Analysis of the Binding Sites of Porcine Sialoadhesin Receptor with PRRSV
Abstract
:1. Introduction
2. Results
2.1. Bioinformatics Analyses
2.1.1. Superimposition of mSN Templates with Ligand and Multiple Sequence Alignment of SN Protein
2.1.2. Identification of Amino Acid Binding Sites in mSN and pSN
2.1.3. Analysis of Signal Peptide and Glycosylation
2.1.4. Model Building by Superposition and Template Alignment
2.1.5. Refinement of MD and Optimization of Homology Model
2.1.6. Assessment of Model Quality
2.1.7. Prediction of an Obvious Cavity in the pSN Surface Models for PRRSV Sialic Acid Binding
2.1.8. Analysis of the Effect of pSN S107 on the Formation of the Binding Cavity
2.2. Experimental Validation
2.2.1. SN-GFP Chimera Protein Expression in 293T Cell and Confirmation Using WB
2.2.2. Analysis of the Binding Activity of SN-GFP Chimeric Proteins with PRRSV-GP5 Using FAR-WB
2.2.3. Analysis of PRRSV Binding to SN-GFP Chimeric Proteins Using ELISA
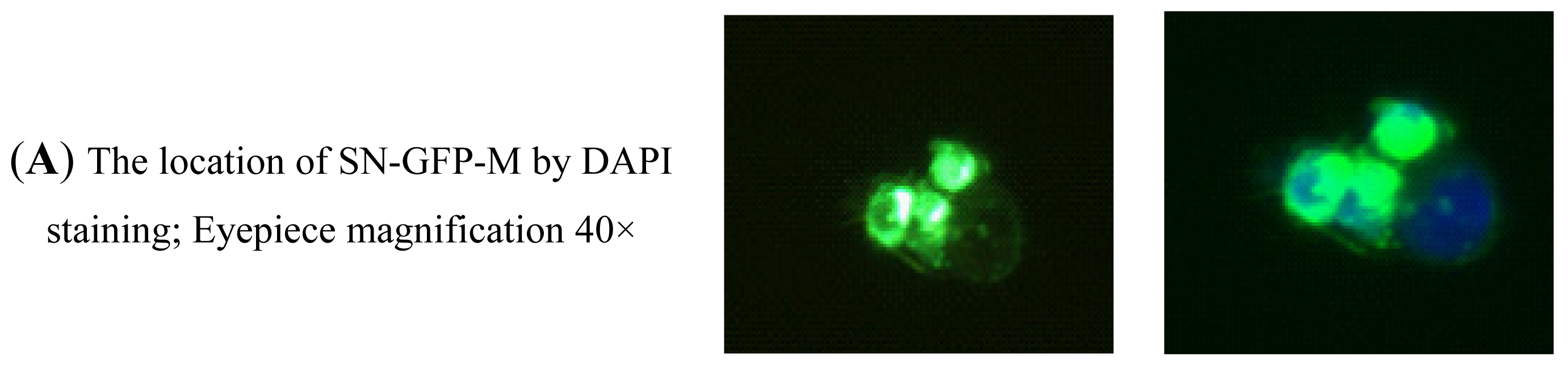
2.2.4. Analysis of the Binding Activity of SN-GFP-M Chimeric Proteins with PRRSV Using Immunofluorescence
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Bioinformatics Analysis
4.2.1. Methods and Software
4.2.2. Analysis of the Interaction of mSN with Sialic Acid
4.2.3. Modeling of pSN and cSN Structures without Ligand
4.2.4. Analysis of the SN Protein Hydrophobic Surface and Prediction of Amino Acid Binding with Sialic Acid on PRRSV
4.3. Experimental Methods
4.3.1. PRRSV and TCID50 Assay
4.3.2. RNA Extraction, cDNA Synthesis, Mutation of pSN and cSN and Construction of Expression Vector
4.3.3. Ultra-Filtration (UF) for Collection and Purification of SN-GFP Chimera Proteins from Cell Medium
4.3.4. Non-Reducing PAGE, WB and FAR-WB Detection of PRRSV-GP5 Binding Activity and SN-GFP Chimera Proteins
4.3.5. ELISA for Detection of Interactions between PRRSV and SN-GFP Chimeric Proteins
4.3.6. Immunofluorescence Assay to Test the Interaction between PRRSV and SN-GFP-M Chimeric Proteins
5. Conclusions
Supplementary Information
ijms-14-23955-s001.pdfAcknowledgments
Abbreviations
| PRRS | Porcine Reproductive and Respiratory Syndrome |
| SN | Sialoadhesin |
| pSN | porcine Sialoadhesin |
| cSN | cattle Sialoadhesin |
| mSN | mouse Sialoadhesin |
| SRBCR | Sheep Red Blood Coagulation Receptor |
| IG | Immuno Globulin |
| PDB | Protein Data Bank |
| UF | Ultra-filtration |
| RMSD | Root Mean Square Deviation |
