Ultraviolet B (UVB) Irradiation-Induced Apoptosis in Various Cell Lineages in Vitro
Abstract
:1. Introduction
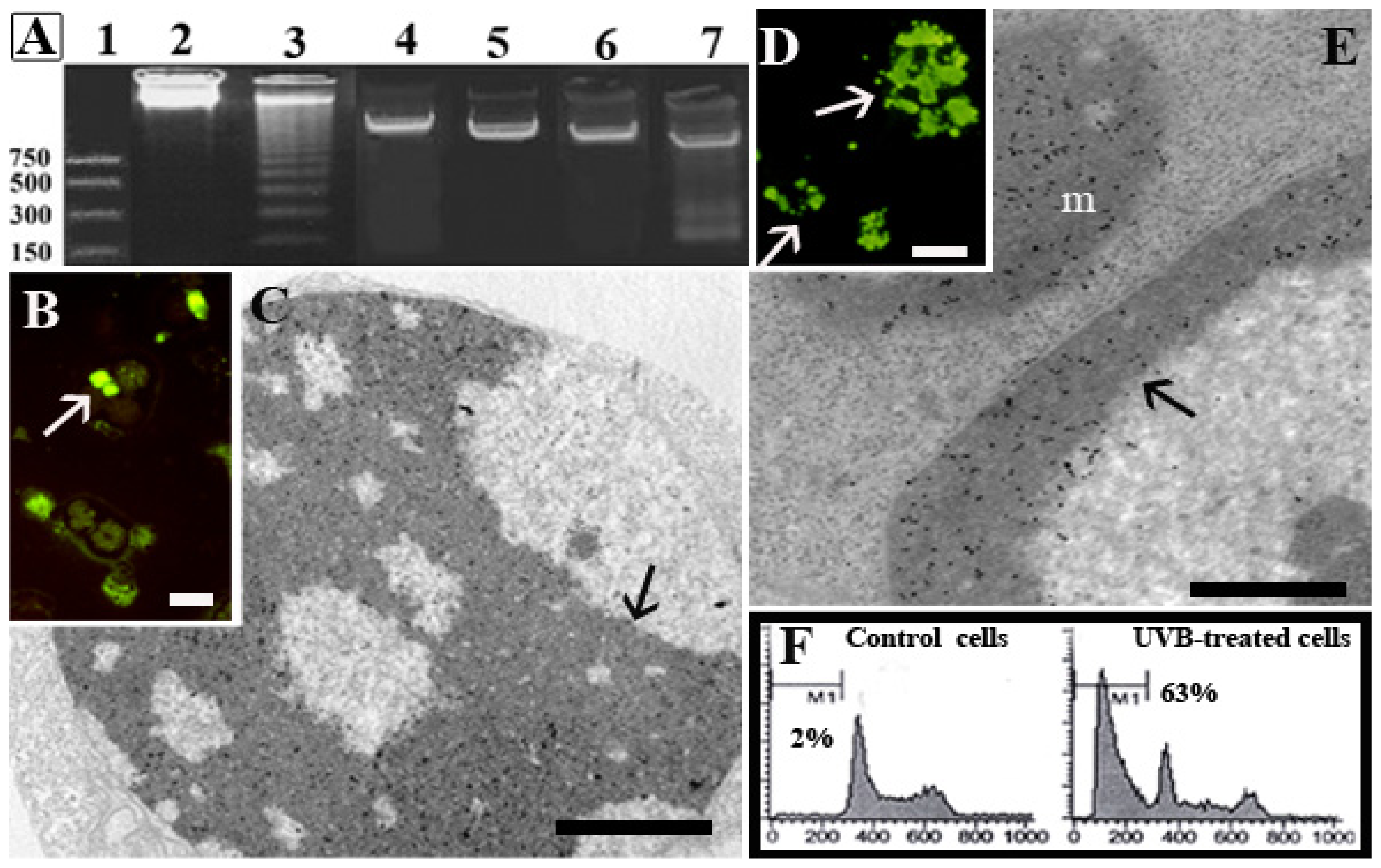
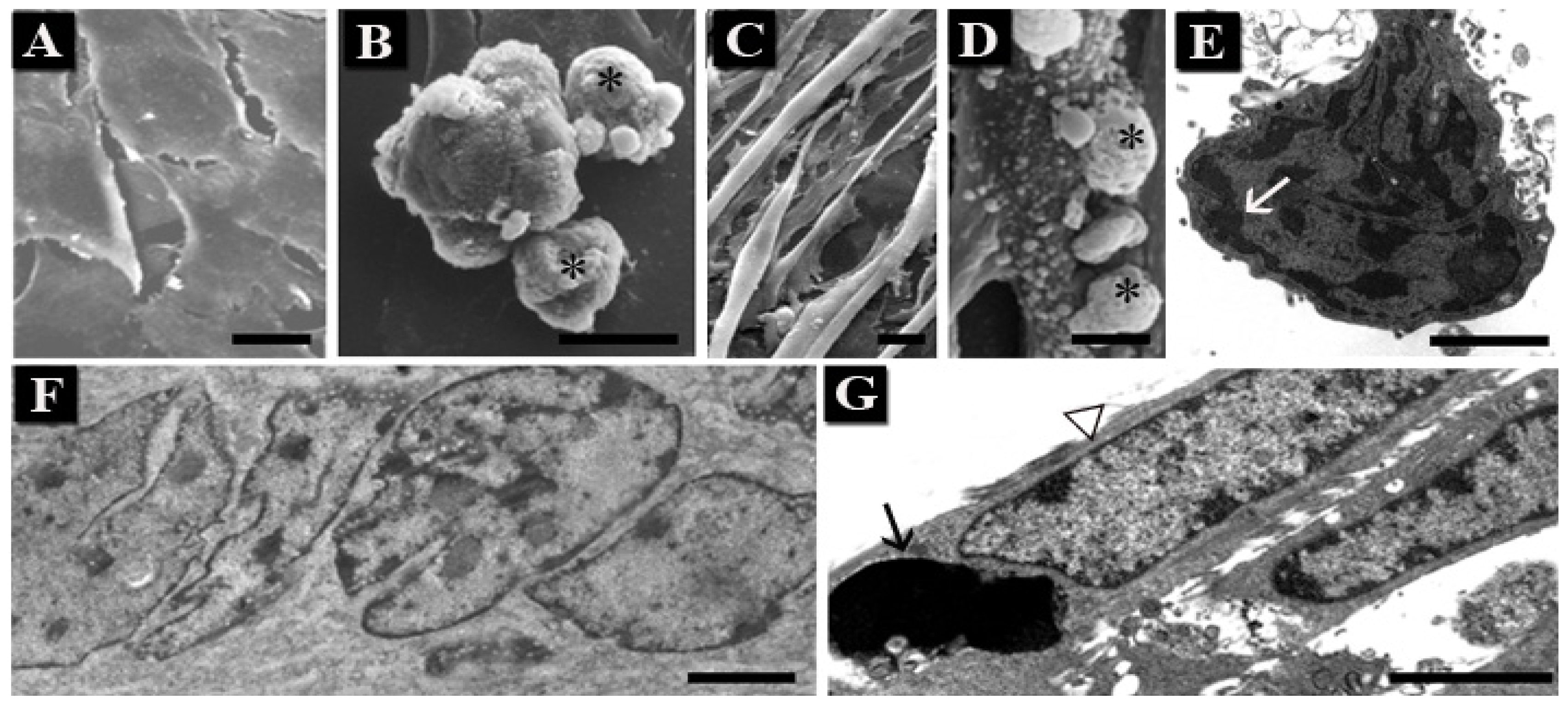
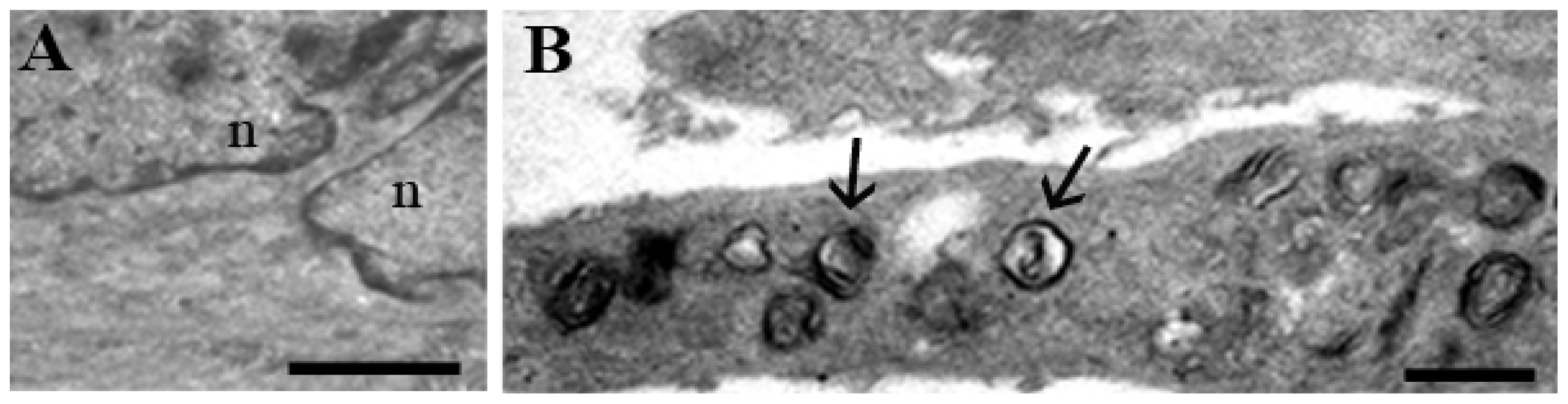
1.1. Human Hemopoietic Cells
1.2. Chondrocytes
1.3. Skeletal Muscle Cells
2. Conclusions
Acknowledgements
- Conflict of InterestThe authors declare no conflict of interest.
References
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Ultraviolet radiation and skin cancer. Int. J. Dermatol 2010, 49, 978–986. [Google Scholar]
- Jackson, S.; Harwood, C.; Thomas, M.; Banks, L.; Storey, A. Role of Bak in UV-induced apoptosis in skin cancer and abrogation by HPV E6 proteins. Genes Dev 2000, 14, 3065–3073. [Google Scholar]
- Katiyar, S.K.; Mantena1, S.K.; Meeran1, S.M. Silymarin protects epidermal keratinocytes from ultraviolet radiation-induced apoptosis and DNA damage by nucleotide excision repair mechanism. PLoS One 2011, 6, e21410. [Google Scholar]
- Kulms, D.; Pöppelmann, B.; Yarosh, D.; Luger, T.A.; Krutmann, J.; Schwarz, T. Nuclear and cell membrane effects contribute independently to the induction of apoptosis in human cells exposed to UVB radiation. Proc. Natl. Acad. Sci. USA 1999, 96, 7974–7979. [Google Scholar]
- Stege, H.; Roza, L.; Vink, A.A.; Grewe, M.; Ruzicka, T.; Grether-Beck, S.; Krutmann, J. Enzyme plus light therapy to repair DNA damage in ultraviolet-B-irradiated human skin. Proc. Natl. Acad. Sci. USA 2000, 97, 1790–1795. [Google Scholar]
- Aragane, Y.; Kulms, D.; Metze, D.; Wilkes, G.; Pöppelmann, B.; Luger, T.A.; Schwarz, T. Ultraviolet light induces apoptosis via direct activation of CD95 (Fas/APO-1) independently of its ligand CD95L. J. Cell Biol 1998, 140, 171–182. [Google Scholar]
- Kulms, D.; Zeise, E.; Pöppelmann, B.; Schwarz, T. DNA damage, death receptor activation and reactive oxygen species contribute to ultraviolet radiation-induced apoptosis in an essential and independent way. Oncogene 2002, 21, 5844–5851. [Google Scholar]
- Wölfle, U.; Esser, P.R.; Simon-Haarhaus, B.; Martin, S.F.; Lademann, J.; Schempp, C.M. UVB-induced DNA damage, generation of reactive oxygen species and inflammation are effectively attenuated by the flavonoid luteolin in vitro and in vivo. Free Radic. Biol. Med 2011, 50, 1081–1093. [Google Scholar]
