A.; Lockley, S.W. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J. Clin. Endocrinol. Metab 2011, 96, E463–E472. [Google Scholar]
- Santhi, N.; Thorne, H.C.; van der Veen, D.R.; Johnsen, S.; Mills, S.L.; Hommes, V.; Schlangen, L.J.; Archer, S.N.; Dijk, D.J. The spectral composition of evening light and individual differences in the suppression of melatonin and delay of sleep in humans. J. Pineal Res 2012, 53, 47–59. [Google Scholar]
- Campbell, S.S.; Dawson, D.; Anderson, M.W. Alleviation of sleep maintenance insomnia with timed exposure to bright light. J. Am. Geriatr. Soc 1993, 41, 829–836. [Google Scholar]
- Riemersma-van der Lek, R.F.; Swaab, D.F.; Twisk, J.; Hol, E.M.; Hoogendijk, W.J.; van Someren, E.J. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: A randomized controlled trial. J. Am. Med. Assoc 2008, 299, 2642–2655. [Google Scholar]
- Van Someren, E.J.; Kessler, A.; Mirmiran, M.; Swaab, D.F. Indirect bright light improves circadian rest-activity rhythm disturbances in demented patients. Biol. Psychiatry 1997, 41, 955–963. [Google Scholar]
- Lieverse, R.; van Someren, E.J.; Nielen, M.M.; Uitdehaag, B.M.; Smit, J.H.; Hoogendijk, W.J. Bright light treatment in elderly patients with nonseasonal major depressive disorder: A randomized placebo-controlled trial. Arch. Gen. Psychiatry 2011, 68, 61–70. [Google Scholar]
- Munch, M.; Scheuermaier, K.D.; Zhang, R.; Dunne, S.P.; Guzik, A.M.; Silva, E.J.; Ronda, J.M.; Duffy, J.F. Effects on subjective and objective alertness and sleep in response to evening light exposure in older subjects. Behav. Brain Res 2011, 224, 272–278. [Google Scholar]
- Lewy, A.J.; Wehr, T.A.; Goodwin, F.K.; Newsome, D.A.; Markey, S.P. Light suppresses melatonin secretion in humans. Science 1980, 210, 1267–1269. [Google Scholar]
- Figueiro, M.G.; Rea, M.S.; Bullough, J.D. Circadian effectiveness of two polychromatic lights in suppressing human nocturnal melatonin. Neurosci. Lett 2006, 406, 293–297. [Google Scholar]
- Hebert, M.; Martin, S.K.; Lee, C.; Eastman, C.I. The effects of prior light history on the suppression of melatonin by light in humans. J. Pineal Res 2002, 33, 198–203. [Google Scholar]
- Herljevic, M.; Middleton, B.; Thapan, K.; Skene, D.J. Light-induced melatonin suppression: Age-related reduction in response to short wavelength light. Exp. Gerontol 2005, 40, 237–242. [Google Scholar]
- Wirz-Justice, A.; Reme, C.; Prunte, A.; Heinen, U.; Graw, P.; Urner, U. Lithium decreases retinal sensitivity, but this is not cumulative with years of treatment. Biol. Psychiatry 1997, 41, 743–746. [Google Scholar]
- Lewy, A.J.; Lefler, B.J.; Emens, J.S.; Bauer, V.K. The circadian basis of winter depression. Proc. Natl. Acad. Sci. USA 2006, 103, 7414–7419. [Google Scholar]
- Terman, J.S.; Terman, M. Photopic and scotopic light detection in patients with seasonal affective disorder and control subjects. Biol. Psychiatry 1999, 46, 1642–1648. [Google Scholar]
- Brown, S.A.; Kunz, D.; Dumas, A.; Westermark, P.O.; Vanselow, K.; Tilmann-Wahnschaffe, A.; Herzel, H.; Kramer, A. Molecular insights into human daily behavior. Proc. Natl. Acad. Sci. USA 2008, 105, 1602–1607. [Google Scholar]