| PS | Picosecond |
| SRCR | Scavenger-Receptor with Cysteine-rich family |
| MD | Molecular Dynamics |
| PRV | Porcine Rota Virus |
| SFV | Swine Fever Virus |
| HIV | Human Acquired Immune Deficiency Syndrome Virus |
| HS | Heparan Sulfate |
| DOPE score | Discrete Optimized Protein Energy score |
| Å | Angstrom |
| PAM | Porcine Alveolar Macrophages |
| PBMC | Porcine Blood Mononuclear Cells |
| PK | Porcine Kidney cells |
| FAR-WB | Far Western Blotting |
| PAGE | Polyacrylamide Gel Electrophoresis |
| MCS | Multiple Cloning Site |
Conflicts of Interest
References
- Meulenberg, J.J. PRRSV, the virus. Vet. Res 2000, 31, 11–21. [Google Scholar]
- Bloemraad, M.; de Kluijver, E.P.; Petersen, A.; Burkhardt, G.E.; Wensvoort, G. Porcine reproductive and respiratory syndrome: Temperature and pH stability of Lelystad virus and its survival in tissue specimens from viraemic pigs. Vet. Microbiol 1994, 42, 361–371. [Google Scholar]
- Forsberg, R.; Storgaard, T.; Nielsen, H.S.; Oleksiewicz, M.B.; Cordioli, P.; Sala, G.; Hein, J.; Bøtner, A. The genetic diversity of European type PRRSV is similar to that of the North American type but is geographically skewed within Europe. Virology 2002, 299, 38–47. [Google Scholar]
- Yoon, K.-J.; Wu, L.-L.; Zimmerman, J.J.; Hill, H.T.; Platt, K.B. Antibody-dependent enhancement (ADE) of porcine reproductive and respiratory syndrome virus (PRRSV) infection in pigs. Viral Immunol 1996, 9, 51–63. [Google Scholar]
- Duan, X.; Nauwynck, H.J.; Favoreel, H.W.; Pensaert, M.B. Identification of a putative receptor for porcine reproductive and respiratory syndrome virus on porcine alveolar macrophages. J. Virol 1998, 72, 4520–4523. [Google Scholar]
- Kim, H.; Kwang, J.; Yoon, I.; Joo, H.; Frey, M. Enhanced replication of porcine reproductive and respiratory syndrome (PRRS) virus in a homogeneous subpopulation of MA-104 cell line. Arch. Virol 1993, 133, 477–483. [Google Scholar]
- Graversen, J.H.; Madsen, M.; Moestrup, S.K. CD163: A signal receptor scavenging haptoglobin-hemoglobin complexes from plasma. Int. J. Biochem. Cell Biol 2002, 34, 309. [Google Scholar]
- Van Gorp, H.; van Breedam, W.; Delputte, P.L.; Nauwynck, H.J. Sialoadhesin and CD163 join forces during entry of the porcine reproductive and respiratory syndrome virus. J. Gen. Virol 2008, 89, 2943–2953. [Google Scholar]
