Characterization of Apoptosis Induced by Emodin and Related Regulatory Mechanisms in Human Neuroblastoma Cells
Abstract
:1. Introduction
2. Results
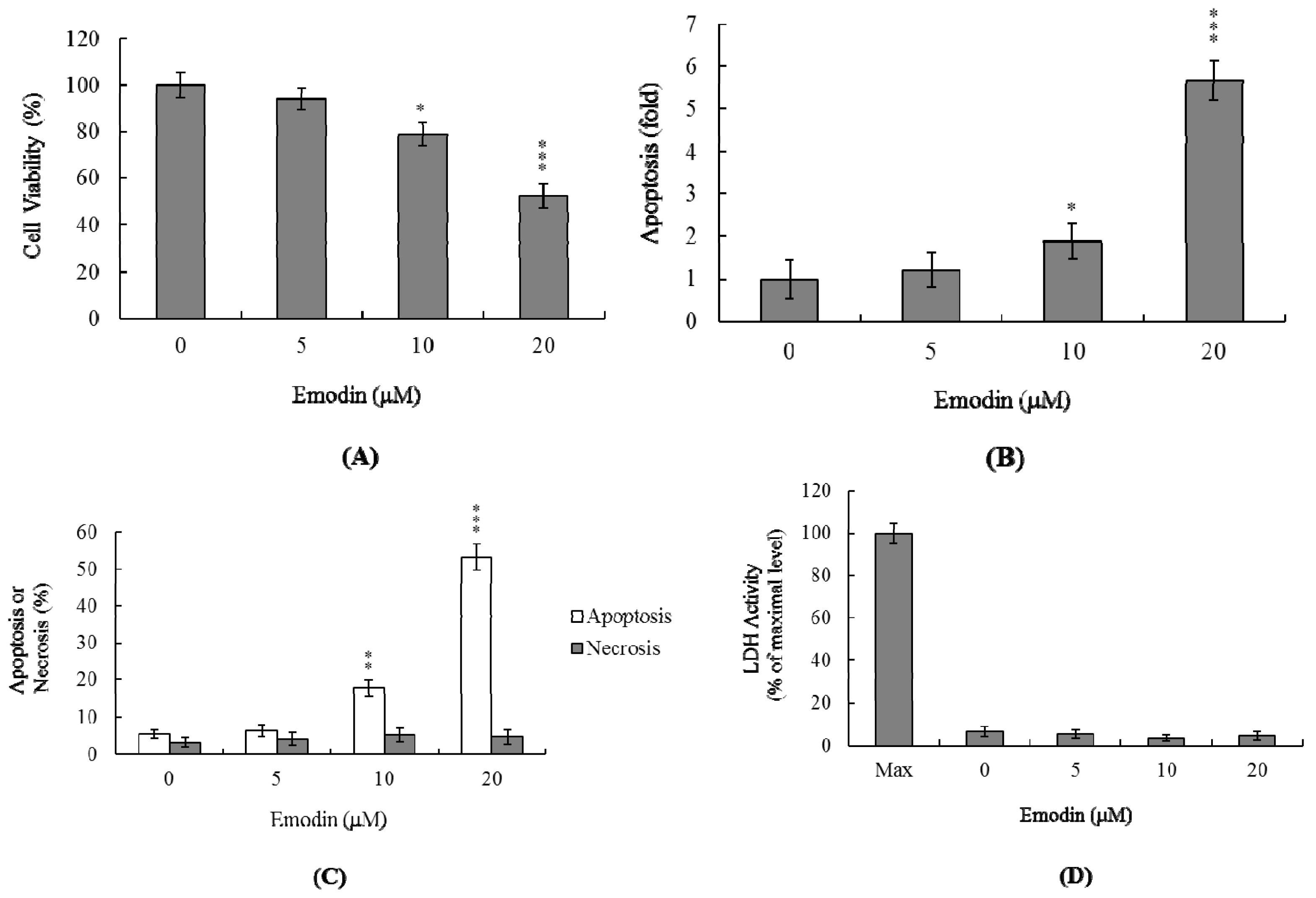
2.1 Cytotoxic Effects of Emodin on IMR-32 Cells
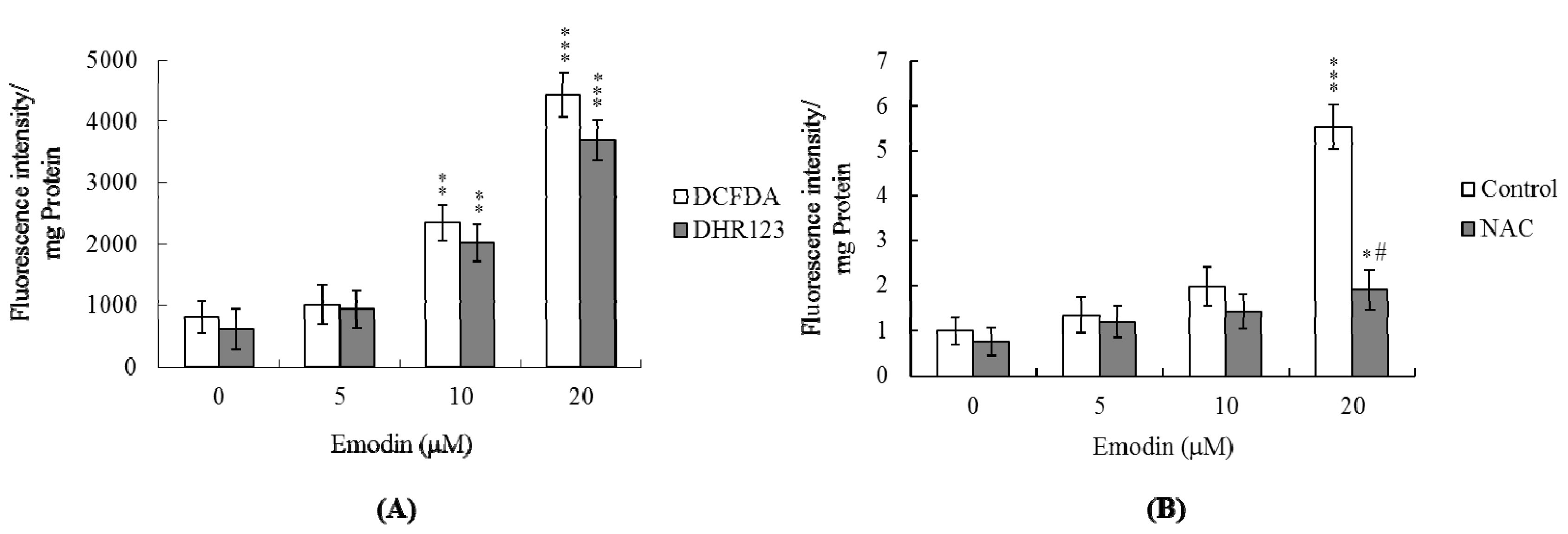
2.2 ROS Levels Are Increased in IMR-32 Cells Treated with Emodin
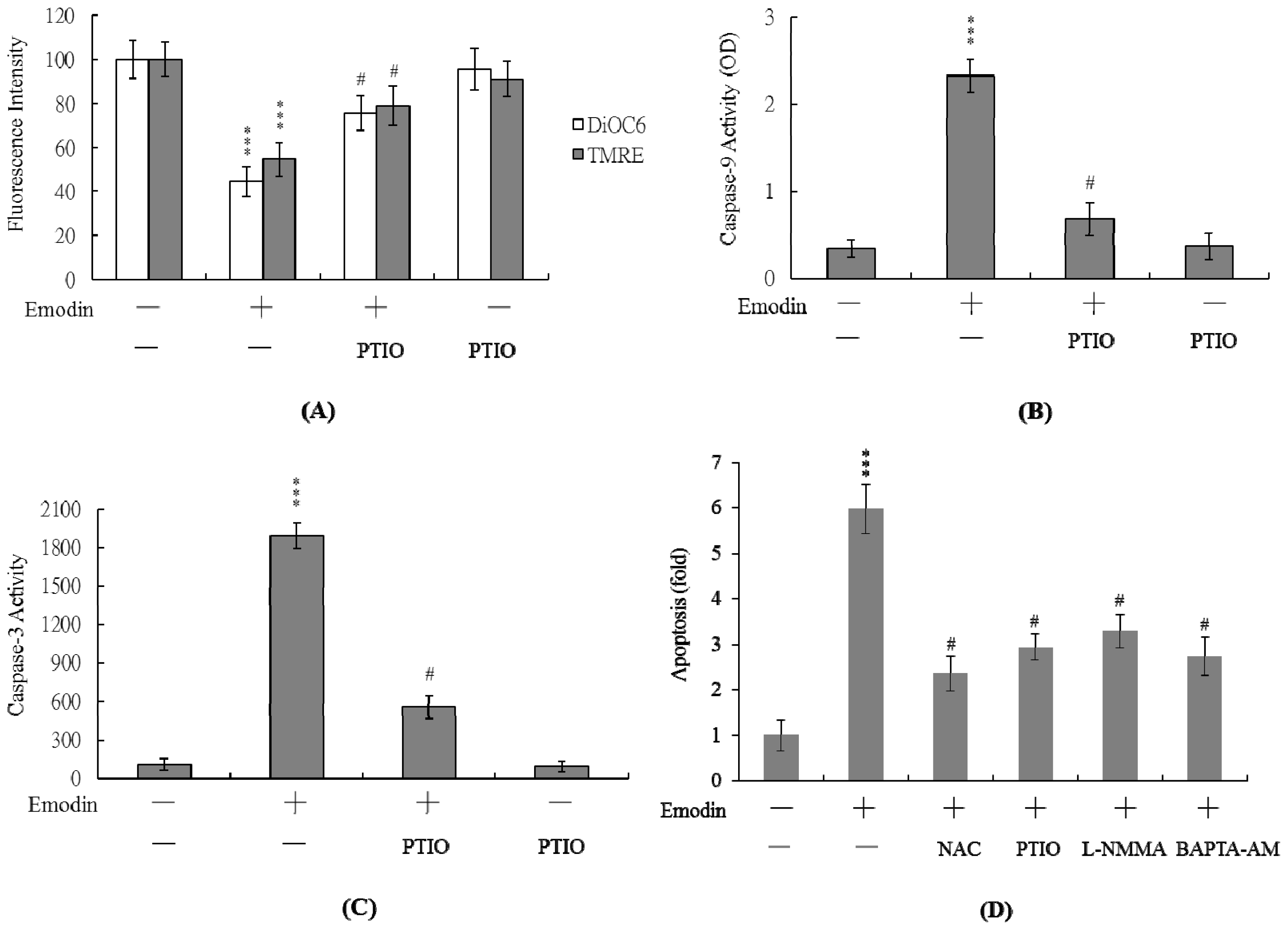
2.3 Changes in Intracellular Ca2+ and NO Levels Are Involved in Emodin-Induced Cell Apoptosis
2.4 PTIO Inhibits Mitochondrial Membrane Potential (MMP) Changes and Caspase Activation during Emodin-Induced Cell Apoptosis
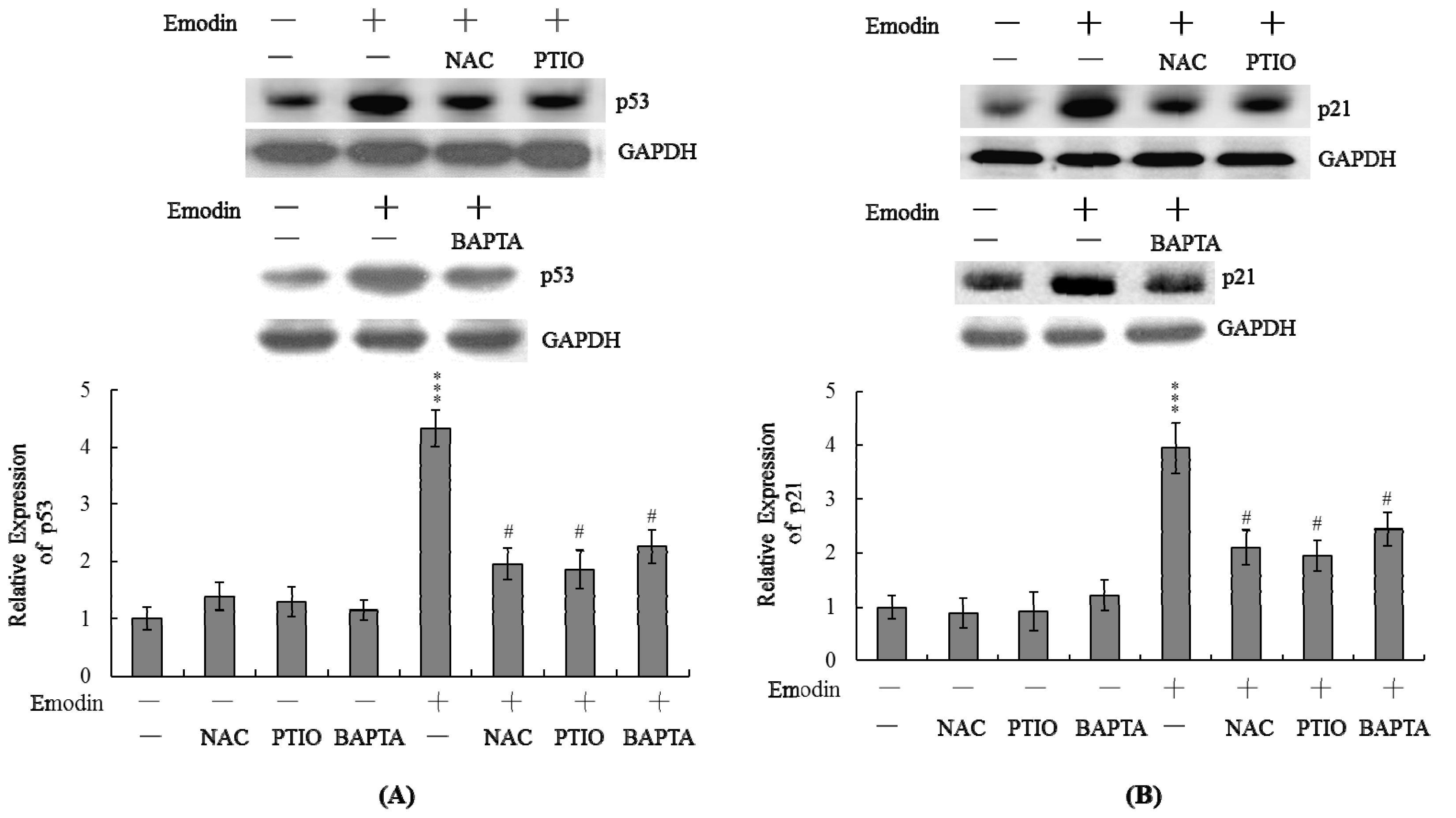
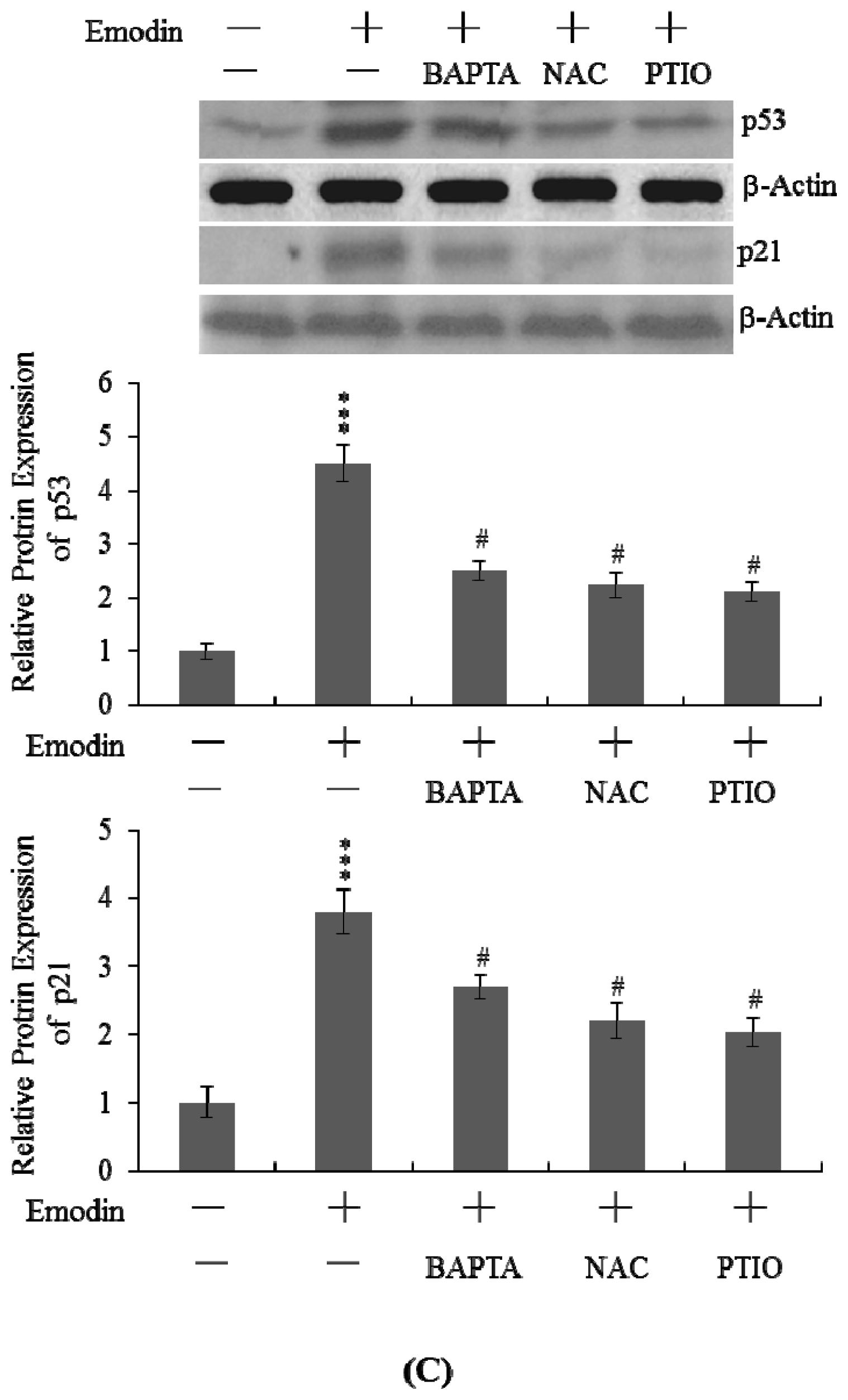
2.5. Changes in p53 and p21 Expression Levels Following Emodin Treatment of IMR-32 Cells
2.6. Treatment of IMR-32 Cells with p53 siRNA Blocks Emodin-Induced Apoptosis
3. Discussion
4. Experimental Section
4.1. Chemicals and Reagents
4.2. Cell Culture and Emodin Treatment
4.3. MTT Assay
4.4. Assessment of Necrosis and Apoptosis
4.5. ROS Assay
4.6. Detection of Intracellular Calcium Concentration ([Ca2+]i )
4.7. Detection of Intracellular NO Content
4.8. Caspase Activity Assays
4.9. Real-Time RT-PCR Assay
4.10. siRNA Knockdown
4.11. Immunoblots
4.12. Statistics
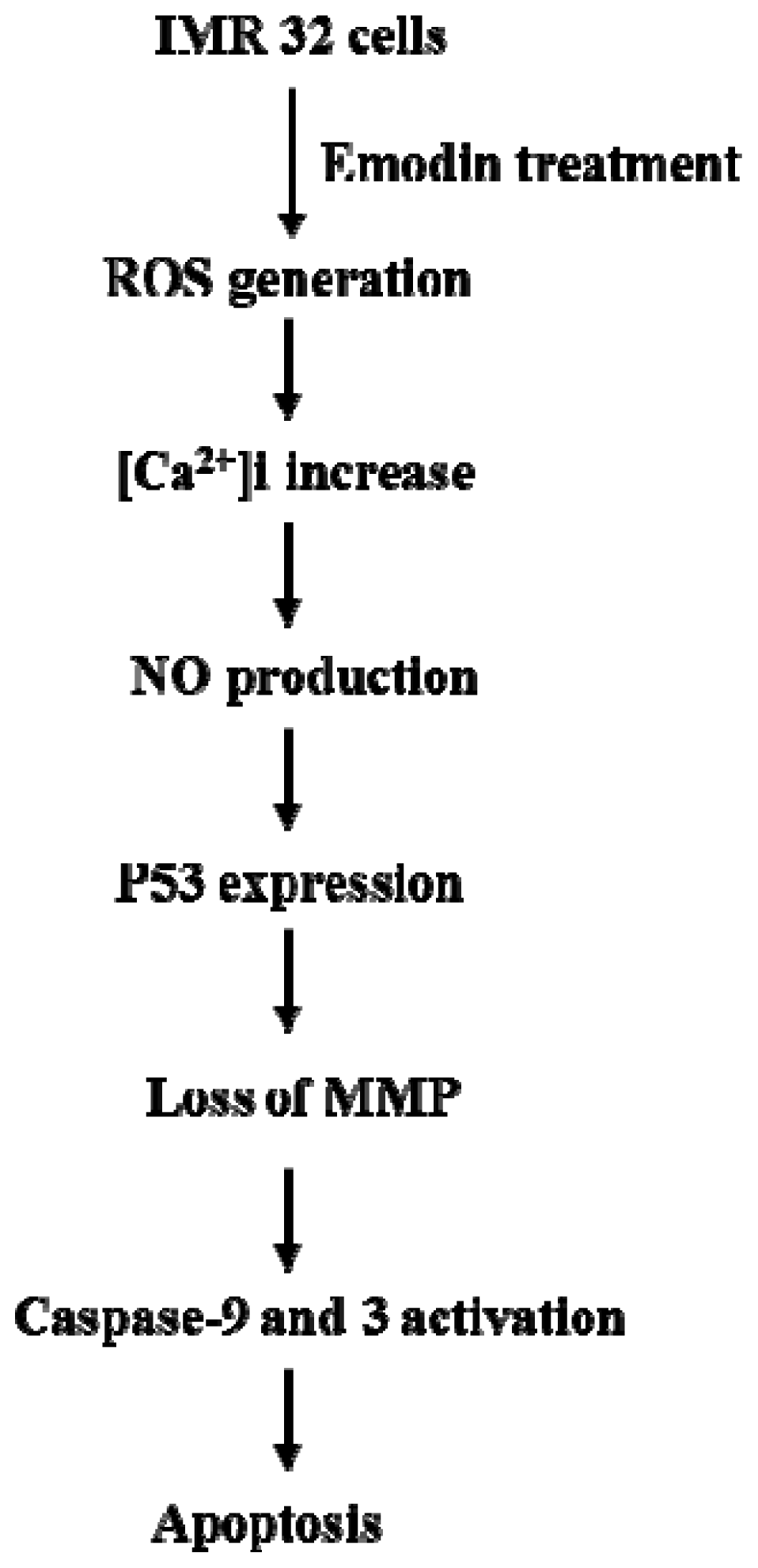
5. Conclusions


Acknowledgments
Conflicts of interest
References
- Yim, H.; Lee, Y.H.; Lee, C.H.; Lee, S.K. Emodin, an anthraquinone derivative isolated from the rhizomes of Rheum palmatum, selectively inhibits the activity of casein kinase II as a competitive inhibitor. Planta Med 1999, 65, 9–13. [Google Scholar]
