Ameliorating Effects of Exogenously Applied Proline on Seed Composition, Seed Oil Quality and Oil Antioxidant Activity of Maize (Zea mays L.) under Drought Stress
Abstract
:1. Introduction
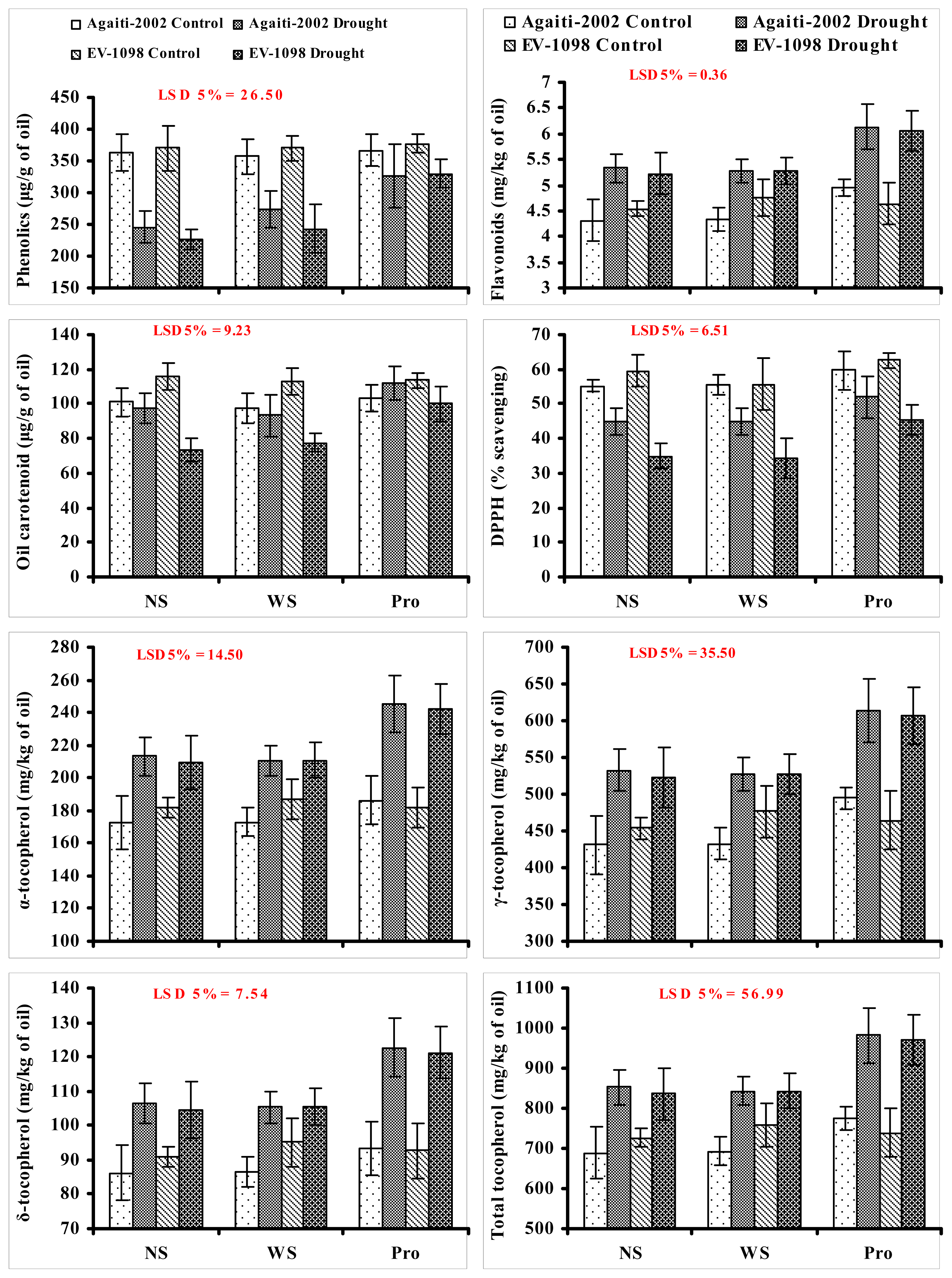
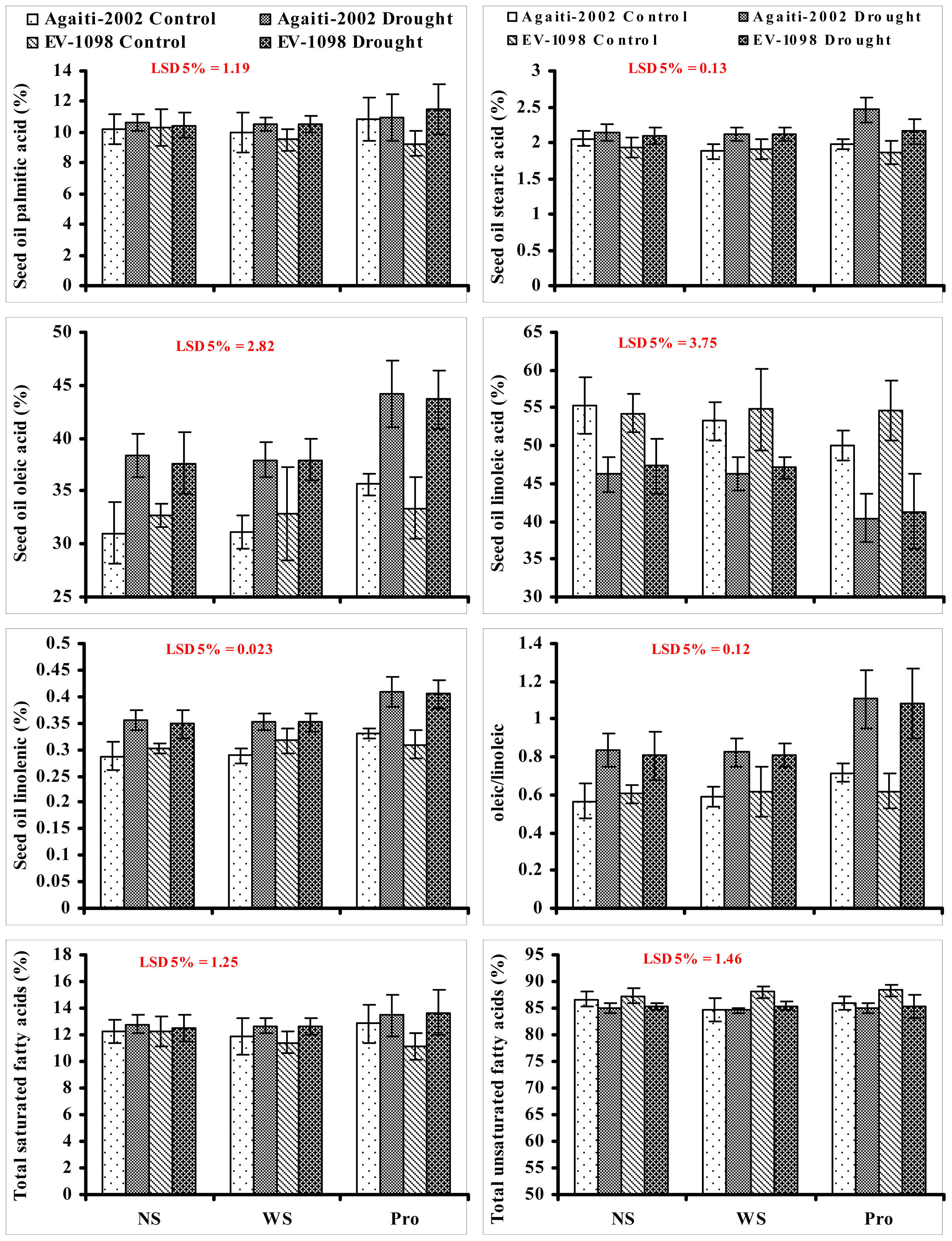
2. Results and Discussion
3. Experimental Section
3.1. Characterization of Maize kernels and Kernel Oil
Proximate Analysis
3.2. Oil Extraction
3.3. Chemical Parameters of Oil
3.4. Fatty Acid Composition of Seed Oil
3.5. Tocopherol Content
3.6. Total Phenolic Contents
3.7. Total Flavonoids
3.8. Total Carotenoid Content
3.9. DPPH Radical Scavenging Activity
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
- Conflict of InterestThe authors declare no conflict of interest.