- Yasui, H.; Hakozaki, T.; Date, A.; Yoshii, T.; Sakurai, H. Real-time chemiluminescent imaging and detection of reactive oxygen species generated in the UVB-exposed human skin equivalent model. Biochem. Biophys. Res. Commun 2006, 347, 83–88. [Google Scholar]
- Perluigi, M.; Di Domenico, F.; Blarzino, C.; Foppoli, C.; Cini, C.; Giorgi, A.; Grillo, C.; De Marco, F.; Butterfield, D.A.; Schininà, M.E.; et al. Effects of UVB-induced oxidative stress on protein expression and specific protein oxidation in normal human epithelial keratinocytes: A proteomic approach. Proteome Sci 2010, 8, 13–27. [Google Scholar]
- Hanson, C.J.; Bootman, M.D.; Distelhorst, C.W.; Maraldi, T.; Roderick, H.L. The cellular concentration of Bcl-2 determines its pro- or anti-apoptotic effect. Cell Calcium 2008, 44, 243–258. [Google Scholar]
- Muthusamy, V.; Piva, T.J. The UV response of the skin: A review of the MAPK, NFkappaB and TNFalpha signal transduction pathways. Arch. Dermatol. Res 2010, 302, 5–17. [Google Scholar]
- Maverakis, E.; Miyamura, Y.; Bowen, M.P.; Correa, G.; Ono, Y.; Goodarzi, H. Light, including ultraviolet. J. Autoimmun 2010, 34, J247–J257. [Google Scholar]
- Filip, A.; Daicoviciu, D.; Clichici, S.; Mocan, T.; Muresan, A.; Postescu, I.D. Photoprotective effects of two naturals products on ultraviolet B-induced oxidative stress and apoptosis in SKH-1 mouse skin. J. Med. Food 2011, 14, 761–766. [Google Scholar]
- Halliday, G.M. Common links among the pathways leading to UV-induced immunosuppression. J. Invest. Dermatol 2010, 130, 1209–1212. [Google Scholar]
- Su, J.; Pearce, D.J.; Feldman, S.R. The role of commercial tanning beds and ultraviolet A light in the treatment of psoriasis. J. Dermatol. Treat 2005, 16, 324–326. [Google Scholar]
- Bao, Y.; Shen, X. Chromatin remodeling in DNA double-strand break repair. Curr. Opin. Genet. Dev 2007, 17, 126–131. [Google Scholar]
- Luchetti, F.; Betti, M.; Canonico, B.; Arcangeletti, M.; Ferri, P.; Galli, F.; Papa, S. ERK MAPK activation mediates the antiapoptotic signaling of melatonin in UVB-stressed U937 cells. Free Radic. Biol. Med 2009, 46, 339–351. [Google Scholar]
