IL-6 Receptor Is a Possible Target against Growth of Metastasized Lung Tumor Cells in the Brain
Abstract
:1. Introduction
2. Results and Discussion
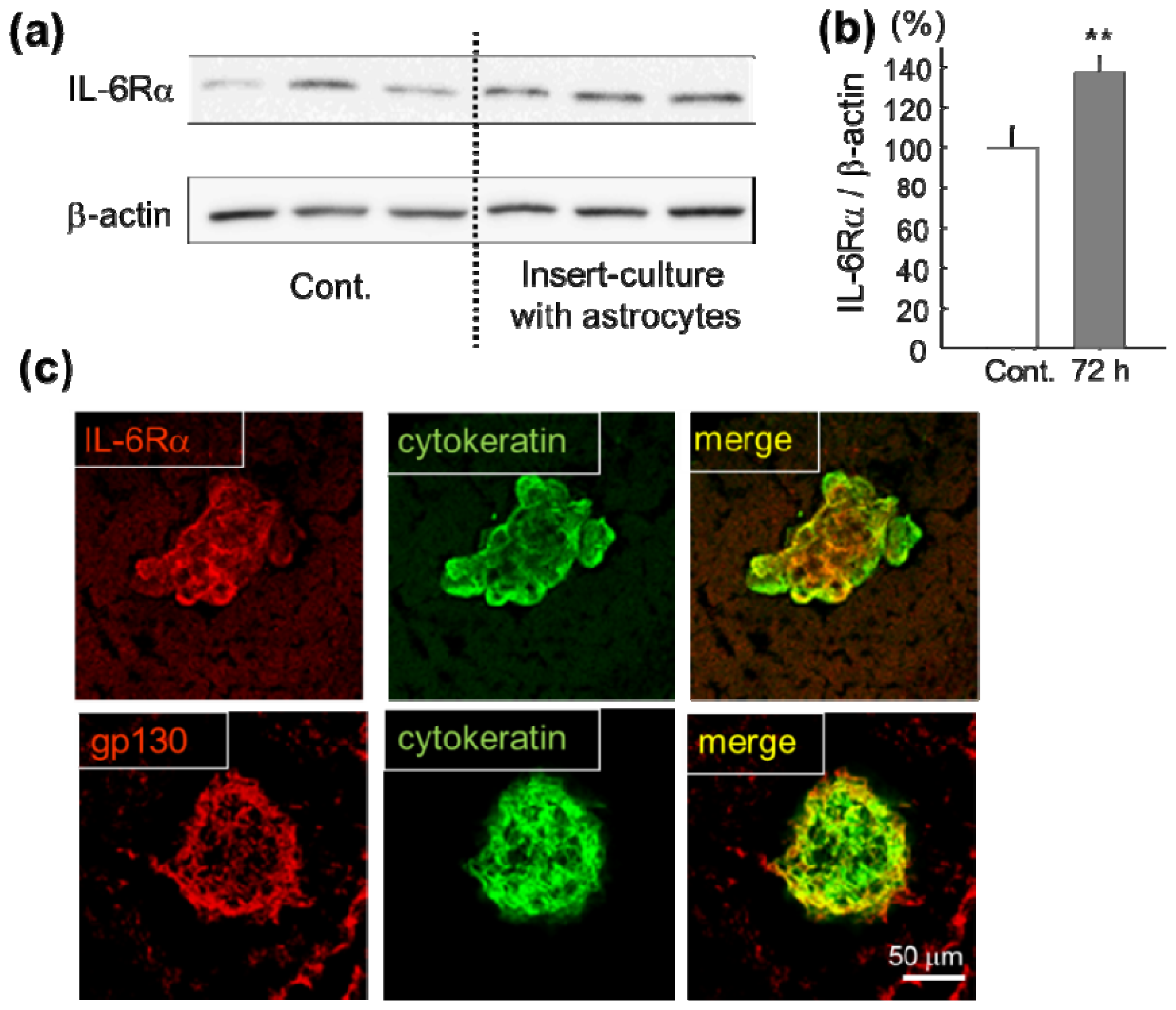
2.1. Increase of IL-6 Receptor in Lung Cancer Cells in Vitro and in Vivo