References
- Anwar, F.; Zafar, S.N.; Rashid, U. Characterization of Moringa oleifera seed oil from drought and irrigated regions of Punjab, Pakistan. Grasas Y Aceites 2006, 57, 160–168. [Google Scholar]
- Ali, Q.; Ashraf, M.; Anwar, F. Physicochemical attributes of seed oil from drought stressed sunflower (Helianthus annuus L.) plants. Grasas Y Aceites 2009, 60, 475–481. [Google Scholar]
- Ali, Q.; Ashraf, M.; Anwar, F. Seed composition and seed oil antioxidant activity of maize under water stress. J. Am. Oil Chem. Soc 2010, 87, 1179–1187. [Google Scholar]
- Lásztity, R. Cereal Chemistry; Akadémiai Kiadó: Budapest, Hungary, 1999; pp. 11–51. [Google Scholar]
- Baye, T.M.; Pearson, T.C.; Settles, A.M. Development of a calibration to predict maize seed composition using single kernel near infrared spectroscopy. J. Cereal Sci 2006, 43, 236–243. [Google Scholar]
- Ali, Q.; Ashraf, M. Exogenously applied glycinebetaine enhances seed and seed oil quality of maize (Zea mays L.) under water deficit conditions. Environ. Exp. Bot 2011, 71, 249–259. [Google Scholar]
- Schussler, R.; Westgate, M.E. Maize kernel set at low water potential sensitivity to reduced assimilates during early kernel growth. Crop Sci 1991, 31, 1189–1195. [Google Scholar]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem 2006, 99, 191–203. [Google Scholar]
- Yancey, P.H. Compatible and Counteracting Solutes. In Cellular and Molecular Physiology of Cell Volume Regulation; Strange, K., Ed.; CRC Press: Boca Raton, FL, USA, 1994; pp. 81–109. [Google Scholar]
- Ashraf, M.; Foolad, M.R. Roles of glycinebetaine and proline in improving plant abiotic stress tolerance. Environ. Exp. Bot 2007, 59, 206–216. [Google Scholar]
- Nawaz, K.; Ashraf, M. Exogenous application of glycinebetaine modulates activities of antioxidants in maize plants subjected to salt stress. J. Agron. Crop Sci 2010, 196, 28–37. [Google Scholar]
- Ashraf, M.Y.; Azmi, A.R.; Khan, A.H.; Ala, S.A. Effect of water stress on total phenol, peroxidase activity and chlorophyll contents in wheat (Triticum aestivum L.). Acta Physiol. Plant 1994, 16, 185–191. [Google Scholar]
- Öztürk, L.; Demir, Y. In vivo and in vitro protective role of proline. Plant Growth Regul 2002, 38, 259–264. [Google Scholar]
- Kavi-Kishor, P.B.; Sangam, S.; Amrutha, R.N.; Sri, L.P.; Naidu, K.R.; Rao, K.R.S.S.; Rao, S.; Reddy, K.J.; Theriappan, P.; Sreeniv, N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci 2005, 88, 424–438. [Google Scholar]
- Mattioli, R. The proline biosynthetic genes P5CS1 and P5CS2 play overlapping roles in Arabidopsis flower transition but not in embryo development. Physiol. Plant 2009, 137, 72–85. [Google Scholar]
- Mohammadkhani, N.; Heidari, R. Effects of drought stress on soluble proteins in two maize varieties. Turk. J. Biol 2008, 32, 23–30. [Google Scholar]
- Ketchum, R.E.B.; Warren, R.C.; Klima, L.J.; Lopez-Gutierrez, F.; Nabors, M.W. The mechanism and regulation of proline accumulation in suspension cultures of the halophytic grass Distichlis spicata L. J. Plant Physiol 1991, 137, 368–374. [Google Scholar]