- Vincent, A.; Thacker, B.; Halbur, P.; Rothschild, M.; Thacker, E. An investigation of susceptibility to porcine reproductive and respiratory syndrome virus between two genetically diverse commercial lines of pigs. J. Anim. Sci 2006, 84, 49–57. [Google Scholar]
- Crocker, P.R.; Gordon, S. Properties and distribution of a lectin-like hemagglutinin differentially expressed by murine stromal tissue macrophages. J. Exp. Med 1986, 164, 1862–1875. [Google Scholar]
- Durocher, J.R.; Payne, R.C.; Conrad, M.E. Role of sialic acid in erythrocyte survival. Blood 1975, 45, 11–20. [Google Scholar]
- Revilla, C.; Poderoso, T.; Martínez, P.; Álvarez, B.; López-Fuertes, L.; Alonso, F.; Ezquerra, A.; Domínguez, J. Targeting to porcine sialoadhesin receptor receptor improves antigen presentation to T cells. Vet. Res 2008, 40. [Google Scholar] [CrossRef]
- Wang, F.; Qiu, H.; Zhang, Q.; Peng, Z.; Liu, B. Association of two porcine reproductive and respiratory syndrome virus (PRRSV) receptor genes, CD163 and SN with immune traits. Mol. Biol. Rep 2012, 39, 3971–3976. [Google Scholar]
- Calvert, J.G.; Slade, D.E.; Shields, S.L.; Jolie, R.; Mannan, R.M.; Ankenbauer, R.G.; Welch, S.-K.W. CD163 expression confers susceptibility to porcine reproductive and respiratory syndrome viruses. J. Virol 2007, 81, 7371–7379. [Google Scholar]
- An, T.-Q.; Tian, Z.-J.; He, Y.-X.; Xiao, Y.; Jiang, Y.-F.; Peng, J.-M.; Zhou, Y.-J.; Liu, D.; Tong, G.-Z. Porcine reproductive and respiratory syndrome virus attachment is mediated by the N-terminal domain of the sialoadhesin receptor. Vet. Microbiol 2010, 143, 371–378. [Google Scholar]
- Delputte, P.L.; Nauwynck, H.J. Porcine arterivirus infection of alveolar macrophages is mediated by sialic acid on the virus. J. Virol 2004, 78, 8094–8101. [Google Scholar]
- Dokland, T. The structural biology of PRRSV. Virus Res 2010, 154, 86–97. [Google Scholar]
- Van Breedam, W.; Delputte, P.L.; van Gorp, H.; Misinzo, G.; Vanderheijden, N.; Duan, X.; Nauwynck, H.J. Porcine reproductive and respiratory syndrome virus entry into the porcine macrophage. J. Gen. Virol 2010, 91, 1659–1667. [Google Scholar]
- Van Breedam, W.; van Gorp, H.; Zhang, J.Q.; Crocker, P.R.; Delputte, P.L.; Nauwynck, H.J. The M/GP5 glycoprotein complex of porcine reproductive and respiratory syndrome virus binds the sialoadhesin receptor in a sialic acid-dependent manner. PLoS Pathogens 2010, 6, e1000730. [Google Scholar]
- May, A.; Robinson, R.; Vinson, M.; Crocker, P.; Jones, E. Crystal structure of the N-terminal domain of sialoadhesin in complex with 3′sialyllactose at 1.85 Å resolution. Mol. Cell 1998, 1, 719–728. [Google Scholar]