- Yang, F.; Zhang, T.; Tian, G.; Cao, H.; Liu, Q.; Ito, Y. Preparative isolation and purification of hydroxyanthraquinones from Rheum officinale Baill by high-speed counter-current chromatography using pH-modulated stepwise elution. J. Chromatogr. A 1999, 858, 103–107. [Google Scholar]
- Huang, H.C.; Chu, S.H.; Chao, P.D. Vasorelaxants from Chinese herbs, emodin and scoparone, possess immunosuppressive properties. Eur. J. Pharmacol 1991, 198, 211–213. [Google Scholar]
- Zhou, X.M.; Chen, Q.H. Biochemical study of Chinese rhubarb. XXII. Inhibitory effect of anthraquinone derivatives on Na+-K+-ATPase of the rabbit renal medulla and their diuretic action. Yao Xue Xue Bao 1988, 23, 17–20. [Google Scholar]
- Koyama, M.; Kelly, T.R.; Watanabe, K.A. Novel type of potential anticancer agents derived from chrysophanol and emodin. Some structure-activity relationship studies. J. Med. Chem 1988, 31, 283–284. [Google Scholar]
- Zhang, L.; Lau, Y.K.; Xia, W.; Hortobagyi, G.N.; Hung, M.C. Tyrosine kinase inhibitor emodin suppresses growth of HER-2/neu-overexpressing breast cancer cells in athymic mice and sensitizes these cells to the inhibitory effect of paclitaxel. Clin. Cancer Res 1999, 5, 343–353. [Google Scholar]
- Shieh, D.E.; Chen, Y.Y.; Yen, M.H.; Chiang, L.C.; Lin, C.C. Emodin-induced apoptosis through p53-dependent pathway in human hepatoma cells. Life Sci 2004, 74, 2279–2290. [Google Scholar]
- Wang, C.-G.; Yang, J.-Q.; Liu, B.-Z.; Jin, D.-T.; Wang, C.; Zhong, L.; Zhu, D.; Wu, Y. Anti-tumor activity of emodin against human chronic myelocytic leukemia K562 cell lines in vitro and in vivo. Eur. J. Pharmacol. 2010, 627, 33–41. [Google Scholar]