- Flowers, T.J. Improving crop salt tolerance. J. Exp. Bot 2004, 55, 1–13. [Google Scholar]
- Ali, Q.; Ashraf, M.; Athar, H.R. Exogenously applied proline at different growth stages enhances growth of two maize cultivars grown under water deficit conditions. Pak. J. Bot 2007, 39, 1133–1144. [Google Scholar]
- Kaul, S.; Sharma, S.S.; Mehta, I.K. Free radical scavenging potential of l-proline: evidence from in vitro assays. Amino Acids 2008, 34, 315–320. [Google Scholar]
- Banu, N.A.; Hoque, A.; Watanab-e-Sugimoto, M.; Matsuoka, K.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Proline and glycinebetaine induce antioxidant defense gene expression and suppress cell death in cultured tobacco cells under salt stress. J. Plant Physiol 2009, 166, 146–156. [Google Scholar]
- Csonka, L.N.; Hanson, A.D. Prokaryotic osmoregulation: Genetics and physiology. Annu. Rev. Microbiol 1991, 45, 569–606. [Google Scholar]
- Athar, H.R.; Ashraf, M.; Wahid, A.; Jamil, A. Inducing salt tolerance in canola (Brassica napus L.) by exogenous application of glycinebetaine and proline: response at the initial growth stages. Pak. J. Bot 2009, 41, 1311–1319. [Google Scholar]
- Islam, M.M. Exogenous proline and glycinebetaine increase antioxidant enzyme activities and confer tolerance to cadmium stress in cultured tobacco cells. J. Plant Physiol 2009, 166, 1587–1597. [Google Scholar]
- Yildiz-Aktas, L.; Dagnon, S.; Gurel, A.; Gesheva, E.; Edreva, A. Drought tolerance in cotton: involvement of nonenzymatic ROS-scavenging compounds. J. Agron. Crop Sci 2009, 195, 247–253. [Google Scholar]
- Mansour, M.M.F. Protection of plasma membrane of onion epidermal cells by glycinebetaine and proline against NaCl stress. Plant Physiol. Biochem 1998, 36, 767–772. [Google Scholar]
- Takagi, H. Proline as a stress protectant in yeast: Physiological functions, metabolic regulations, and biotechnological applications. Appl. Microbiol. Biotechnol 2008, 81, 211–223. [Google Scholar]
- Brdar, M.D.; Kraljevíc-Balalíc, M.M.; Kobiljski, B.D. The parameters of grain filling and yield components in common wheat (Triticum aestivum L.) and durum wheat (Triticum turgidum L. var. durum). Cent. Eur. J. Biol 2008, 3, 75–82. [Google Scholar]
- Carvalho, I.S.; Ricardo, C.P.; Chaves, M. Quality and distribution of assimilates within the whole plant of lupins (L. albus and L. mutabilis) influenced by water stress. J. Agron. Crop Sci 2004, 190, 205–210. [Google Scholar]
- Ali, R.M.; Abbas, H.M.; Kamal, R.K. The effects of treatment with polyamines on dry matter, oil and flavonoid contents in salinity stressed chamomile and sweet marjoram. Plant Soil Environ 2007, 53, 529–543. [Google Scholar]
- Mäkelä, P.; Munns, R.; Colmer, T.D.; Condon, A.G.; Peltonen-Sainio, P. Effects of foliar applications of glycinebetaine on stomatal conductance, abscisic acid and solute concentrations in leaves of salt- or drought-stressed tomato. Aust. J. Plant Physiol 1998, 25, 655–663. [Google Scholar]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 2002, 7, 405–410. [Google Scholar]