2.2. Effect of IL-6 Receptor Antibody on Lung Cancer Cells
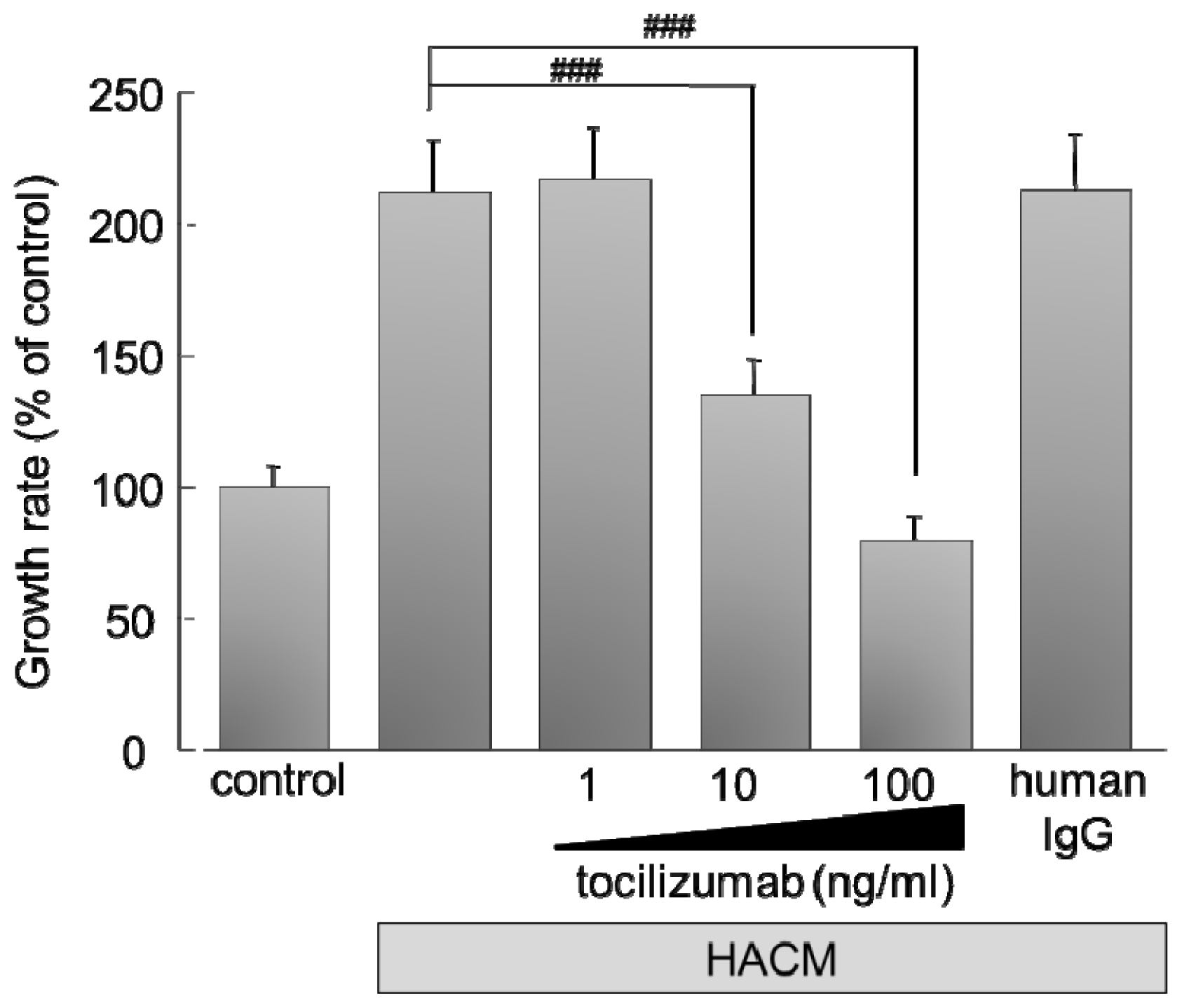
2.2.1. Effect of Monoclonal Antibody against Human IL-6 Receptor (Tocilizumab) on the Growth of HARA-B Cells in Vitro
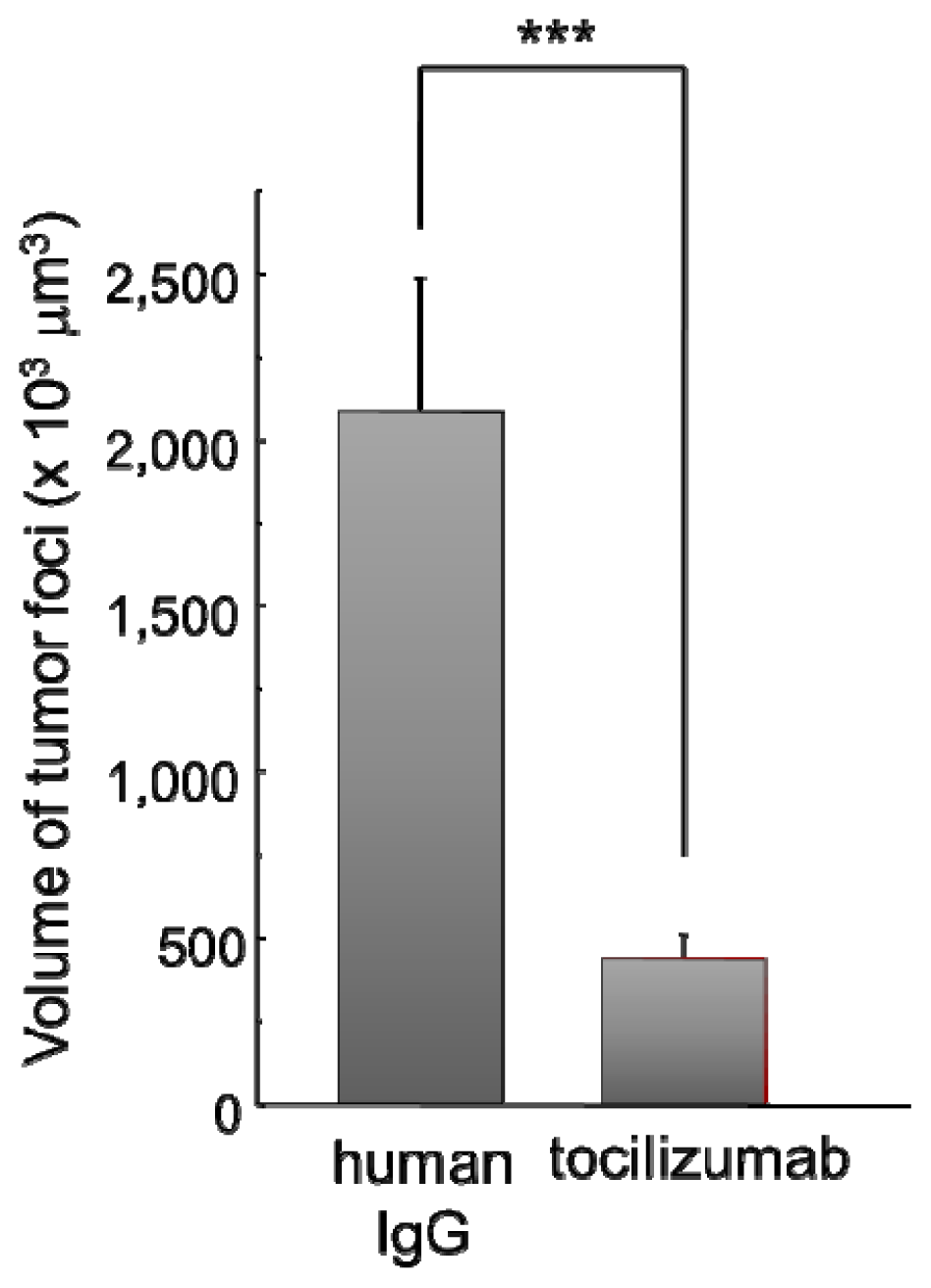