- Su, Y.T.; Chang, H.L.; Shyue, S.K.; Hsu, S.L. Emodin induces apoptosis in human lung adenocarcinoma cells through a reactive oxygen species-dependent mitochondrial signaling pathway. Biochem. Pharmacol 2005, 70, 229–241. [Google Scholar]
- Lin, S.Y.; Lai, W.W.; Ho, C.C.; Yu, F.S.; Chen, G.W.; Yang, J.S.; Liu, K.C.; Lin, M.L.; Wu, P.P.; Fan, M.J.; et al. Emodin induces apoptosis of human tongue squamous cancer SCC-4 cells through reactive oxygen species and mitochondria-dependent pathways. Anticancer Res 2009, 29, 327–335. [Google Scholar]
- Kuo, T.C.; Yang, J.S.; Lin, M.W.; Hsu, S.C.; Lin, J.J.; Lin, H.J.; Hsia, T.C.; Liao, C.L.; Yang, M.D.; Fan, M.J.; et al. Emodin has cytotoxic and protective effects in rat C6 glioma cells: Roles of Mdr1a and nuclear factor kappaB in cell survival. J. Pharmacol. Exp. Ther 2009, 330, 736–744. [Google Scholar]
- Chang, M.H.; Huang, F.J.; Chan, W.H. Emodin induces embryonic toxicity in mouse blastocysts through apoptosis. Toxicology 2012, 299, 25–32. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M. Role of free radicals and catalytic metal ions in human disease: An overview. Methods Enzymol 1990, 186, 1–85. [Google Scholar]
- Chan, W.H. Ginkgolide B induces apoptosis and developmental injury in mouse embryonic stem cells and blastocysts. Hum. Reprod 2006, 21, 2985–2995. [Google Scholar]
- Chan, W.H.; Shiao, N.H.; Lu, P.Z. CdSe quantum dots induce apoptosis in human neuroblastoma cells via mitochondrial-dependent pathways and inhibition of survival signals. Toxicol. Lett 2006, 167, 191–200. [Google Scholar]
