Synthesis, Structure-Activity Relationships (SAR) and in Silico Studies of Coumarin Derivatives with Antifungal Activity
Abstract
:1. Introduction
2. Results and Discussion
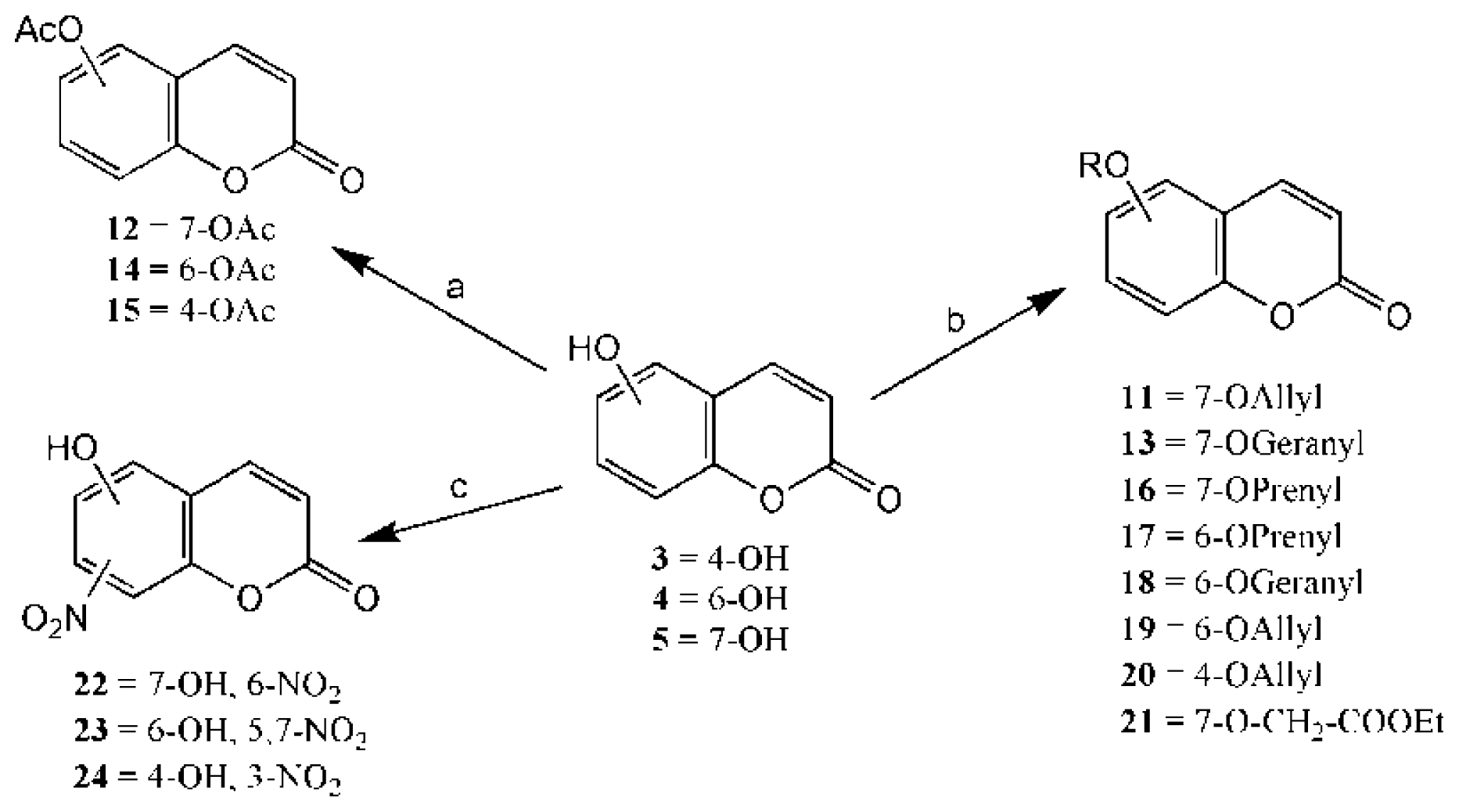
2.1. Synthesis