2.2.2. Effect of Tocilizumab on the Brain Metastasis of HARA-B Cells in Vivo
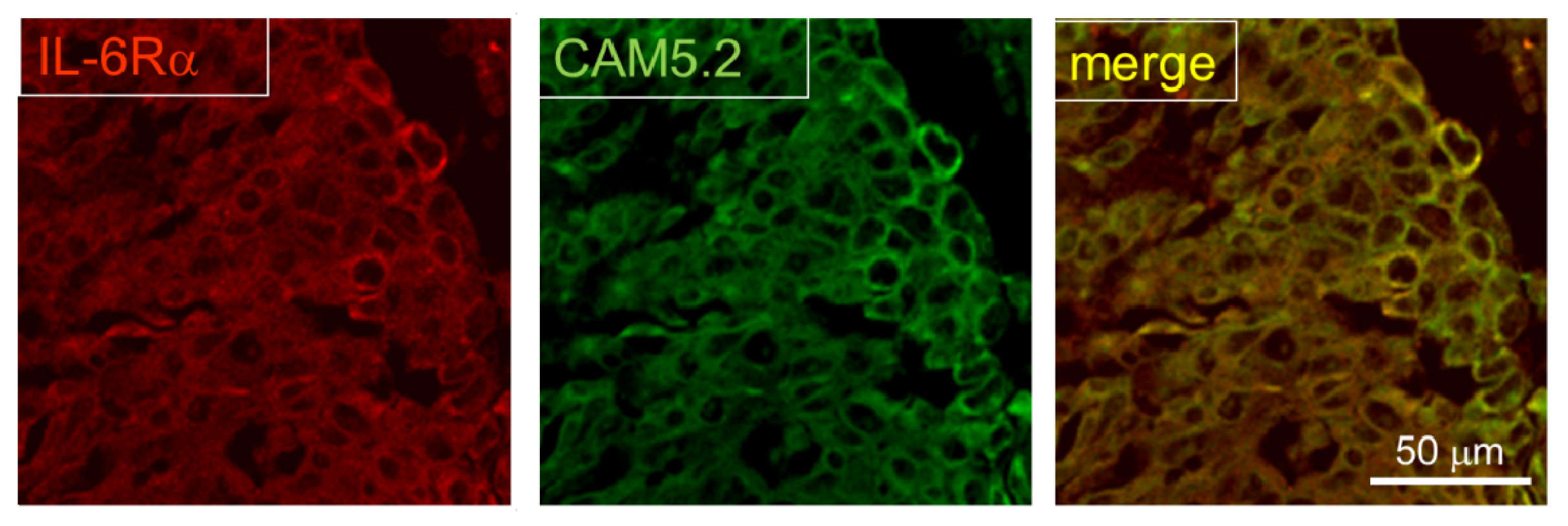
2.3. Expression of IL-6R in Postmortem Human Brain
3. Experimental Section
3.1. Cell Culture
3.2. Western Blotting
3.3. Experimental Model for Brain Metastasis
3.4. Human Tissue Samples
3.5. Immunohistochemistry
3.6. Cell Proliferation Assay
3.7. In Vivo Anti-Tumor Activity
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
- Conflict of InterestThe authors declare no conflict of interest.