- Ekmekcioglu, S.; Tang, C.H.; Grimm, E.A. NO news is not necessarily good news in cancer. Curr. Cancer Drug Targets 2005, 5, 103–115. [Google Scholar]
- Zhou, J.; Brune, B. NO and transcriptional regulation: From signaling to death. Toxicology 2005, 208, 223–233. [Google Scholar]
- Rao, C.V. Nitric oxide signaling in colon cancer chemoprevention. Mutat. Res 2004, 555, 107–119. [Google Scholar]
- Lu, Z.; Tao, Y.; Zhou, Z.; Zhang, J.; Li, C.; Ou, L.; Zhao, B. Mitochondrial reactive oxygen species and nitric oxide-mediated cancer cell apoptosis in 2-butylamino-2-demethoxyhypocrellin B photodynamic treatment. Free Radic. Biol. Med 2006, 41, 1590–1605. [Google Scholar]
- Nazarewicz, R.R.; Zenebe, W.J.; Parihar, A.; Larson, S.K.; Alidema, E.; Choi, J.; Ghafourifar, P. Tamoxifen induces oxidative stress and mitochondrial apoptosis via stimulating mitochondrial nitric oxide synthase. Cancer Res 2007, 67, 1282–1290. [Google Scholar]
- Xu, Y.; Liu, B.; Zweier, J.L.; He, G. Formation of hydrogen peroxide and reduction of peroxynitrite via dismutation of superoxide at reperfusion enhances myocardial blood flow and oxygen consumption in postischemic mouse heart. J. Pharmacol. Exp. Ther 2008, 327, 402–410. [Google Scholar]
- Ghafourifar, P.; Cadenas, E. Mitochondrial nitric oxide synthase. Trends Pharmacol. Sci 2005, 26, 190–195. [Google Scholar]
- Brookes, P.S. Mitochondrial nitric oxide synthase. Mitochondrion 2004, 3, 187–204. [Google Scholar]