- Zaccai, N.R.; Maenaka, K.; Maenaka, T.; Crocker, P.R.; Brossmer, R.; Kelm, S.; Jones, E.Y. Structure-guided design of sialic acid-based siglec inhibitors and crystallographic analysis in complex with sialoadhesin. Structure 2003, 11, 557–567. [Google Scholar]
- Bukrinsky, J.T.; St Hilaire, P.M.; Meldal, M.; Crocker, P.R.; Henriksen, A. Complex of sialoadhesin with a glycopeptide ligand. Biochim. Biophys. Acta 2004, 1702, 173–179. [Google Scholar]
- Delputte, P.L.; van Breedam, W.; Delrue, I.; Oetke, C.; Crocker, P.R.; Nauwynck, H.J. Porcine arterivirus attachment to the macrophage-specific receptor sialoadhesin is dependent on the sialic acid-binding activity of the N-terminal immunoglobulin domain of sialoadhesin. J. Virol 2007, 81, 9546–9550. [Google Scholar]
- Eswar, N.; Webb, B.; Marti-Renom, M.A.; Madhusudhan, M.; Eramian, D.; Shen, M.Y.; Pieper, U.; Sali, A. Comparative protein structure modeling using Modeller. Curr. Protoc. Bioinforma 2006. [Google Scholar] [CrossRef]
- Guex, N.; Peitsch, M.C. SWISS-MODEL and the Swiss-Pdb Viewer: An environment for comparative protein modelling. Electrophoresis 1997, 18, 2714–2723. [Google Scholar]
- Pal, D.; Chakrabarti, P. On residues in the disallowed region of the Ramachandran map. Biopolymers 2002, 63, 195–206. [Google Scholar]
- Crook, M. The determination of plasma or serum sialic acid. Clin. Biochem 1993, 26, 31–38. [Google Scholar]
- De Baere, M.I.; van Gorp, H.; Delputte, P.L.; Nauwynck, H.J. Interaction of the European genotype porcine reproductive and respiratory syndrome virus (PRRSV) with sialoadhesin (CD169/Siglec-1) inhibits alveolar macrophage phagocytosis. Vet. Res 2012, 43, 47. [Google Scholar]
- Delputte, P.; Costers, S.; Nauwynck, H. Analysis of porcine reproductive and respiratory syndrome virus attachment and internalization: Distinctive roles for heparan sulphate and sialoadhesin. J. Gen. Virol 2005, 86, 1441–1445. [Google Scholar]
- Richards, F.; Richmond, T. Solvents, interfaces and protein structure. Mol. Interact. Act. Proteins 2009, 916, 23. [Google Scholar]
- Fersht, A.R. The hydrogen bond in molecular recognition. Trends Biochem. Sci 1987, 12, 301–304. [Google Scholar]