- Liu, S.; Mizu, H.; Yamauchi, H. Molecular response to phototoxic stress of UVB-irradiated ketoprofen through arresting cell cycle in G2/M phase and inducing apoptosis. Biochem. Biophys. Res. Commun 2007, 364, 650–655. [Google Scholar]
- Pozzi, D.; Grimaldi, P.; Gaudenzi, S.; Di Giambattista, L.; Silvestri, I.; Morrone, S.; Congiu Castellano, A. UVB-radiation-induced apoptosis in Jurkat cells: A coordinated fourier transform infrared spectroscopy-flow cytometry study. Radiat. Res 2007, 168, 698–705. [Google Scholar]
- Paz, M.L.; González Maglio, D.H.; Weill, F.S.; Bustamante, J.; Leoni, J. Mitochondrial dysfunction and cellular stress progression after ultraviolet B irradiation in human keratinocytes. Photodermatol. Photoimmunol. Photomed 2008, 24, 115–122. [Google Scholar]
- Sandri, M.; El Meslemani, A.H.; Sandri, C.; Schjerling, P.; Vissing, K.; Andersen, J.L.; Rossini, K.; Carraro, U.; Angelini, C. Caspase 3 expression correlates with skeletal muscle apoptosis in Duchenne and facioscapulo human muscular dystrophy. A potential target for pharmacological treatment? J. Neuropathol. Exp. Neurol 2001, 60, 302–312. [Google Scholar]
- Ji, C.; Yang, B.; Yang, Z.; Tu, Y.; Yang, Y.L.; He, L.; Bi, Z.G. Ultra-violet B (UVB)-induced skin cell death occurs through a cyclophilin D intrinsic signaling pathway. Biochem. Biophys. Res. Commun 2012, 425, 825–829. [Google Scholar]
- Hilder, T.L.; Carlson, G.M.; Haystead, T.A.; Krebs, E.G.; Graves, L.M. Caspase-3 dependent cleavage and activation of skeletal muscle phosphorylase b kinase. Mol. Cell. Biochem 2005, 275, 233–242. [Google Scholar]
- D’Emilio, A.; Biagiotti, L.; Burattini, S.; Battistelli, M.; Canonico, B.; Evangelisti, C.; Ferri, P.; Papa, S.; Martelli, A.M.; Falcieri, E. Morphological and biochemical patterns in skeletal muscle apoptosis. Histol. Histopathol 2010, 25, 21–32. [Google Scholar]
- Nys, K.; van Laethem, A.; Michiels, C.; Rubio, N.; Piette, J.G.; Garmyn, M.; Agostinis, P.A. p38(MAPK)/HIF-1 pathway initiated by UVB irradiation is required to induce Noxa and apoptosis of human keratinocytes. J. Invest. Dermatol 2010, 130, 2269–2276. [Google Scholar]