- Dennis, J.; Bennett, J.P., Jr. Interactions among nitric oxide and Bcl-family proteins after MPP+ exposure of SH-SY5Y neural cells I: MPP+ increases mitochondrial NO and Bax protein. J. Neurosci. Res 2003, 72, 76–88. [Google Scholar]
- Taylor, C.T.; Moncada, S. Nitric oxide, cytochrome C oxidase, and the cellular response to hypoxia. Arterioscler. Thromb. Vasc. Biol 2010, 30, 643–647. [Google Scholar]
- Elfering, S.L.; Sarkela, T.M.; Giulivi, C. Biochemistry of mitochondrial nitric-oxide synthase. J. Biol. Chem 2002, 277, 38079–38086. [Google Scholar]
- Dedkova, E.N.; Ji, X.; Lipsius, S.L.; Blatter, L.A. Mitochondrial calcium uptake stimulates nitric oxide production in mitochondria of bovine vascular endothelial cells. Am. J. Physiol. Cell Physiol 2004, 286, C406–415. [Google Scholar]
- Chan, W.H. Effect of resveratrol on high glucose-induced stress in human leukemia K562 cells. J. Cell Biochem 2005, 94, 1267–1279. [Google Scholar]
- Hsuuw, Y.D.; Chang, C.K.; Chan, W.H.; Yu, J.S. Curcumin prevents methylglyoxal-induced oxidative stress and apoptosis in mouse embryonic stem cells and blastocysts. J. Cell Physiol 2005, 205, 379–386. [Google Scholar]
- Lin, M.L.; Lu, Y.C.; Su, H.L.; Lin, H.T.; Lee, C.C.; Kang, S.E.; Lai, T.C.; Chung, J.G.; Chen, S.S. Destabilization of CARP mRNAs by aloe-emodin contributes to caspase-8-mediated p53-independent apoptosis of human carcinoma cells. J. Cell Biochem 2011, 112, 1176–1191. [Google Scholar]
- Li, C.Q.; Robles, A.I.; Hanigan, C.L.; Hofseth, L.J.; Trudel, L.J.; Harris, C.C.; Wogan, G.N. Apoptotic signaling pathways induced by nitric oxide in human lymphoblastoid cells expressing wild-type or mutant p53. Cancer Res 2004, 64, 3022–3029. [Google Scholar]