- Corrada, D.; D’ursi, P.; Botti, S.; Luperini, A.; Milanesi, L.; Rovida, E. 3D-Structure Prediction of the Modular Protein Sialoadhesin Using a Multi-step Modelling Strategy. In Proceedings of the Sysbiohealth Symposium Medicine and Disease: A System Biology Perspective; Bononia University Press: Bologna, Italy, 2008; pp. 15–26. [Google Scholar]
- Stehle, T.; Harrison, S.C. High-resolution structure of a polyomavirus VP1-oligosaccharide complex: Implications for assembly and receptor binding. EMBO J 1997, 16, 5139–5148. [Google Scholar]
- Jiang, W.; Jiang, P.; Li, Y.; Tang, J.; Wang, X.; Ma, S. Recombinant adenovirus expressing GP5 and M fusion proteins of porcine reproductive and respiratory syndrome virus induce both humoral and cell-mediated immune responses in mice. Vet. Immunol. Immunopathol 2006, 113, 169–180. [Google Scholar]
- Lee, Y.J.; Park, C.-K.; Nam, E.; Kim, S.-H.; Lee, O.-S.; Lee, D.S.; Lee, C. Generation of a porcine alveolar macrophage cell line for the growth of porcine reproductive and respiratory syndrome virus. J. Virol. Methods 2010, 163, 410–415. [Google Scholar]
- Bowers, K.J.; Chow, E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; Klepeis, J.L.; Kolossvary, I.; Moraes, M.A.; Sacerdoti, F.D. Scalable Algorithms for Molecular Dynamics Simulations on Commodity Clusters. Proceedings of the ACM/IEEE SC 2006 Conference, Tampa, FL, USA, 11–17 November 2006; Wiley Online Library: Hoboken, NJ, USA, 2006; pp. 43–43. [Google Scholar]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem 2004, 25, 1605–1612. [Google Scholar]
- Bendtsen, J.D.; Nielsen, H.; von Heijne, G.; Brunak, S. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol 2004, 340, 783–795. [Google Scholar]
- Blom, N.; Sicheritz-Pontén, T.; Gupta, R.; Gammeltoft, S.; Brunak, S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics 2004, 4, 1633–1649. [Google Scholar]
- Dundas, J.; Ouyang, Z.; Tseng, J.; Binkowski, A.; Turpaz, Y.; Liang, J. CASTp: Computed atlas of surface topography of proteins with structural and topographical mapping of functionally annotated residues. Nucleic Acids Res 2006, 34, W116–W118. [Google Scholar]
- Larkin, M.; Blackshields, G.; Brown, N.; Chenna, R.; McGettigan, P.; McWilliam, H.; Valentin, F.; Wallace, I.; Wilm, A.; Lopez, R. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar]
- Overend, C.; Mitchell, R.; He, D.; Rompato, G.; Grubman, M.; Garmendia, A. Recombinant swine beta interferon protects swine alveolar macrophages and Marc-145 cells from infection with porcine reproductive and respiratory syndrome virus. J. Gen. Virol 2007, 88, 925–931. [Google Scholar]