- Kulms, D.; Schwarz, T. Molecular mechanisms of UV-induced apoptosis. Photodermatol. Photoimmunol. Photomed 2000, 16, 195–201. [Google Scholar]
- Svobodová, A.R.; Galandáková, A.; Sianská, J.; Doležal, D.; Lichnovská, R.; Ulrichová, J.; Vostálová, J. DNA damage after acute exposure of mice skin to physiological doses of UVB and UVA light. Arch. Dermatol. Res 2012, 304, 407–412. [Google Scholar]
- Caricchio, R.; McPhie, L.; Cohen, P.L. Ultraviolet B radiation-induced cell death: Critical role of ultraviolet dose in inflammation and lupus autoantigen redistribution. J. Immunol 2003, 171, 5778–5786. [Google Scholar]
- Abu-Yousif, A.O.; Smith, K.A.; Getsios, S.; Green, K.J.; van Dross, R.T.; Pelling, J.C. Enhancement of UVB-induced apoptosis by apigenin in human keratinocytes and organotypic keratinocyte cultures. Cancer Res 2008, 68, 3057–3065. [Google Scholar]
- Huynh, T.T.; Chan, K.S.; Piva, T.J. Effect of ultraviolet radiation on the expression of pp38MAPK and furin in human keratinocyte-derived cell lines. Photodermatol. Photoimmunol. Photomed 2009, 25, 20–29. [Google Scholar]
- Bivik, C.A.; Larsson, P.K.; Kågedal, K.M.; Rosdahl, I.K.; Ollinger, K.M. UVA/B-induced apoptosis in human melanocytes involves translocation of cathepsins and Bcl-2 family members. J. Invest. Dermatol 2006, 126, 1119–1127. [Google Scholar]
- Arrebola, F.; Fernández-Segura, E.; Campos, A.; Crespo, P.V.; Skepper, J.N.; Warley, A. Changes in intracellular electrolyte concentrations during apoptosis induced by UV irradiation of human myeloblastic cells. Am. J. Physiol. Cell Physiol 2006, 290, C638–C649. [Google Scholar]
- Elkholi, R.; Floros, K.V.; Chipuk, J.E. The role of BH3-only proteins in tumor cell development, signaling and treatment. Genes Cancer 2011, 2, 523–537. [Google Scholar]
- Olivotto, E.; Vitellozzi, R.; Fernandez, P.; Falcieri, E.; Battistelli, M.; Burattini, S.; Facchini, A.; Flamigni, F.; Santi, S.; Facchini, A.; et al. Chondrocyte hypertrophy and apoptosis induced by GROalpha require three-dimensional interaction with the extracellular matrix and a co-receptor role of chondroitin sulfate and are associated with the mitochondrial splicing variant of cathepsin B. J. Cell. Physiol 2007, 210, 417–427. [Google Scholar]