- Okada, H.; Mak, T.W. Pathways of apoptotic and non-apoptotic death in tumour cells. Nat. Rev. Cancer 2004, 4, 592–603. [Google Scholar]
- Wei, W.T.; Chen, H.; Ni, Z.L.; Liu, H.B.; Tong, H.F.; Fan, L.; Liu, A.; Qiu, M.X.; Liu, D.L.; Guo, H.C.; et al. Antitumor and apoptosis-promoting properties of emodin, an anthraquinone derivative from Rheum officinale Baill, against pancreatic cancer in mice via inhibition of Akt activation. Int. J. Oncol 2011, 39, 1381–1390. [Google Scholar]
- Buttke, T.M.; Sandstrom, P.A. Oxidative stress as a mediator of apoptosis. Immunol. Today 1994, 15, 7–10. [Google Scholar]
- Slater, A.F.; Nobel, C.S.; Maellaro, E.; Bustamante, J.; Kimland, M.; Orrenius, S. Nitrone spin traps and a nitroxide antioxidant inhibit a common pathway of thymocyte apoptosis. Biochem. J 1995, 306, 771–778. [Google Scholar]
- Chan, W.H.; Wu, C.C.; Yu, J.S. Curcumin inhibits UV irradiation-induced oxidative stress and apoptotic biochemical changes in human epidermoid carcinoma A431 cells. J. Cell Biochem 2003, 90, 327–338. [Google Scholar]
- Almeida, R.D.; Manadas, B.J.; Carvalho, A.P.; Duarte, C.B. Intracellular signaling mechanisms in photodynamic therapy. Biochim. Biophys. Acta 2004, 1704, 59–86. [Google Scholar]
- Inanami, O.; Yoshito, A.; Takahashi, K.; Hiraoka, W.; Kuwabara, M. Effects of BAPTA-AM and forskolin on apoptosis and cytochrome c release in photosensitized Chinese hamster V79 cells. Photochem. Photobiol 1999, 70, 650–655. [Google Scholar]