- Arendt, J. Melatonin and the Mammalian Pineal Gland; University Press: Cambridge, UK, 1995; pp. 201–285. [Google Scholar]
- Kunz, D.; Mahlberg, R. Melatonin: A Chronobiotic that not only Shifts Rhythms. In Melatonin: Biological Basis of its Function in Health and Dieases; Pandi-Perumal, S.R., Cardinali, D.P., Eds.; Landes Biosciences: Austin, TX, USA, 2004; pp. 1–11. [Google Scholar]
- Hardeland, R. Melatonin in aging and disease—Multiple consequences of reduced secretion, options and limits of treatment. Aging Dis 2012, 3, 194–225. [Google Scholar]
- Mahlberg, R.; Tilmann, A.; Salewski, L.; Kunz, D. Normative data on the daily profile of urinary 6-sulfatoxymelatonin in healthy subjects between the ages of 20 and 84. Psychoneuroendocrinology 2006, 31, 634–641. [Google Scholar]
- Riemann, D.; Klein, T.; Rodenbeck, A.; Feige, B.; Horny, A.; Hummel, R.; Weske, G.; Al-Shajlawi, A.; Voderholzer, U. Nocturnal cortisol and melatonin secretion in primary insomnia. Psychiatry Res 2002, 113, 17–27. [Google Scholar]
- Rodenbeck, A.; Huether, G.; Ruther, E.; Hajak, G. Nocturnal melatonin secretion and its modification by treatment in patients with sleep disorders. Adv. Exp. Med. Biol 1999, 467, 89–93. [Google Scholar]
- Hajak, G.; Rodenbeck, A.; Staedt, J.; Bandelow, B.; Huether, G.; Ruther, E. Nocturnal plasma melatonin levels in patients suffering from chronic primary insomnia. J. Pineal Res 1995, 19, 116–122. [Google Scholar]
- Mahlberg, R.; Walther, S.; Kalus, P.; Bohner, G.; Haedel, S.; Reischies, F.M.; Kuhl, K.P.; Hellweg, R.; Kunz, D. Pineal calcification in Alzheimer’s disease: An in vivo study using computed tomography. Neurobiol. Aging 2008, 29, 203–209. [Google Scholar]
- Skene, D.J.; Swaab, D.F. Melatonin rhythmicity: Effect of age and Alzheimer’s disease. Exp. Gerontol 2003, 38, 199–206. [Google Scholar]
- Pandi-Perumal, S.R.; Bahammam, A.S.; Brown, G.M.; Spence, D.W.; Bharti, V.K.; Kaur, C.; Hardeland, R.; Cardinali, D.P. Melatonin Antioxidative Defense: Therapeutical Implications for Aging and Neurodegenerative Processes. Neurotox. Res. 2012. [Google Scholar] [CrossRef]
- Kunz, D. Chronobiotic protocol and circadian sleep propensity index: New tools for clinical routine and research on melatonin and sleep. Pharmacopsychiatry 2004, 37, 139–146. [Google Scholar]
- Tzischinsky, O.; Shlitner, A.; Lavie, P. The association between the nocturnal sleep gate and nocturnal onset of urinary 6-sulfatoxymelatonin. J. Biol. Rhythms 1993, 8, 199–209. [Google Scholar]
- Wirz-Justice, A.; Armstrong, S.M. Melatonin: Nature’s soporific? J. Sleep Res 1996, 5, 137–141. [Google Scholar]
- Vandewalle, G.; Balteau, E.; Phillips, C.; Degueldre, C.; Moreau, V.; Sterpenich, V.; Albouy, G.; Darsaud, A.; Desseilles, M.; Dang-Vu, T.T.; et al. Daytime light exposure dynamically enhances brain responses. Curr. Biol 2006, 16, 1616–1621. [Google Scholar]
- International Agency for Research on Cancer, World Health Organization, Graveyard Shift Work Linked to Cancer; The Asoociated Press: London, UK, 2007.