- Smirnoff, N. Ascorbate, Tcopherol and Carotenoids: Metabolism, Pathway Engineering and Function. In Antioxidants and Reactive Oxygen Species; Smirnoff, N., Ed.; Blackwell publishing Ltd: Oxford, UK, 2005; pp. 53–86. [Google Scholar]
- Ashraf, M. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol. Adv 2009, 27, 84–93. [Google Scholar]
- Taize, L.; Zeiger, E. Plant Physiologym, 4th ed; Sinauer Associates, Inc: Sunderland, MA, USA, 2006. [Google Scholar]
- Bailly, C.; Audigier, C.; Ladonne, F.; Wagner, M.H.; Coste, F.; Corbineau, F.; Côme, D. Changes in oligosachride content and antioxidant enzyme activities in developing been seeds as related to acquisition of drying tolerance and seed quality. J. Exp. Bot 2001, 52, 701–708. [Google Scholar]
- Goffman, F.D.; Böhme, T. Relationship between fatty acid profile and vitamin E content in maize hybrids (Zea mays L.). J. Agric. Food Chem 2001, 49, 4990–4994. [Google Scholar]
- Britz, S.J.; Kremer, D.F. Warm temperature and drought during seed maturation increase free α-tocopherol in the seeds of soybean. J. Agric. Food Chem. 2002, 50, 6058–6063. [Google Scholar]
- Havaux, M.; Bonfils, J.P.; Lütz, C.; Niyogi, K.K. Photodamage of the photosynthetic apparatus and its dependence on the leaf developmental stage in the npq1 Arabidopsis mutant deficient in the xanthophyll cycle enzyme violaxanthin de-epoxidase. Plant Physiol 2000, 124, 273–284. [Google Scholar]
- Munné-Bosch, S.; Alegre, L. Changes in carotenoids, tocopherols and diterpenes during drought and recovery, and the biological significance of chlorophyll loss in Rosmarinus officinalis plants. Planta 2000, 210, 925–931. [Google Scholar]
- Collakova, E.; DellaPenna, D. The role of homogentisate phytyltransferase and other tocopherol pathway enzymes in the regulation of tocopherol synthesis during abiotic stress. Plant Physiol 2003, 133, 930–940. [Google Scholar]
- Kamal-Eldin, A.; Appelqvist, L.A. The chemistry and antioxidants properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar]
- Kriese, U.; Schumann, E.; Weber, W.E.; Beyer, M.; Bruhl, D.; Matthäus, B. Oil content, tocopherol composition and fatty acid patterns of the seeds of 51 Cannabis sativa L. genotypes. Euphytica 2004, 137, 339–351. [Google Scholar]
- Ali, Q.; Ashraf, M. Induction of drought tolerance in maize (Zea mays L.) due to exogenous application of trehalose: growth, photosynthesis, water relations, and oxidative defense mechanism. J. Agron. Crop Sci 2011, 197, 258–271. [Google Scholar]
- Ali, R.M.; Abbas, H.M. Response of salt stressed barley seedlings to phenylurea. Plant Soil Environ 2003, 4, 158–162. [Google Scholar]
- Kumar, V.; Rani, A.; Dixit, A.K.; Bhatnagar, D.; Chauhan, G.S. Relative changes in tocopherols, isoflavones, total phenolic content, and antioxidative activity in soybean seeds at different reproductive stages. J. Agric. Food Chem 2009, 57, 2705–2710. [Google Scholar]