- Chan, W.H. Citrinin induces apoptosis in mouse embryonic stem cells. IUBMB Life 2008, 60, 171–179. [Google Scholar]
- Hsieh, M.S.; Chan, W.H. Impact of methylglyoxal and high glucose co-treatment on human mononuclear cells. Int. J. Mol. Sci 2009, 10, 1445–1464. [Google Scholar]
- Lin, S.Z.; Wei, W.T.; Chen, H.; Chen, K.J.; Tong, H.F.; Wang, Z.H.; Ni, Z.L.; Liu, H.B.; Guo, H.C.; Liu, D.L. Antitumor activity of emodin against pancreatic cancer depends on its dual role: Promotion of apoptosis and suppression of angiogenesis. PLoS One 2012, 7, e42146. [Google Scholar]
- Chen, T.G.; Chen, J.Z.; Wang, X.X. Effects of rapamycin on number activity and eNOS of endothelial progenitor cells from peripheral blood. Cell Prolif 2006, 39, 117–125. [Google Scholar]
- Li, C.Q.; Wogan, G.N. Nitric oxide as a modulator of apoptosis. Cancer Lett 2005, 226, 1–15. [Google Scholar]
- Gomes, E.R.; Almeida, R.D.; Carvalho, A.P.; Duarte, C.B. Nitric oxide modulates tumor cell death induced by photodynamic therapy through a cGMP-dependent mechanism. Photochem. Photobiol 2002, 76, 423–430. [Google Scholar]
- Wu, H.J.; Chan, W.H. Genistein protects methylglyoxal-induced oxidative DNA damage and cell injury in human mononuclear cells. Toxicol. In Vitro 2007, 21, 335–342. [Google Scholar]
- Chan, W.H.; Chang, Y.J. Dosage effects of resveratrol on ethanol-induced cell death in the human K562 cell line. Toxicol Lett 2006, 161, 1–9. [Google Scholar]
- Behl, C.; Davis, J.B.; Lesley, R.; Schubert, D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell 1994, 77, 817–827. [Google Scholar]