References
- Fidler, I.J.; Yano, S.; Zhang, R.D.; Fujimaki, T.; Bucana, C.D. The seed and soil hypothesis: Vascularisation and brain metastases. Lancet Oncol 2002, 3, 53–57. [Google Scholar]
- Steeg, P.S.; Camphausen, K.A.; Smith, Q.R. Brain metastases as preventive and therapeutic targets. Nat. Rev. Cancer 2011, 11, 352–363. [Google Scholar]
- Aloisi, F.; Ria, F.; Adorini, L. Regulation of t-cell responses by cns antigen-presenting cells: Different roles for microglia and astrocytes. Immunol. Today 2000, 21, 141–147. [Google Scholar]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to virchow? Lancet 2001, 357, 539–545. [Google Scholar]
- Fitzgerald, D.P.; Palmieri, D.; Hua, E.; Hargrave, E.; Herring, J.M.; Qian, Y.; Vega-Valle, E.; Weil, R.J.; Stark, A.M.; Vortmeyer, A.O.; et al. Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clin. Exp. Metastasis 2008, 25, 799–810. [Google Scholar]
- Noda, M. The brain microenvironment. In Brain and Central Nervous System Metasitasis, the Biological Basis and Clinical Considerations; Palmieri, D., Ed.; Springer: New York, NY, USA, 2012. [Google Scholar]
- Heyn, C.; Ronald, J.A.; Ramadan, S.S.; Snir, J.A.; Barry, A.M.; MacKenzie, L.T.; Mikulis, D.J.; Palmieri, D.; Bronder, J.L.; Steeg, P.S.; et al. In vivo mri of cancer cell fate at the single-cell level in a mouse model of breast cancer metastasis to the brain. Magn. Reson. Med 2006, 56, 1001–1010. [Google Scholar]
- Seike, T.; Fujita, K.; Yamakawa, Y.; Kido, M.A.; Takiguchi, S.; Teramoto, N.; Iguchi, H.; Noda, M. Interaction between lung cancer cells and astrocytes via specific inflammatory cytokines in the microenvironment of brain metastasis. Clin. Exp. Metastasis 2011, 28, 13–25. [Google Scholar]
- Maini, R.N.; Taylor, P.C.; Szechinski, J.; Pavelka, K.; Broll, J.; Balint, G.; Emery, P.; Raemen, F.; Petersen, J.; Smolen, J.; et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in european patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum 2006, 54, 2817–2829. [Google Scholar]
- Mihara, M.; Nishimoto, N.; Ohsugi, Y. The therapy of autoimmune diseases by anti-interleukin-6 receptor antibody. Expert Opin. Biol. Ther 2005, 5, 683–690. [Google Scholar]
- Ding, C.; Jones, G. Anti-interleukin-6 receptor antibody treatment in inflammatory autoimmune diseases. Rev. Recent Clin. Trials 2006, 1, 193–200. [Google Scholar]
- Yokota, S.; Miyamae, T.; Imagawa, T.; Iwata, N.; Katakura, S.; Mori, M.; Woo, P.; Nishimoto, N.; Yoshizaki, K.; Kishimoto, T. Therapeutic efficacy of humanized recombinant anti-interleukin-6 receptor antibody in children with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum 2005, 52, 818–825. [Google Scholar]
- Yokota, S. Interleukin 6 as a therapeutic target in systemic-onset juvenile idiopathic arthritis. Curr. Opin. Rheumatol 2003, 15, 581–586. [Google Scholar]
- Straub, R.H.; Harle, P.; Yamana, S.; Matsuda, T.; Takasugi, K.; Kishimoto, T.; Nishimoto, N. Anti-interleukin-6 receptor antibody therapy favors adrenal androgen secretion in patients with rheumatoid arthritis: A randomized, double-blind, placebo-controlled study. Arthritis Rheum 2006, 54, 1778–1785. [Google Scholar]
- Plushner, S.L. Tocilizumab: An interleukin-6 receptor inhibitor for the treatment of rheumatoid arthritis. Ann. Pharmacother 2008, 42, 1660–1668. [Google Scholar]
- Nishimoto, N.; Yoshizaki, K.; Miyasaka, N.; Yamamoto, K.; Kawai, S.; Takeuchi, T.; Hashimoto, J.; Azuma, J.; Kishimoto, T. Treatment of rheumatoid arthritis with humanized anti-interleukin-6 receptor antibody: A multicenter, double-blind, placebo-controlled trial. Arthritis Rheum 2004, 50, 1761–1769. [Google Scholar]