- Kanno, T.; Huettel, B.; Mette, M.F.; Aufsatz, W.; Jaligot, E.; Daxinger, L.; Kreil, D.P.; Matzke, M.; Matzke, A.J. Atypical RNA polymerase subunits required for RNA-directed DNA methylation. Nat. Genet 2005, 37, 761–765. [Google Scholar]
- The protocol of the first Strand cDNA Synthesis Kit, Version; Fermentas Company, Thermo Scientific: Boston, MA, USA, 2010.
- Schanstra, J.P.; Rink, R.; Pries, F.; Janssen, D.B. Construction of an expression and site-directed mutagenesis system of haloalkane dehalogenase in Escherichia coli. Protein Expr. Purif 1993, 4, 479–489. [Google Scholar]
- Li, Y.; Xie, M.; Zhang, K. Diagnosis of mareks disease by paper immunogold-silver staining (P-IGSS). Chin. J. Anim. Poult. Infect. Dis 1991, 3, 15–16. [Google Scholar]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int 2004, 11, 36–42. [Google Scholar]
- Kerschbaumer, R.J.; Hirschl, S.; Kaufmann, A.; Ibl, M.; Koenig, R.; Himmler, G. Single-chain Fv fusion proteins suitable as coating and detecting reagents in a double antibody sandwich enzyme-linked immunosorbent assay. Anal. Biochem 1997, 249, 219–227. [Google Scholar]
- Spitzenberg, V.; Grun, M. Efficient transfection of neuroblastoma cell lines using FuGENE® HD transfection reagent. Biochem. Mannh 2007, 1, 11. [Google Scholar]
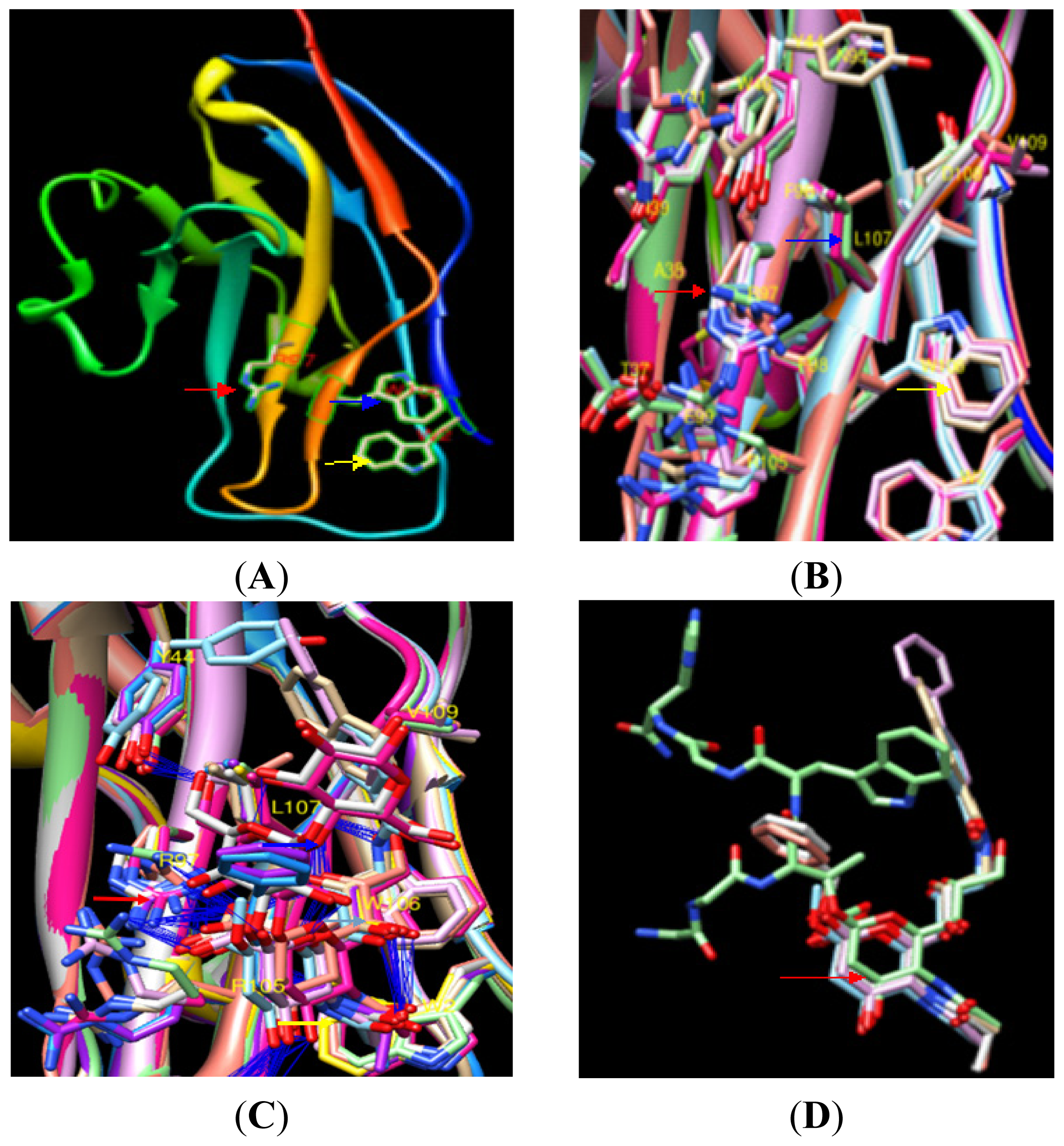


| RMSD | PDB:ID | 1OD7 | 1OD9 | 1ODA | 1QFOa | 1QFOb | 1QFOc | 1URL | 2BVEa | 2BVEb |
|---|---|---|---|---|---|---|---|---|---|---|
| 1OD7 | ||||||||||
| 1OD9 | 0.447 Å | |||||||||
| 1ODA | 0.434 Å | 0.439 Å | ||||||||
| 1QFOa | 0.468 Å | 0.421 Å | 0.454 Å | |||||||
| 1QFOb | 0.591 Å | 0.585 Å | 0.476 Å | 0.418 Å | ||||||
| 1QFOc | 0.407 Å | 0.331 Å | 0.470 Å | 0.400 Å | 0.394 Å | |||||
| 1URL | 0.551 Å | 0.541 Å | 0.571 Å | 0.518 Å | 0.570 Å | 0.491 Å | ||||
| 2BVEa | 0.509 Å | 0.455 Å | 0.561 Å | 0.417 Å | 0.437 Å | 0.447 Å | 0.562 Å | |||
| 2BVEb | 0.438 Å | 0.441 Å | 0.449 Å | 0.339 Å | 0.347 Å | 0.356 Å | 0.564 Å | 0.375 Å |
| PDB:ID | Subunit | Amino acids binding to sialic acid | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1ODA | W2 | N95 | R97 | S103 | R105 | W106 | L107 | D108 | V109 | ||
| 1OD9 | W2 | Y44 | R97 | S103 | R105 | W106 | L107 | V109 | |||
| 1OD7 | W2 | Y44 | S45 | R97 | S103 | R105 | W106 | L107 | D108 | V109 | |
| 1URL | W2 | R97 | R105 | R106 | L107 | V109 | |||||
| 2BVE | A | W2 | R97 | S103 | R105 | R106 | L107 | V109 | |||