- Kleindt, C.K.; Stracke, R.; Mehrtens, F.; Weisshaar, B. Expression analysis of flavonoid biosynthesis genes during Arabidopsis thaliana silique and seed development with a primary focus on the proanthocyanidin biosynthetic pathway. BMC Res 2010, 3, 1–12. [Google Scholar]
- Lansac, A.R.; Sullivan, C.Y.; Johnson, B.E. Accumulation of free proline in sorghum (Sorghum bicolor) pollen. Can. J. Bot 1996, 74, 40–45. [Google Scholar]
- Francisco, A.; Tomtis-Barberhn; Manuela, M.; Garcia-Grau; Tomtis-Lorente, F. Flavonoid concentration changes in maturing broad bean pods. J. Agric. Food Chem. 1991, 39, 255–258. [Google Scholar]
- Siger, A.; Nogala-kalucka, M.; Lampart-szczapa, E. The content and antioxidant activity of phenolic compounds in cold-pressed plant oils. J. Food Lipids 2008, 15, 137–149. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med 1996, 20, 933–956. [Google Scholar]
- Dykes, L.; Rooney, L.W. Phenolic compounds in cereal grains and their health benefits. Cereal Foods World 2007, 52, 105–111. [Google Scholar]
- Greven, M.; Neal, S.; Green, S.; Dichio, B.; Clothier, B. The effects of drought on the water use, fruit development and oil yield from young olive trees. Agric. Water Manag 2009, 96, 1525–1531. [Google Scholar]
- Bouchereau, A.; Clossais-Besnard, N.; Bensaoud, A.; Leport, L.; Renard, M. Water stress effects on rapeseed quality. Eur. J. Agron 1996, 5, 19–30. [Google Scholar]
- Karjalainen, R.; Lehtinen, A.; Hietaniemi, V.; Pihlava, J.M.; Jokinen, K.; Keinänen, M.; Julkunen-Tiito, R. Benzothiadiazole and glycine betaine treatments enhance phenolic compound production in strawberry. Acta Hortic 2002, 567, 353–356. [Google Scholar]
- Nemeskéri, E. Breeding strategy for improvement of colour quality and carotenoid levels in dry pea seeds. Commun. Biometry Crop Sci 2006, 1, 49–55. [Google Scholar]
- Flagella, Z.; Rotunno, T.; Tarantino, E.; Caterina, R.; Caro, A. Changes in seed yield and oil fatty acid composition of high oleic sunflower (Helianthus annuus L.) hybrids in relation to the sowing date and the water regime. Eur. J. Agron 2002, 17, 221–230. [Google Scholar]
- Dewis, J.; Freitas, F. Physical and Chemical Methods of Soil and Water Analysis; FAO Soil Bulletin No. 10; Food and Agriculture Organization of the United Nations, (FAO): Rome, Italy, 1970; pp. 39–51. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice-Hall, Inc: Englewood Cliffs, NJ, USA, 1962. [Google Scholar]
- Approved Methods of the American Association of Cereal Chemists, 10th ed; AACC: St. Paul, MN, USA, 2000.
- American Oil Chemist’s Society (AOCS), Official and Recommended Practices of the American Oil Chemists’ Society, 5th ed; AOCS Press: Champaign, IL, USA, 1997.
- International Union of Pure and Applied Chemistry (IUPAC), Standard Methods for the Analysis of Oils, Fats and Derivatives, 7th ed; Paquot, C.; Hautfenne, A. (Eds.) Blackwell: London, UK, 1987.
- Lee, B.I.; New, A.L.; Ong, C.N. Simultaneous determination of tocotrienols, tocopherols, retinols and major carotenoids in human plasma. Clin. Chem 2003, 49, 2056–2066. [Google Scholar]