- Nishimoto, N.; Yoshizaki, K.; Maeda, K.; Kuritani, T.; Deguchi, H.; Sato, B.; Imai, N.; Suemura, M.; Kakehi, T.; Takagi, N.; et al. Toxicity, pharmacokinetics, and dose-finding study of repetitive treatment with the humanized anti-interleukin 6 receptor antibody mra in rheumatoid arthritis. Phase i/ii clinical study. J. Rheumatol 2003, 30, 1426–1435. [Google Scholar]
- Mihara, M.; Kotoh, M.; Nishimoto, N.; Oda, Y.; Kumagai, E.; Takagi, N.; Tsunemi, K.; Ohsugi, Y.; Kishimoto, T.; Yoshizaki, K.; et al. Humanized antibody to human interleukin-6 receptor inhibits the development of collagen arthritis in cynomolgus monkeys. Clin. Immunol 2001, 98, 319–326. [Google Scholar]
- Choy, E.H.; Isenberg, D.A.; Garrood, T.; Farrow, S.; Ioannou, Y.; Bird, H.; Cheung, N.; Williams, B.; Hazleman, B.; Price, R.; et al. Therapeutic benefit of blocking interleukin-6 activity with an anti-interleukin-6 receptor monoclonal antibody in rheumatoid arthritis: A randomized, double-blind, placebo-controlled, dose-escalation trial. Arthritis Rheum 2002, 46, 3143–3150. [Google Scholar]
- Ito, H. Novel therapy for crohn’s disease targeting il-6 signalling. Expert Opin. Ther. Targets 2004, 8, 287–294. [Google Scholar]
- Nishimoto, N.; Kanakura, Y.; Aozasa, K.; Johkoh, T.; Nakamura, M.; Nakano, S.; Nakano, N.; Ikeda, Y.; Sasaki, T.; Nishioka, K.; et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric castleman disease. Blood 2005, 106, 2627–2632. [Google Scholar]
- Nishimoto, N. Clinical studies in patients with castleman’s disease, crohn’s disease, and rheumatoid arthritis in japan. Clin. Rev. Allergy Immunol 2005, 28, 221–230. [Google Scholar]
- Kawabe, T.; Phi, J.H.; Yamamoto, M.; Kim, D.G.; Barfod, B.E.; Urakawa, Y. Treatment of brain metastasis from lung cancer. Prog. Neurol. Surg 2012, 25, 148–155. [Google Scholar]
- Xu, Z.; Elsharkawy, M.; Schlesinger, D.; Sheehan, J. Gamma knife radiosurgery for resectable brain metastasis. World Neurosurg. 2012, in press. [Google Scholar]
- Padovani, L.; Muracciole, X.; Regis, J. Gamma knife radiosurgery of brain metastasis from breast cancer. Prog. Neurol. Surg 2012, 25, 156–162. [Google Scholar]
- Marchan, E.M.; Sheehan, J. Stereotactic radiosurgery of brain metastasis from melanoma. Prog. Neurol. Surg 2012, 25, 176–189. [Google Scholar]
- Kelly, P.J.; Lin, Y.B.; Yu, A.Y.; Alexander, B.M.; Hacker, F.; Marcus, K.J.; Weiss, S.E. Stereotactic irradiation of the postoperative resection cavity for brain metastasis: A frameless linear accelerator-based case series and review of the technique. Int. J. Radiat. Oncol. Biol., Phys 2012, 82, 95–101. [Google Scholar]
- Momiyama, M.; Suetsugu, A.; Kimura, H.; Kishimoto, H.; Aki, R.; Yamada, A.; Sakurada, H.; Chishima, T.; Bouvet, M.; Endo, I.; et al. Imaging the efficacy of uvc irradiation on superficial brain tumors and metastasis in live mice at the subcellular level. J. Cell. Biochem 2012, 114, 428–434. [Google Scholar]
- Huang, J.; Dong, X.R.; Lu, H.D.; Liu, L. A case report: Successful treatment of meningeal metastasis with concurrent whole brain radiotherapy and erlotinib in a patient with non-small cell lung cancer. West Indian Med. J 2012, 61, 106–108. [Google Scholar]
- Li, G.H.; Liu, Y.; Tang, J.L.; Zhang, D.; Zhou, P.; Yang, D.Q.; Ma, C.K. Pulsed reduced dose-rate radiotherapy as re-irradiation for brain metastasis in a patient with lung squamous-celled carcinoma. Jpn. J.Clin. Oncol 2012, 42, 856–860. [Google Scholar]