| B | W2 | R97 | S103 | N104 | R105 | W106 | L107 | V109 | |||
| 1QFO | A | W2 | Y44 | R97 | S103 | R105 | W106 | L107 | V109 | ||
| B | W2 | Y44 | R97 | S103 | R105 | W106 | L107 | V109 | |||
| C | W2 | R97 | S103 | R105 | R106 | L107 | V109 | ||||
| 1QFP | |||||||||||
| Model name | DOPE score |
|---|---|
| Cattle.01 | −12,062 |
| Cattle.02 | −12,184 |
| Cattle.03 | −12,156 |
| Cattle.04 | −12,079 |
| Cattle.05 | −12,142 |
| Cattle.06 | −12,238 |
| Cattle.07 | −12,052 |
| Cattle.08 | −12,217 |
| Cattle.09 | −12,151 |
| Porcine.01 | −12,425 |
| Porcine.02 | −12,506 |
| Porcine.03 | −12,270 |
| Porcine.04 | −12,503 |
| Porcine.05 | −12,543 |
| Porcine.06 | −12,570 |
| Porcine.07 | −12,509 |
| Porcine.08 | −12,599 |
| Porcine.09 | −12,486 |
| SN | 5′ terminal primer | 3′ terminal primer | Annealing temperature |
|---|---|---|---|
| pSN T3G | GGCCTGGCCTCGTGG ggc GTTTCCAGCCCCGAGA | TCTCGGGGCTGGAAAC gcc CCACGAGGCCAGGCC | 54.0 °C |
| pSN T78V | CAGGTTGAACAGAGG gtg TGCAGCCTGCTGCTG | CAGCAGCAGGCTGCA cac CCTCTGTTCAACCTG | 54.0 °C |
| pSN S107V | GAGGGCAACCGCTGG gta GATGTCAAAGGCACAG | CTGTGCCTTTGACATC tac CCAGCGGTTGCCCTC | 54.5 °C |
| pSN Wild-type | GTAGATCTTC ATGGACTTCCTGCTCCTGCTCCTC | CAACCGGTAA CAAGGCAATGGTGGGCACGCTGG | 55.0 °C |
| cSN Wild-type | GTAGATCTTC ATGGACTTCCTGCTCCAGCTCCTC | CAACCGGTAA AGAGGCAACCGTGGGCATGATGAG | 56.0 °C |
| SN membrane domain BsrG I/Xba I | CTTGTACAAGCTTCTCTGGTTCCTGG | GCTCTAGACTACCAGACCCCCAGGCC | 53.0 °C |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jiang, Y.; Khan, F.A.; Pandupuspitasari, N.S.; Kadariya, I.; Cheng, Z.; Ren, Y.; Chen, X.; Zhou, A.; Yang, L.; Kong, D.; et al. Analysis of the Binding Sites of Porcine Sialoadhesin Receptor with PRRSV. Int. J. Mol. Sci. 2013, 14, 23955-23979. https://doi.org/10.3390/ijms141223955
Jiang Y, Khan FA, Pandupuspitasari NS, Kadariya I, Cheng Z, Ren Y, Chen X, Zhou A, Yang L, Kong D, et al. Analysis of the Binding Sites of Porcine Sialoadhesin Receptor with PRRSV. International Journal of Molecular Sciences. 2013; 14(12):23955-23979. https://doi.org/10.3390/ijms141223955
Chicago/Turabian StyleJiang, Yibo, Faheem Ahmed Khan, Nuruliarizki Shinta Pandupuspitasari, Ishwari Kadariya, Zhangrui Cheng, Yuwei Ren, Xing Chen, Ao Zhou, Liguo Yang, Dexin Kong, and et al. 2013. "Analysis of the Binding Sites of Porcine Sialoadhesin Receptor with PRRSV" International Journal of Molecular Sciences 14, no. 12: 23955-23979. https://doi.org/10.3390/ijms141223955





