Determination of Homoarginine, Arginine, NMMA, ADMA, and SDMA in Biological Samples by HPLC-ESI-Mass Spectrometry
Abstract
:1. Introduction
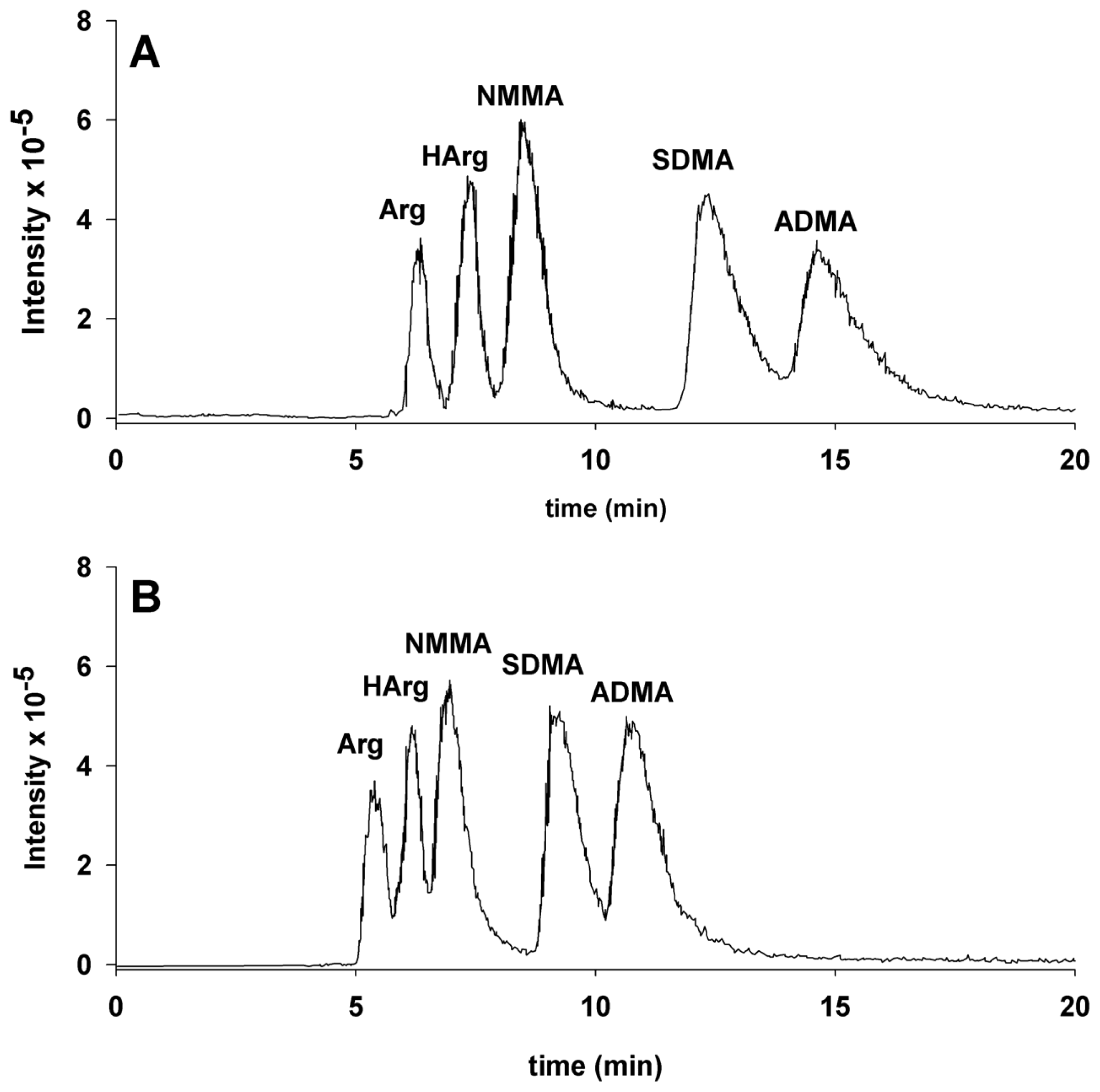
2. Results and Discussion
3. Experimental Section
3.1. Reagents
3.2. Sample Preparation
3.3. HPLC–ESI–MS/MS Instrumental Conditions
4. Conclusions
| Agilent LC–MSD SL quadrupole ion trap settings | |
|---|---|
| MS acquisition | ESI in positive ion mode |
| Nebulizer pressure | 30 psi |
| Drying temperature | 350 °C |
| Drying gas | 7 L/min |
| Ion Charge Control (ICC), target set at 30,000, maximum accumulation time at 20 ms | |
Acknowledgments
Conflicts of Interest
References
- Caplin, B.; Leiper, J. Endogenous nitric oxide synthase inhibitors in the biology of disease: Markers, mediators, and regulators? Arterioscler. Thromb. Vasc. Biol 2012, 32, 1343–1353. [Google Scholar]
- Cooke, J.P.; Dzau, V.J. Nitric oxide synthase: Role in the genesis of vascular disease. Annu. Rev. Med 1997, 48, 489–509. [Google Scholar]
- Moncada, S.; Higgs, A. The l-arginine-nitric oxide pathway. N. Engl. J. Med 1993, 329, 2002–2012. [Google Scholar]
- Bedford, M.T.; Clarke, S.G. Protein arginine methylation in mammals: Who, what, and why. Mol. Cell 2009, 33, 1–13. [Google Scholar]
- Moncada, S.; Higgs, E.A. The discovery of nitric oxide and its role in vascular biology. Br. J. Pharmacol 2006, 147, S193–S201. [Google Scholar]
- Tsikas, D.; Sandmann, J.; Savva, A.; Luessen, P.; Böger, R.H.; Gutzki, F.M.; Mayer, B.; Frölich, J,C. Assessment of nitric oxide synthase activity in vitro and in vivo by gas chromatography-mass spectrometry. J. Chromatogr. B 2000, 742, 143–153. [Google Scholar]
- Visser, M.; Vermeulen, M.A.; Richir, M.C.; Teerlink, T.; Houdijk, A.P.; Kostense, P.J.; Wisselink, W.; de Mol, B.A.; van Leeuwen, P.A.; Oudemans-van Straaten, H.M. Imbalance of arginine and asymmetric dimethylarginine is associated with markers of circulatory failure, organ failure and mortality in shock patients. Br. J. Nutr 2012, 107, 1458–1465. [Google Scholar]
- Zairis, M.N.; Patsourakos, N.G.; Tsiaousis, G.Z.; Theodossis Georgilas, A.; Melidonis, A.; Makrygiannis, S.S.; Velissaris, D.; Batika, P.C.; Argyrakis, K.S.; Tzerefos, S.P.; et al. Plasma asymmetric dimethylarginine and mortality in patients with acute decompensation of chronic heart failure. Heart 2012, 98, 860–864. [Google Scholar]