- Khan, E.; Ismail, S.; Muirhead, R. Incidence of symptomatic brain metastasis following radical radiotherapy for non-small cell lung cancer: Is there a role for prophylactic cranial irradiation? Bri. J. Radiol. 2012. [Google Scholar] [CrossRef]
- Preusser, M.; Berghoff, A.S.; Schadendorf, D.; Lin, N.U.; Stupp, R. Brain metastasis: Opportunity for drug development? Curr. Opin. Neurol 2012, 25, 786–794. [Google Scholar]
- Chen, L.T.; Xu, S.D.; Xu, H.; Zhang, J.F.; Ning, J.F.; Wang, S.F. Microrna-378 is associated with non-small cell lung cancer brain metastasis by promoting cell migration, invasion and tumor angiogenesis. Med. Oncol 2012, 29, 1673–1680. [Google Scholar]
- Hwang, S.J.; Seol, H.J.; Park, Y.M.; Kim, K.H.; Gorospe, M.; Nam, D.H.; Kim, H.H. Microrna-146a suppresses metastatic activity in brain metastasis. Mol. Cells 2012, 34, 329–334. [Google Scholar]
- Soffietti, R.; Trevisan, E.; Ruda, R. Targeted therapy in brain metastasis. Curr. Opin. Oncol 2012, 24, 679–686. [Google Scholar]
- Lee, S.H. Role of chemotherapy on brain metastasis. Prog. Neurol. Surg 2012, 25, 110–114. [Google Scholar]
- Lyons, S.A.; Kettenmann, H. Oligodendrocytes and microglia are selectively vulnerable to combined hypoxia and hypoglycemia injury in vitro. J. Cereb. Blood Flow Metab 1998, 18, 521–530. [Google Scholar]
- Noda, M.; Nakanishi, H.; Nabekura, J.; Akaike, N. Ampa-kainate subtypes of glutamate receptor in rat cerebral microglia. J. Neurosci 2000, 20, 251–258. [Google Scholar]
- Kim, S.; Gaber, M.W.; Zawaski, J.A.; Zhang, F.; Richardson, M.; Zhang, X.A.; Yang, Y. The inhibition of glioma growth in vitro and in vivo by a chitosan/ellagic acid composite biomaterial. Biomaterials 2009, 30, 4743–4751. [Google Scholar]
- Li, Q.; Pan, P.Y.; Gu, P.; Xu, D.; Chen, S.H. Role of immature myeloid gr-1+ cells in the development of antitumor immunity. Cancer Res 2004, 64, 1130–1139. [Google Scholar]
- Schwartz, L.H.; Ginsberg, M.S.; DeCorato, D.; Rothenberg, L.N.; Einstein, S.; Kijewski, P.; Panicek, D.M. Evaluation of tumor measurements in oncology: Use of film-based and electronic techniques. J. Clin. Oncol 2000, 18, 2179–2184. [Google Scholar]
- Kim, S.A.; Kim, Y.C.; Kim, S.W.; Lee, S.H.; Min, J.J.; Ahn, S.G.; Yoon, J.H. Antitumor activity of novel indirubin derivatives in rat tumor model. Clin. Cancer Res 2007, 13, 253–259. [Google Scholar]
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Noda, M.; Yamakawa, Y.; Matsunaga, N.; Naoe, S.; Jodoi, T.; Yamafuji, M.; Akimoto, N.; Teramoto, N.; Fujita, K.; Ohdo, S.; et al. IL-6 Receptor Is a Possible Target against Growth of Metastasized Lung Tumor Cells in the Brain. Int. J. Mol. Sci. 2013, 14, 515-526. https://doi.org/10.3390/ijms14010515
Noda M, Yamakawa Y, Matsunaga N, Naoe S, Jodoi T, Yamafuji M, Akimoto N, Teramoto N, Fujita K, Ohdo S, et al. IL-6 Receptor Is a Possible Target against Growth of Metastasized Lung Tumor Cells in the Brain. International Journal of Molecular Sciences. 2013; 14(1):515-526. https://doi.org/10.3390/ijms14010515
Chicago/Turabian StyleNoda, Mami, Yukiko Yamakawa, Naoya Matsunaga, Satoko Naoe, Taishi Jodoi, Megumi Yamafuji, Nozomi Akimoto, Norihiro Teramoto, Kyota Fujita, Shigehiro Ohdo, and et al. 2013. "IL-6 Receptor Is a Possible Target against Growth of Metastasized Lung Tumor Cells in the Brain" International Journal of Molecular Sciences 14, no. 1: 515-526. https://doi.org/10.3390/ijms14010515