- Ferreira, R.; Neuparth, M.J.; Vitorino, R.; Appell, H.J.; Amado, F.; Duarte, J.A. Evidences of apoptosis during the early phases of soleus muscle atrophy in hindlimb suspended mice. Physiol. Res 2008, 57, 601–611. [Google Scholar]
- Adhihetty, P.J.; O’Leary, M.F.N.; Hood, D.A. Mitochondria in skeletal muscle: Adaptable rheostats of apoptotic susceptibility. Exerc. Sport Sci. Rev. 2009, 36, 116–121. [Google Scholar]
- Falcieri, E.; Martelli, A.M.; Bareggi, R.; Cataldi, A.; Cocco, L. The protein kinase inhibitor staurosporine induces morphological changes typical of apoptosis in MOLT-4 cells without concomitant DNA fragmentation. Biochem. Biophys. Res. Commun 1993, 193, 19–25. [Google Scholar]
- Luchetti, F.; Burattini, S.; Ferri, P.; Papa, S.; Falcieri, E. Actin involvement in apoptotic chromatin changes of hemopoietic cells undergoing hyperthermia. Apoptosis 2002, 7, 143–152. [Google Scholar]
- Salucci, S.; Battistelli, M.; Burattini, S.; Squillace, C.; Canonico, B.; Gobbi, P.; Papa, S.; Falcieri, E. C2C12 myoblast sensitivity to different apoptotic chemical triggers. Micron 2010, 41, 966–973. [Google Scholar]
- Maraldi, T.; Prata, C.; Caliceti, C.; Dalla Sega, F.V.; Zambonin, L.; Fiorentini, D.; Hakim, G. VEGF-induced ROS generation from NAD(P)H oxidase protects human leukemic cells from apoptosis. Int. J. Oncol 2010, 36, 1581–1589. [Google Scholar]
- Falcieri, E.; Burattini, S.; Bortul, R.; Luchetti, F.; Tabellini, G.; Tazzari, P.L.; Cappellini, A.; Cocco, L.; Martelli, A.M. Intranucleolar localization of DNA topoisomerase IIalpha is a distinctive feature of necrotic, but not of apoptotic, Jurkat T-cells. Microsc. Res. Tech 2003, 62, 192–200. [Google Scholar]
- Renò, F.; Tontini, A.; Burattini, S.; Papa, S.; Falcieri, E.; Tarzia, G. Mimosine induces apoptosis in the HL60 human tumor cell line. Apoptosis 1999, 4, 469–477. [Google Scholar]
- Burattini, S.; Ferri, P.; Battistelli, M.; D’Emilio, A.; Biagiotti, L.; Sestili, P.; Rocchi, M.B.; Falcieri, E. Apoptotic DNA fragmentation can be revealed in situ: An ultrastructural approach. Microsc. Res. Tech 2009, 72, 913–923. [Google Scholar]