- Aoshima, H.; Satoh, T.; Sakai, N.; Yamada, M.; Enokido, Y.; Ikeuchi, T.; Hatanaka, H. Generation of free radicals during lipid hydroperoxide-triggered apoptosis in PC12h cells. Biochim. Biophys. Acta 1997, 1345, 35–42. [Google Scholar]
- Nakatsubo, N.; Kojima, H.; Kikuchi, K.; Nagoshi, H.; Hirata, Y.; Maeda, D.; Imai, Y.; Irimura, T.; Nagano, T. Direct evidence of nitric oxide production from bovine aortic endothelial cells using new fluorescence indicators: Diaminofluoresceins. FEBS Lett 1998, 427, 263–266. [Google Scholar]
- Hsieh, Y.J.; Wu, C.C.; Chang, C.J.; Yu, J.S. Subcellular localization of Photofrin determines the death phenotype of human epidermoid carcinoma A431 cells triggered by photodynamic therapy: When plasma membranes are the main targets. J. Cell Physiol 2003, 194, 363–375. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Huang, F.-J.; Hsuuw, Y.-D.; Chan, W.-H. Characterization of Apoptosis Induced by Emodin and Related Regulatory Mechanisms in Human Neuroblastoma Cells. Int. J. Mol. Sci. 2013, 14, 20139-20156. https://doi.org/10.3390/ijms141020139
Huang F-J, Hsuuw Y-D, Chan W-H. Characterization of Apoptosis Induced by Emodin and Related Regulatory Mechanisms in Human Neuroblastoma Cells. International Journal of Molecular Sciences. 2013; 14(10):20139-20156. https://doi.org/10.3390/ijms141020139
Chicago/Turabian StyleHuang, Fu-Jen, Yan-Der Hsuuw, and Wen-Hsiung Chan. 2013. "Characterization of Apoptosis Induced by Emodin and Related Regulatory Mechanisms in Human Neuroblastoma Cells" International Journal of Molecular Sciences 14, no. 10: 20139-20156. https://doi.org/10.3390/ijms141020139