- Celik, M.; Iyisoy, A.; Celik, T.; Yilmaz, M.I.; Yuksel, U.C.; Yaman, H. The relationship between l-arginine/ADMA ratio and coronary collateral development in patients with low glomerular filtration rate. Cardiol. J 2012, 19, 29–35. [Google Scholar]
- Furuki, K.; Adachi, H.; Matsuoka, H.; Enomoto, M.; Satoh, A.; Hino, A.; Hirai, Y.; Imaizumi, T. Plasma levels of asymmetric dimethylarginine (ADMA) are related to intima-media thickness of the carotid artery: An epidemiological study. Atherosclerosis 2007, 191, 206–210. [Google Scholar]
- Servillo, L.; Giovane, A.; Cautela, D.; Castaldo, D.; Balestrieri, M.L. The methylarginines NMMA, ADMA, and SDMA are ubiquitous constituents of the main vegetables of human nutrition. Nitric Oxide 2013, 30, 43–48. [Google Scholar]
- Moali, C.; Boucher, J.L.; Sari, M.A.; Stuehr, D.J.; Mansuy, D. Substrate specificity of NO synthases: Detailed comparison of l-arginine, homo-l-arginine, their N omega-hydroxy derivatives, and N omega-hydroxynor-l-arginine. Biochemistry 1998, 37, 10453–10460. [Google Scholar]
- März, W.; Meinitzer, A.; Drechsler, C.; Pilz, S.; Krane, V.; Kleber, M.E.; Fischer, J.; Winkelmann, B.R.; Bohm, B.O.; Ritz, E.; et al. Homoarginine, cardiovascular risk, and mortality. Circulation 2010, 122, 967–975. [Google Scholar]
- Drechsler, C.; Meinitzer, A.; Pilz, S.; Krane, V.; Tomaschitz, A.; Ritz, E.; März, W.; Wanner, C. Homoarginine, heart failure, and sudden cardiac death in haemodialysis patients. Eur. J. Heart. Fail 2011, 13, 852–859. [Google Scholar]
- Pilz, S.; Meinitzer, A.; Tomaschitz, A.; Drechsler, C.; Ritz, E.; Krane, V.; Wanner, C.; Böhm, B.O.; März, W. Low homoarginine concentration is a novel risk factor for heart disease. Heart 2011, 97, 1222–1227. [Google Scholar]
- Bishop, M.J.; Crow, B.; Norton, D.; Paliakov, E.; George, J.; Bralley, J.A. Direct analysis of un-derivatized asymmetric dimethylarginine (ADMA) and Larginine from plasma using mixed-mode ion-exchange liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci 2007, 859, 164–169. [Google Scholar]
- D’Apolito, O.; Paglia, G.; Tricarico, F.; Garofalo, D.; Pilotti, A.; Lamacchia, O.; Cignarelli, M.; Corso, G. Development and validation of a fast quantitative method for plasma dimethylarginines analysis using liquid chromatography-tandem mass spectrometry. Clin. Biochem 2008, 41, 1391–1395. [Google Scholar]