- Zamai, L.; Burattini, S.; Luchetti, F.; Canonico, B.; Ferri, P.; Melloni, E.; Gonelli, A.; Guidotti, L.; Papa, S.; Falcieri, E. In vitro apoptotic cell death during erythroid differentiation. Apoptosis 2004, 9, 235–246. [Google Scholar]
- Luchetti, F.; Canonico, B.; Curci, R.; Battistelli, M.; Mannello, F.; Papa, S.; Tarzia, G.; Falcieri, E. Melatonin prevents apoptosis induced by UV-B treatment in U937 cell line. J. Pineal Res 2006, 40, 158–167. [Google Scholar]
- Martin, S.J.; Cotter, T.G. Ultraviolet B irradiation of human leukemia HL-60 cells in vitro induces apoptosis. Int. J. Radiat. Biol 1991, 59, 1001–1016. [Google Scholar]
- Renò, F.; Burattini, S.; Rossi, S.; Luchetti, F.; Columbaro, M.; Santi, S.; Papa, S.; Falcieri, E. Phospholipid rearrangement of apoptotic membrane does not depend on nuclear activity. Histochem. Cell Biol. 1998, 110, 467–476. [Google Scholar]
- Shin, S.W.; Park, C.I.; Yang, C.H.; Park, J.W. Protective effect of Rehmannia glutinosa on the UV induced apoptosis in U937 cells. Am. J. Chin. Med 2008, 36, 1159–1170. [Google Scholar]
- Kawashima, K.; Doi, H.; Ito, Y.; Shibata, M.A.; Yoshinaka, R.; Otsuki, Y. Evaluation of cell death and proliferation in psoriatic epidermis. J. Dermatol. Sci 2004, 35, 207–214. [Google Scholar]
- Ito, Y.; Shibata, M.A.; Kusakabe, K.; Otsuki, Y. Method of specific detection of apoptosis using formamide-induced DNA denaturation assay. J. Histochem. Cytochem. 2006, 54, 683–692. [Google Scholar]
- Biagiotti, L.; Ferri, P.; D’Emilio, A.; Rocchi, M.B.L.; Falcieri, E.; Burattini, S. Light and electron microscopy of apoptotic DNA fragmentation. Microscopie 2008, 1, 45–52. [Google Scholar]
- Liu, Y.Q.; You, S.; Zhang, C.L.; Tashiro, S.; Onodera, S.; Ikejima, T. Oridonin enhances phagocytosis of UV-irradiated apoptotic U937 cells. Biol. Pharm. Bull 2005, 28, 461–467. [Google Scholar]
- Luchetti, F.; Canonico, B.; Mannello, F.; Masoni, C.; D’Emilio, A.; Battistelli, M.; Papa, S.; Falcieri, E. Melatonin reduces early changes in intramitochondrial cardiolipin during apoptosis in U937 cell line. Toxicol In Vitro 2007, 21, 293–301. [Google Scholar]