- Chaovanalikit, A.; Wrolstad, R.E. Total anthocyanins and total phenolics of fresh and processed cherries and their antioxidant properties. Food Chem. Toxicol 2004, 69, 67–72. [Google Scholar]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem 2002, 50, 3010–3014. [Google Scholar]
- Gao, X.; Ohlander, M.; Jeppsson, N.; Björk, L.; Trajkovski, V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophaë rhamnoides L.) during maturation. J. Agric. Food Chem 2000, 48, 1485–1490. [Google Scholar]
- Sultana, B.; Anwar, F.; Przybylski, R. Antioxidant potential of corn cob extracts for stabilization of corn oil subjected to microwave heating. Food Chem. 2007, 104, 997–1005. [Google Scholar]
- Iqbal, S.; Bhanger, M.I. Stabilization of sunflower oil by garlic extract during accelerated storage. Food Chem 2007, 100, 246–254. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics; McGraw Hill Book Co., Inc: New York, NY, USA, 1986. [Google Scholar]
| Cultivar | Drought treatment | Treatments | Seed oil content | Protein content | Starch content | Sugar content | Ash content | Fiber content | Moisture content |
|---|---|---|---|---|---|---|---|---|---|
| % of dry weight | |||||||||
| EV-1098 | Control | NS | 3.87 ± 0.13 b | 7.92 ± 0.49 a,b | 56.67 ± 8.30 c | 1.60 ± 0.16 c | 1.87 ± 0.24 c | 9.08 ± 0.30 e | 9.07 ± 0.67 b |
| WS | 3.87 ± 0.18 b | 7.87 ± 0.35 a,b,c | 56.67 ± 5.88 c | 1.80 ± 0.02 b | 1.85 ± 0.12 c | 9.52 ± 0.71 d,e | 8.95 ± 1.02 b | ||
| Pro | 4.12 ± 0.15 a | 8.01 ± 0.73 a | 61.33 ± 7.88 a,b,c | 2.21 ± 0.16 a | 1.97 ± 0.22 c | 10.10 ± 0.80 b,c | 10.18 ± 0.49 a | ||
| Drought | NS | 2.95 ± 0.15 d | 6.26 ± 0.67 e | 60.33 ± 2.43 b,c | 1.13 ± 0.02 d | 2.23 ± 0.18 b | 10.45 ± 0.82 b,c | 5.71 ± 0.18 d | |
| WS | 2.87 ± 0.13 d | 6.85 ± 0.75 d,e | 59.67 ± 4.44 b,c | 1.14 ± 0.01 d | 2.29 ± 0.31 b | 10.55 ± 0.53 b,c | 5.70 ± 0.68 d | ||
| Pro | 3.63 ± 0.15 c | 7.23 ± 0.68 b,c,d | 65.67 ± 6.30 a,b | 1.18 ± 0.02 d | 2.55 ± 0.24 a | 12.12 ± 0.77 a | 7.18 ± 0.43 c | ||
| Agaiti- 2002 | Control | NS | 3.72 ± 0.15 b,c | 7.59 ± 0.62 a,b,c | 60.33 ± 6.12 b,c | 1.63 ± 0.08 c | 1.77 ± 0.16 c | 8.62 ± 0.81 f | 8.57 ± 0.29 b |
| WS | 3.65 ± 0.13 c | 7.51 ± 0.90 a,b,c | 61.33 ± 9.58 a,b | 1.80 ± 0.02 b | 1.80 ± 0.10 c | 8.65 ± 0.45 f | 9.10 ± 0.92 b | ||
| Pro | 4.16 ± 0.14 a | 8.01 ± 0.66 a | 68.00 ± 7.22 a | 2.18 ± 0.16 a | 1.95 ± 0.15 c | 9.89 ± 0.30 c,d | 10.24 ± 0.51 a | ||
| Drought | NS | 2.36 ± 0.08 e | 6.59 ± 0.48 d,e | 63.67 ± 3.41 a,b | 1.14 ± 0.01 d | 2.27 ± 0.23 b | 10.65 ± 0.57 b | 5.35 ± 0.31 d | |
| WS | 2.42 ± 0.09 e | 6.76 ± 0.81 d,e | 61.67 ± 9.59 a,b | 1.14 ± 0.01 d | 2.23 ± 0.18 b | 10.54 ± 0.46 b,c | 5.46 ± 0.13 d | ||
| Pro | 2.82 ± 0.12 d | 7.18 ± 0.54 c,d | 68.33 ± 3.81 a | 1.16 ± 0.01 d | 2.45 ± 0.28 a,b | 12.27 ± 0.86 a | 6.95 ± 0.31 c | ||
| LSD at 5% | 0.15 | 0.73 | 7.40 | 0.10 | 0.23 | 0.72 | 0.63 | ||
| Cultivar | Drought treatment | Treatments | Saponification value (mg of KOH g−1 of oil) | Un-saponifiable matter (%) | Iodine value (g of I 100g−1 of oil) | FFA (mg of KOH/g of oil) | Peroxide value (meq kg−1) | p-anisidine value | Dienes [ɛ1 cm(λ232 nm)] | Trienes [ɛ1 cm(λ268 nm)] |