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res 1989, 28, 193–213. [Google Scholar]
- Horne, J.A.; Ostberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol 1976, 4, 97–110. [Google Scholar]
- Williams, J.B.W.; Link, M.J.; Rosenthal, N.E.; Amira, L.; Terman, M. Structured Interview Guide for the Hamilton Depression Rating Scale-Seasonal Affective Disorder. Version (Sigh-SAD); New York State Psychiatric Institute: New York, NY, USA, 1992. [Google Scholar]
- Mongrain, V.; Lavoie, S.; Selmaoui, B.; Paquet, J.; Dumont, M. Phase relationships between sleep-wake cycle and underlying circadian rhythms in Morningness-Eveningness. J. Biol. Rhythms 2004, 19, 248–257. [Google Scholar]
- Bond, A.; Lader, M. The use of analogue scales in rating subjective feelings. Br. J. Med. Psychol 1974, 47, 211–218. [Google Scholar]
- Cross, E.M.; Chaffin, W.W. Use of binomial theorem in interpreting results of multiple tests of significance. Educ. Psychol. Meas 1982, 42, 25–34. [Google Scholar]
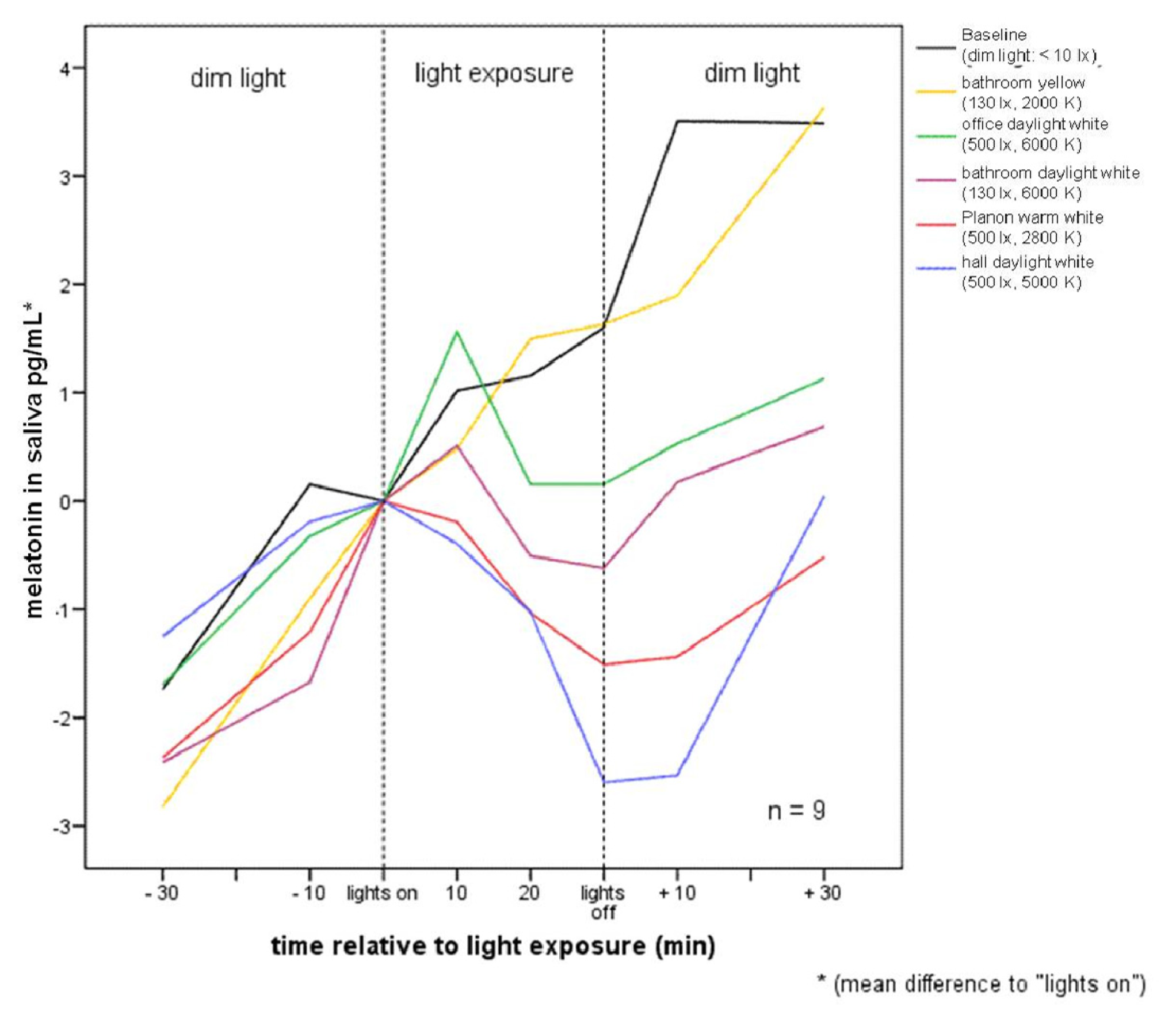

| Pre −60 | Pre −30 | Mean −10 to 0 min before lights on | 10 | 20 | 30 | Post +10 | Post +30 | ||
|---|---|---|---|---|---|---|---|---|---|
| Part 1 | Baseline (n = 9) | (−4.21) ± 1.89 | (−2.26) ± 1.67 | 0 | 0.94 ± 2.47 | 1.08 ± 2.20 | 1.525 ± 1.69 | 3.48 ± 2.34 | 3.41 ± 1.78 |