- Battistelli, M.; Borzì, R.M.; Olivotto, E.; Vitellozzi, R.; Burattini, S.; Facchini, A.; Falcieri, E. Cell and matrix morpho-functional analysis in chondrocyte micromasses. Microsc. Res. Tech. 2005, 67, 286–295. [Google Scholar]
- Falcieri, E.; Zamai, L.; Santi, S.; Cinti, C.; Gobbi, P.; Bosco, D.; Cataldi, A.; Betts, C.; Vitale, M. The behavior of nuclear domains in the course of apoptosis. Histochemistry 1994, 102, 221–231. [Google Scholar]
- Shiokawa, D.; Kobayashi, T.; Tanuma, S. Involvement of DNase gamma in apoptosis associated with myogenic differentiation of C2C12 cells. J. Biol. Chem 2002, 277, 31031–31037. [Google Scholar]
- Burattini, S.; Ferri, P.; Battistelli, M.; Curci, R.; Luchetti, F.; Falcieri, E. C2C12 murine myoblasts as a model of skeletal muscle development: Morpho-functional characterization. Eur. J. Histochem 2004, 48, 223–233. [Google Scholar]
- Primeau, A.J.; Adhihetty, P.J.; Hood, D.A. Apoptosis in heart and skeletal muscle. Can. J. Appl. Physiol 2002, 27, 349–395. [Google Scholar]
- Pustisek, N.; Situm, M. UV-radiation, apoptosis and skin. Coll Antropol 2011, 2, 339–241. [Google Scholar]
- Assefa, Z.; van Laethem, A.; Garmyn, M.; Agostinis, P. Ultraviolet radiation-induced apoptosis in keratinocytes: On the role of cytosolic factors. Biochim. Biophys. Acta 2005, 1755, 90–106. [Google Scholar]
- Herrlich, P.; Karin, M.; Weiss, C. Supreme EnLIGHTenment: Damage recognition and signaling in the mammalian UV response. Mol. Cell 2008, 29, 279–290. [Google Scholar]
- Sitailo, L.A.; Tibudan, S.S.; Denning, M.F. Activation of caspase-9 is required for UV-induced apoptosis of human keratinocytes. J. Biol. Chem 2002, 277, 19346–19352. [Google Scholar]
- Aravamudan, B.; Mantilla, C.B.; Zhan, W.Z.; Sieck, G.C. Denervation effects on myonuclear domain size of rat diaphragm fibers. J. Appl. Physiol 2006, 100, 1617–1622. [Google Scholar]
- Marzetti, E.; Privitera, G.; Simili, V.; Wohlgemuth, S.E.; Aulisa, L.; Pahor, M.; Leeuwenburgh, C. Multiple pathways to the same end: Mechanisms of myonuclear apoptosis in sarcopenia of aging. Scientific World J. 2010, 10, 340–349. [Google Scholar]
- Walker, P.R.; Leblanc, J.; Smith, B.; Pandey, S.; Sikorska, M. Detection of DNA fragmentation and endonucleases in apoptosis. Methods 1999, 17, 329–338. [Google Scholar]