- Davis, M.; Swieringa, E.; Palm, F.; Smith, D.E.C.; Smulders, Y.M.; Scheffer, P.G.; Blom, H.J.; Teerlink, T. Simultaneous determination of asymmetric and symmetric dimethylarginine, l-monomethylarginine, l-arginine, and l-homoarginine in biological samples using stable isotope dilution liquid chromatography tandem mass spectrometry. J. Chromatogr. B 2012, 900, 38–47. [Google Scholar]
- Di Gangi, I.M.; Chiandetti, L.; Gucciardi, A.; Moret, V.; Naturale, M.; Giordano, G. Simultaneous quantitative determination of N(G),N(G)-dimethyl-l-arginine or asymmetric dimethylarginine and related pathway’s metabolites in biological fluids by ultrahigh-performance liquid chromatography/electrospray ionization-tandem mass spectrometry. Anal. Chim. Acta 2010, 677, 140–148. [Google Scholar]
- Martens-Lobenhoffer, J.; Bode-Böger, S.M. Simultaneous detection of arginine, asymmetric dimethylarginine, symmetric dimethylarginine and citrulline in human plasma and urine applying liquid chromatography-mass spectrometry with very straightforward sample preparation. J. Chromatogr. B. Anal. Technol. Biomed. Life Sci 2003, 798, 231–239. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Servillo, L.; Giovane, A.; D'Onofrio, N.; Casale, R.; Cautela, D.; Castaldo, D.; Balestrieri, M.L. Determination of Homoarginine, Arginine, NMMA, ADMA, and SDMA in Biological Samples by HPLC-ESI-Mass Spectrometry. Int. J. Mol. Sci. 2013, 14, 20131-20138. https://doi.org/10.3390/ijms141020131
Servillo L, Giovane A, D'Onofrio N, Casale R, Cautela D, Castaldo D, Balestrieri ML. Determination of Homoarginine, Arginine, NMMA, ADMA, and SDMA in Biological Samples by HPLC-ESI-Mass Spectrometry. International Journal of Molecular Sciences. 2013; 14(10):20131-20138. https://doi.org/10.3390/ijms141020131
Chicago/Turabian StyleServillo, Luigi, Alfonso Giovane, Nunzia D'Onofrio, Rosario Casale, Domenico Cautela, Domenico Castaldo, and Maria Luisa Balestrieri. 2013. "Determination of Homoarginine, Arginine, NMMA, ADMA, and SDMA in Biological Samples by HPLC-ESI-Mass Spectrometry" International Journal of Molecular Sciences 14, no. 10: 20131-20138. https://doi.org/10.3390/ijms141020131