| bathroom yellow (n = 9) | (−4.40) ± 2.68 | (−2.37) ± 2.04 | 0 | 0.93 ± 1.56 | 1.95 ± 0.64 | 2.08 ± 1.15 | 2.075 ± 2.96 | 4.085 ± 4.26 | |
| office daylight white (n = 8) | (−3.33) ± 2.23 | (−1.29) ± 1.88 | 0 | 1.97 ± 2.90 | 0.56 ± 1.55 | 0.56 ± 2.48 | 0.94 ± 1.57 | 1.54 ± 2.23 | |
| bathroom daylight white (n = 8) | (−4.05) ± 1.99 | (−2.03) ± 1.44 | 0 | 0.97 ± 1.15 | (−0.12) ± 1.00 | (−0.13) ± 1.72 | 0.62 ± 2.96 | 2.03 ± 4.28 | |
| Planon warm white (n = 9) | (−3.68) ± 2.94 | (−1.77) ± 1.98 | 0 | 0.41 ± 2.12 | (−0.43) ± 1.72 | (−0.91) ± 1.64 | (−0.83) ± 2.07 | 0.09 ± 2.83 | |
| hall daylight white (n = 8) | (−2.66) ± 1.12 | (−1.15) ± 0.76 | 0 | (−0.30) ± 1.30 | (−1.475) ± 1.42 | (−2.50) ± 2.11 | (−3.23) ± 4.675 | (−0.98) ± 6.09 | |
| Part 2 | n = 6 | ||||||||
| Baseline | (−3.40) ± 1.54 | (−1.88) ± 1.06 | 0 | 1.58 ± 1.87 | 1.42 ± 1.44 | 1.66 ± 1.92 | 3.84 ± 2.48 | 2.67 ± 1.58 | |
| bathroom yellow | (−5.18) ± 2.91 | (−2.76) ± 2.33 | 0 | 0.55 ± 1.80 | 2.02 ± 0.74 | 2.29 ± 1.39 | 1.18 ± 3.30 | 2.38 ± 3.72 | |
| office daylight white | (−2.96) ± 2.39 | (−0.97) ± 2.11 | 0 | 2.45 ± 3.24 | 0.37 ± 1.78 | 0.61 ± 2.94 | 0.885 ± 1.85 | 1.24 ± 2.14 | |
| bathroom daylight white | (−4.295) ± 2.07 | (−2.16) ± 1.65 | 0 | 0.58 ± 0.85 | (−0.11) ± 1.01 | (−0.505) ± 1.86 | (−0.535) ± 1.79 | 0.22 ± 2.47 | |
| Planon warm white | (−2.95) ± 1.43 | (−0.97) ± 1.31 | 0 | 0.98 ± 0.93 | 0.02 ± 0.69 | (−0.89) ± 1.45 | (−0.68) ± 1.17 | 0.27 ± 1.39 | |