- Yanagisawa-Shiota, F.; Sakagami, H.; Kuribayashi, N.; Iida, M.; Sakagami, T.; Takeda, M. Endonuclease activity and induction of DNA fragmentation in human myelogenous leukemic cell lines. Anticancer Res 1995, 15, 259–265. [Google Scholar]
- Yang, Y.; Wang, H.; Wang, S.; Xu, M.; Liu, M.; Liao, M.; Frank, J.A.; Adhikari, S.; Bower, K.A.; Shi, X.; et al. GSK3β signaling is involved in ultraviolet B-induced activation of autophagy in epidermal cells. Int. J. Oncol 2012, 41, 1782–1788. [Google Scholar]
- Wang, Q.; Ye, Y.; Liu, W.; Jiang, S.; Tashiro, S.; Onodera, S.; Gu, F.; Wang, Y.; Ikejima, T. Dual effects of silibinin treatment on autophagy-regulated dermal apoptosis retardation and epidermal apoptosis up-regulation in UVB-induced skin inflammation. J. Asian Nat. Prod. Res 2012, 14, 688–699. [Google Scholar]
- Levine, B.; Klionsky, D.J. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev. Cell 2004, 6, 463–477. [Google Scholar]
- Wang, Y.; Singh, R.; Massey, A.C.; Kane, S.S.; Kaushik, S.; Grant, T.; Xiang, Y.; Cuervo, A.M.; Czaja, M.J. Loss of macroautophagy promotes or prevents fibroblast apoptosis depending on the death stimulus. J. Biol. Chem. 2008, 283, 4766–4777. [Google Scholar]



© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Salucci, S.; Burattini, S.; Battistelli, M.; Baldassarri, V.; Maltarello, M.C.; Falcieri, E. Ultraviolet B (UVB) Irradiation-Induced Apoptosis in Various Cell Lineages in Vitro. Int. J. Mol. Sci. 2013, 14, 532-546. https://doi.org/10.3390/ijms14010532
Salucci S, Burattini S, Battistelli M, Baldassarri V, Maltarello MC, Falcieri E. Ultraviolet B (UVB) Irradiation-Induced Apoptosis in Various Cell Lineages in Vitro. International Journal of Molecular Sciences. 2013; 14(1):532-546. https://doi.org/10.3390/ijms14010532
Chicago/Turabian StyleSalucci, Sara, Sabrina Burattini, Michela Battistelli, Valentina Baldassarri, Maria Cristina Maltarello, and Elisabetta Falcieri. 2013. "Ultraviolet B (UVB) Irradiation-Induced Apoptosis in Various Cell Lineages in Vitro" International Journal of Molecular Sciences 14, no. 1: 532-546. https://doi.org/10.3390/ijms14010532