|---|---|---|---|---|---|---|---|---|---|---|
| EV-1098 | Control | NS | 196.12 ± 10.78 a | 2.11 ± 0.23 d,e | 115.16 ± 5.53 a,b | 1.41 ± 0.08 c | 6.30 ± 0.56 f | 1.87 ± 0.057 f | 1.83 ± 0.005 b | 1.54 ± 0.009 e |
| WS | 193.93 ± 07.42 a,c | 2.25 ± 0.09 b,c,d | 114.37 ± 4.96 b | 1.42 ± 0.08 c | 6.41 ± 0.47 d | 1.89 ± 0.066 f | 1.79 ± 0.005 d | 1.64 ± 0.005 d | ||
| Pro | 201.67 ± 10.31 a | 1.97 ± 0.04 e | 121.56 ± 8.05 a | 1.29 ± 0.05 d | 5.58 ± 0.47 h | 2.12 ± 0.106 d,e | 1.63 ± 0.006 h | 1.63 ± 0.007 d | ||
| Drought | NS | 178.55 ± 09.14 b | 2.35 ± 0.19 a,b | 115.34 ± 3.90 a,b | 1.70 ± 0.11 a,b | 7.60 ± 0.37 a | 2.14 ± 0.072 c,d,e | 1.71 ± 0.008 e | 1.81 ± 0.006 b | |
| WS | 179.74 ± 10.28 b | 2.28 ± 0.16 b,c | 112.95 ± 7.37 c | 1.76 ± 0.08 a | 7.56 ± 0.44 a | 2.14 ± 0.057 c,d | 1.69 ± 0.007 f | 1.68 ± 0.006 c | ||
| Pro | 188.08 ± 12.93 b,c | 2.14 ± 0.09 c | 121.86 ± 7.19 a | 1.48 ± 0.10 c | 6.34 ± 0.42 e,f | 2.24 ± 0.097 b,c | 1.60 ± 0.008 i | 1.55 ± 0.008 e | ||
| Agaiti- 2002 | Control | NS | 191.07 ± 11.27 a | 2.11 ± 0.18 d,e | 113.49 ± 3.40 c | 1.35 ± 0.07 d | 6.15 ± 0.37 g | 2.09 ± 0.091 d,e | 1.88 ± 0.011 a | 1.68 ± 0.005 c |
| WS | 189.26 ± 11.50 b,c | 2.16 ± 0.15 c | 113.75 ± 4.28 c | 1.40 ± 0.06 c | 6.37 ± 0.43 d,e | 2.04 ± 0.189 e | 1.81 ± 0.009 c | 1.64 ± 0.005 d | ||
| Pro | 198.67 ± 07.27 a | 1.95 ± 0.11 e | 115.68 ± 5.28 a,b | 1.28 ± 0.04 d | 5.50 ± 0.56 i | 2.10 ± 0.068 d,e | 1.66 ± 0.011 g | 1.55 ± 0.004 e | ||
| Drought | NS | 175.38 ± 11.03 b | 2.47 ± 0.14 a | 116.60 ± 5.41 a,b | 1.64 ± 0.08 b | 6.59 ± 0.39 c | 2.33 ± 0.091 a,b | 1.71 ± 0.009 e | 1.87 ± 0.006 a | |
| WS | 179.86 ± 03.80 b | 2.30 ± 0.11 b | 113.83 ± 6.29 c | 1.64 ± 0.08 b | 6.79 ± 0.29 b | 2.32 ± 0.040 a,b | 1.64 ± 0.009 h | 1.82 ± 0.010 b | ||
| Pro | 186.14 ± 10.24 b,c | 2.15 ± 0.15 c | 116.64 ± 7.55 a | 1.46 ± 0.08 c | 6.33 ± 0.57 e,f | 2.42 ± 0.070 a | 1.55 ± 0.008 j | 1.55 ± 0.005 e | ||
| LSD at 5% | 11.07 | 0.16 | 6.62 | 0.09 | 0.05 | 0.01 | 0.10 | 0.01 | ||
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ali, Q.; Anwar, F.; Ashraf, M.; Saari, N.; Perveen, R. Ameliorating Effects of Exogenously Applied Proline on Seed Composition, Seed Oil Quality and Oil Antioxidant Activity of Maize (Zea mays L.) under Drought Stress. Int. J. Mol. Sci. 2013, 14, 818-835. https://doi.org/10.3390/ijms14010818
Ali Q, Anwar F, Ashraf M, Saari N, Perveen R. Ameliorating Effects of Exogenously Applied Proline on Seed Composition, Seed Oil Quality and Oil Antioxidant Activity of Maize (Zea mays L.) under Drought Stress. International Journal of Molecular Sciences. 2013; 14(1):818-835. https://doi.org/10.3390/ijms14010818
Chicago/Turabian StyleAli, Qasim, Farooq Anwar, Muhammad Ashraf, Nazamid Saari, and Rashida Perveen. 2013. "Ameliorating Effects of Exogenously Applied Proline on Seed Composition, Seed Oil Quality and Oil Antioxidant Activity of Maize (Zea mays L.) under Drought Stress" International Journal of Molecular Sciences 14, no. 1: 818-835. https://doi.org/10.3390/ijms14010818