| hall daylight white | (−2.31) ± 1.06 | (−1.02) ± 0.83 | 0 | (−0.26) ± 1.53 | (−1.29) ± 1.58 | (−2.09) ± 2.09 | (−1.69) ± 2.05 | 1.04 ± 3.60 | |
| Group | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 |
|---|---|---|---|---|---|---|
| I | Baseline: | hall lighting | office lighting | bathroom | bathroom | Planon |
| II | office lighting | bathroom | bathroom | Planon | hall lighting | |
| II | bathroom | bathroom | Planon | hall lighting | office lighting |
 daylight white
daylight white
 warm white
warm white
 yellow
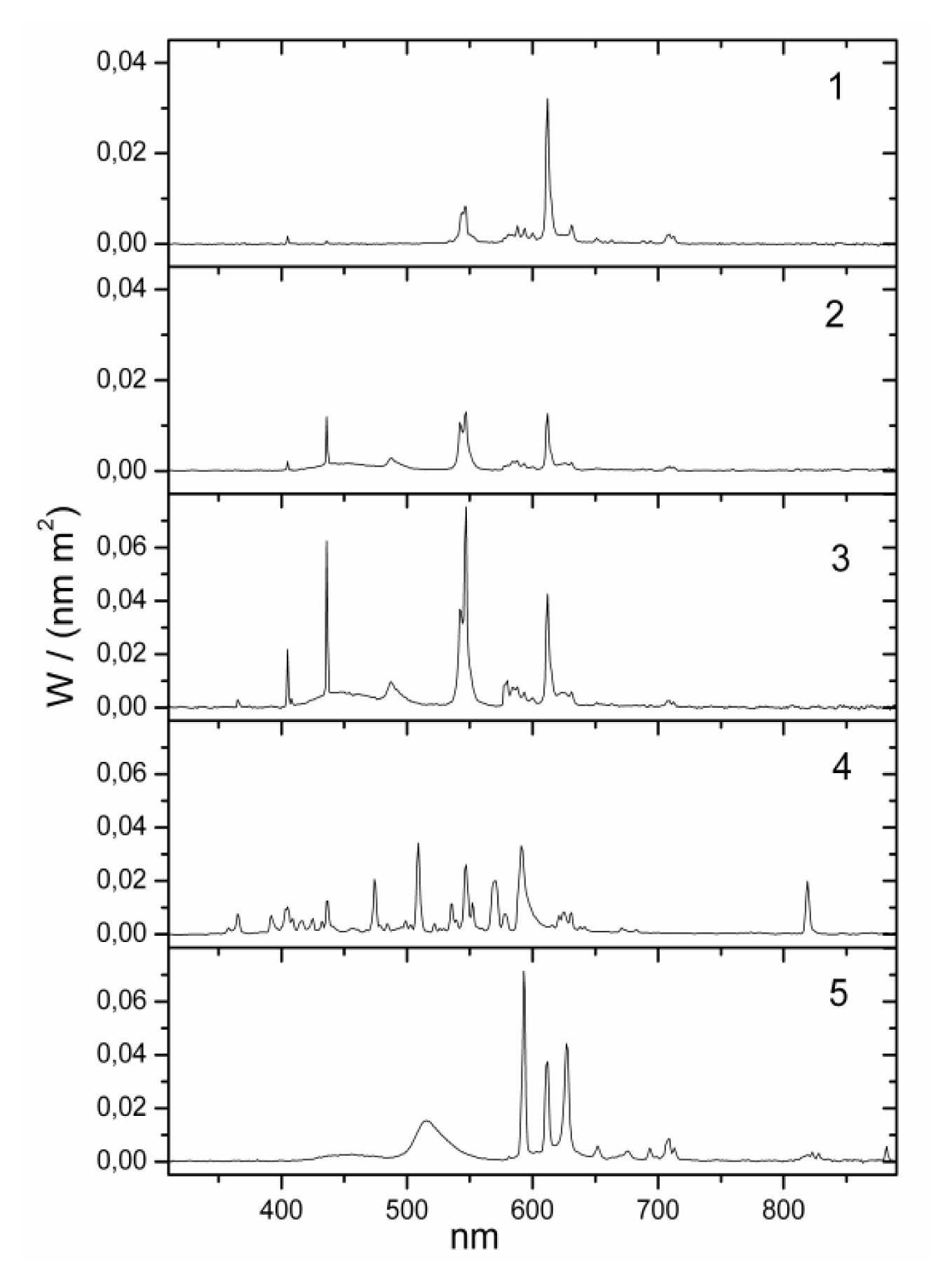
yellow| No. | lamp name | Lux (lx) | Kelvin (K) | Irradiance (W/m2) | Luxm (lxm) 1 | Type | Model | Blue component | Position | Eye (cm) distance 2 | Company |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | baseline (dim light) | <10 | normal | pointing to ceiling | 150 cm | ||||||
| 1 | bathroom yellow | 130 | 2000 | 0.319 | 118 | fluorescent | Custom-made | zero | wall over mirror | 100 cm | Narva BEL |
| 2 | bathroom daylight white | 130 | 6000 | 0.385 | 581 | fluorescent | 6641 L18/W | high | wall over mirror | 100 cm | Narva BEL |
| 3 | office daylight white | 500 | 6000 | 1.437 | 2069 | fluorescent (3 pieces) | T5 54W/860 | high | ceiling | 150 cm | Trilux Narva BEL |
| 4 | hall daylight white | 500 | 5000 | 1.391 | 1724 | metal halogenid | NCT 70W | high | ceiling | 150 cm | Trilux Narva GLE |
| 5 | Planon warm white 3 | 500 | 2800 | 1.432 | 1826 | dielectric inhibited | custom made | low | wall | 100 cm | Osram |
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wahnschaffe, A.; Haedel, S.; Rodenbeck, A.; Stoll, C.; Rudolph, H.; Kozakov, R.; Schoepp, H.; Kunz, D. Out of the Lab and into the Bathroom: Evening Short-Term Exposure to Conventional Light Suppresses Melatonin and Increases Alertness Perception. Int. J. Mol. Sci. 2013, 14, 2573-2589. https://doi.org/10.3390/ijms14022573
Wahnschaffe A, Haedel S, Rodenbeck A, Stoll C, Rudolph H, Kozakov R, Schoepp H, Kunz D. Out of the Lab and into the Bathroom: Evening Short-Term Exposure to Conventional Light Suppresses Melatonin and Increases Alertness Perception. International Journal of Molecular Sciences. 2013; 14(2):2573-2589. https://doi.org/10.3390/ijms14022573
Chicago/Turabian StyleWahnschaffe, Amely, Sven Haedel, Andrea Rodenbeck, Claudia Stoll, Horst Rudolph, Ruslan Kozakov, Heinz Schoepp, and Dieter Kunz. 2013. "Out of the Lab and into the Bathroom: Evening Short-Term Exposure to Conventional Light Suppresses Melatonin and Increases Alertness Perception" International Journal of Molecular Sciences 14, no. 2: 2573-2589. https://doi.org/10.3390/ijms14022573