2.2. In Vitro Antifungal Evaluation and SAR Study
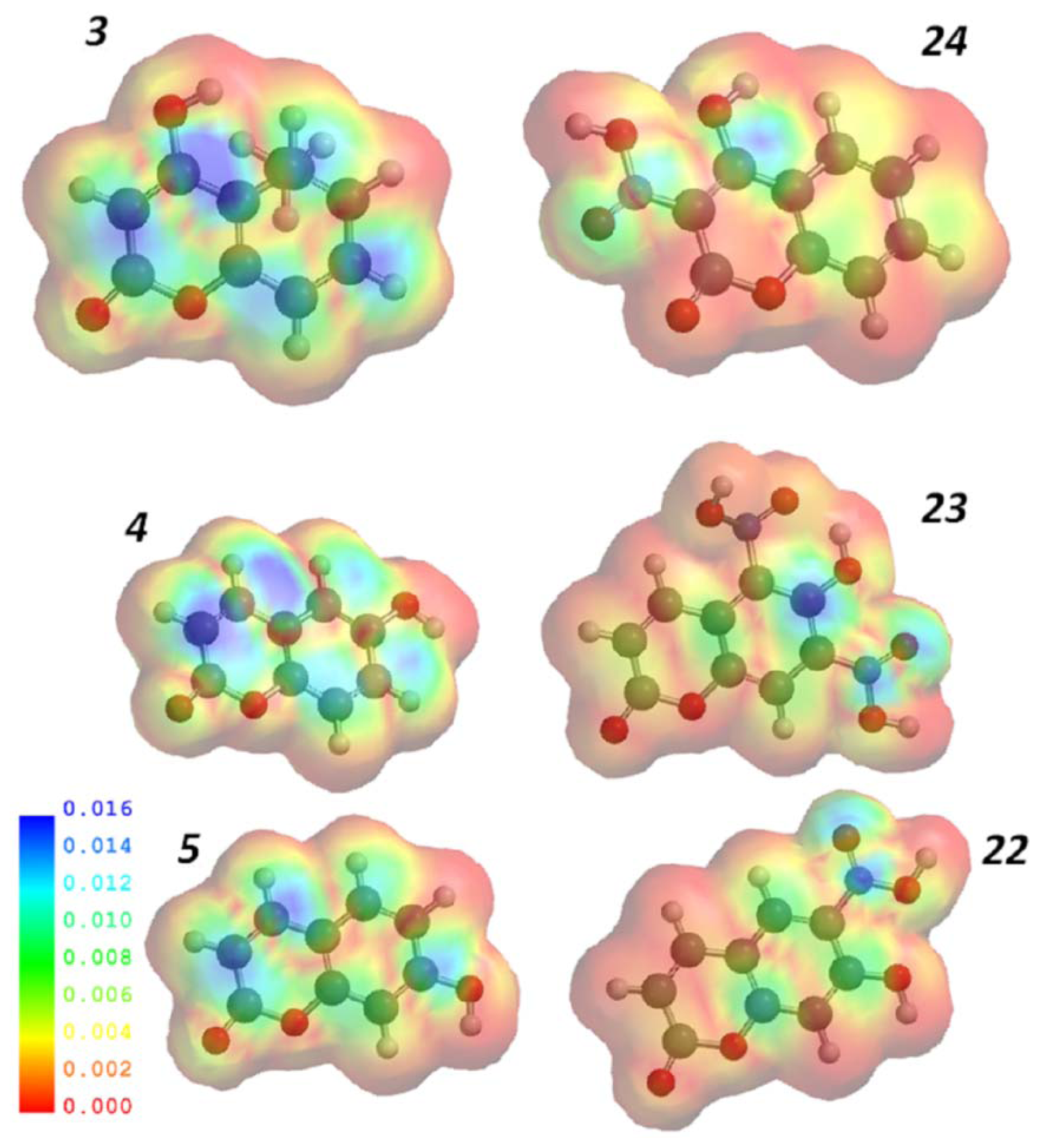
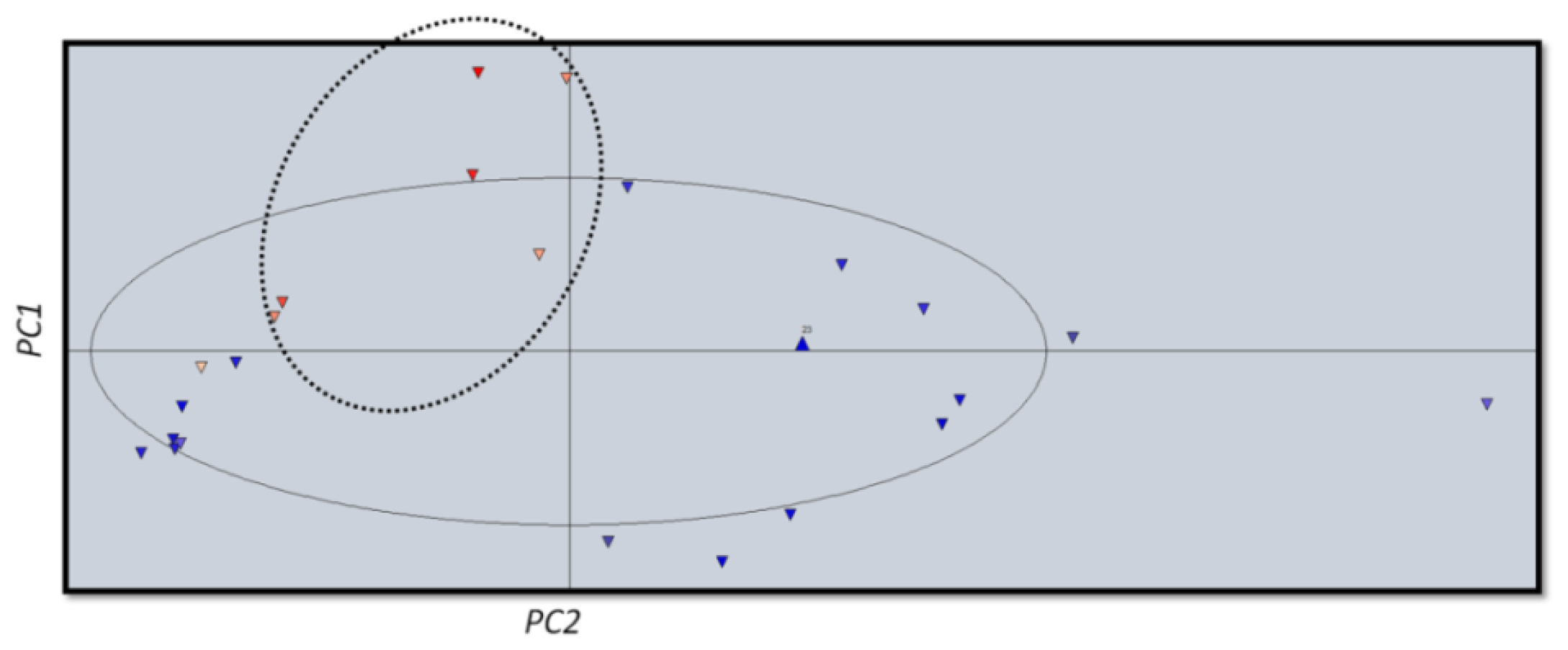
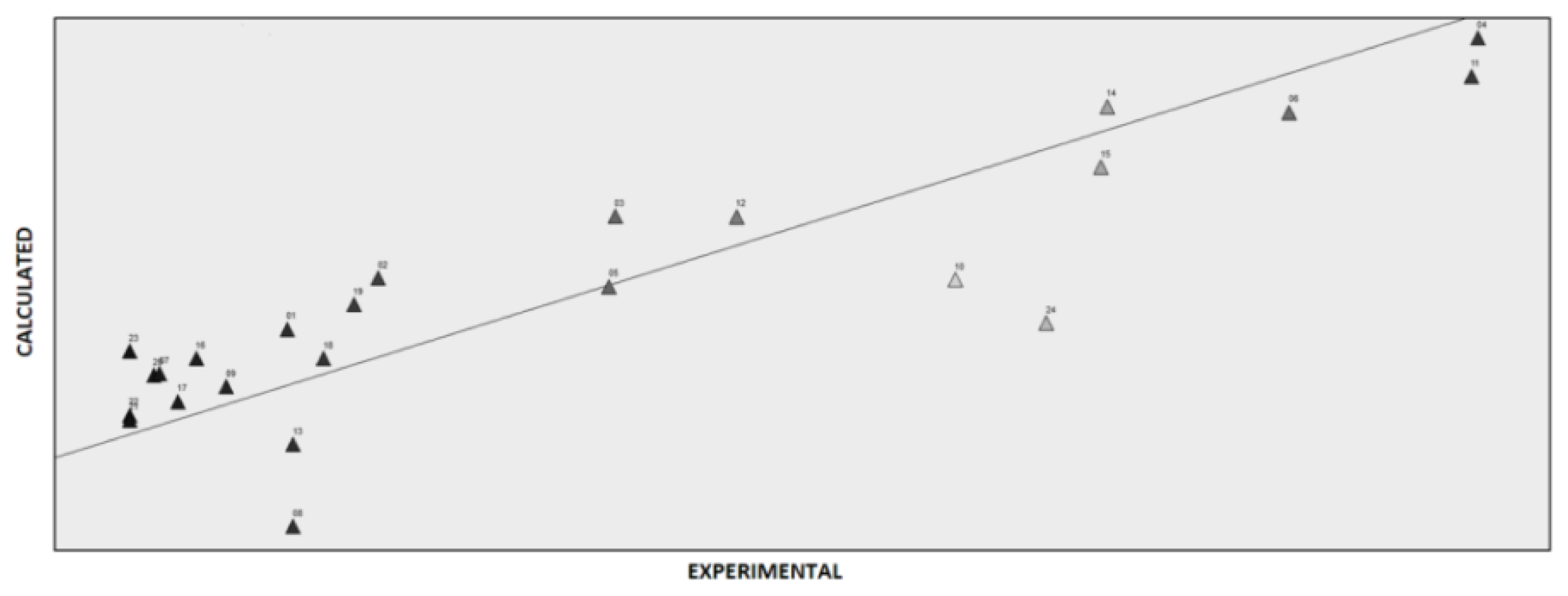
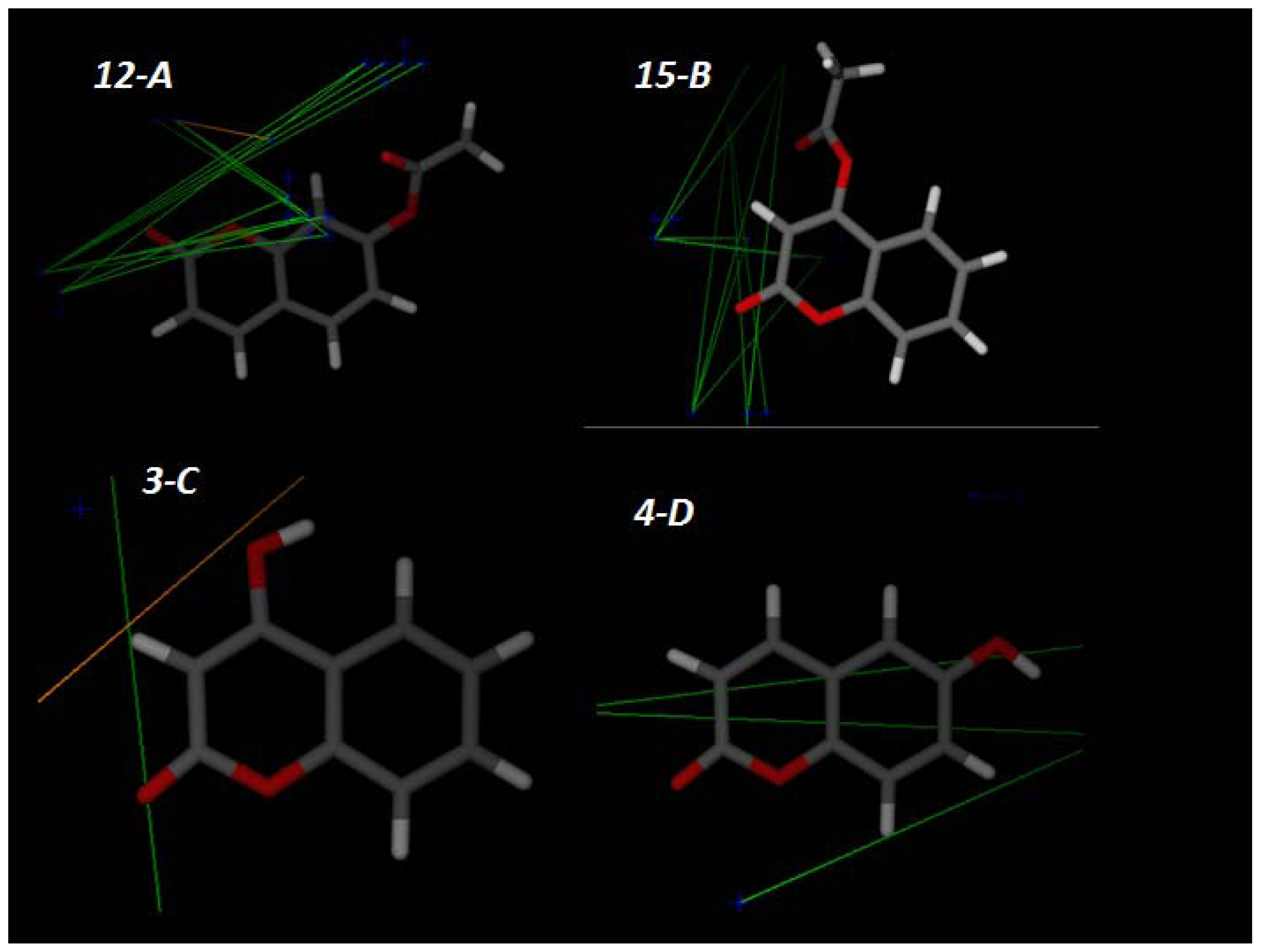
2.3. Computational Studies
Principal Component Analysis (PCA) and Partial Lest Squares Regression (PLS)
3. Material and Methods
3.1. General Methods
3.2. Synthesis
3.2.1. General Alkylation Procedure
3.2.2. General Acetylation Procedure
3.2.3. General Nitration Procedure
3.3. Antifungal Activity
3.4. Molecular Modeling and Electronic Surfaces
4. Conclusions
Acknowledgments
- Sample AvailabilitySamples of the compounds 11–24 are available from the authors.
References
- Mendonça, F.J.B., Junior; Lima-Neto, R.G.; Oliveira, T.B.; Lima, M.C.A.; Pitta, I.R.; Galdino, S.L.; Cruz, R.M.D.; Araújo, R.S.A.; Neves, R.P. Synthesis and evaluation of the antifungal activity of 2-(substituted-amino)-4,5-dialkyl-thiophene-3-carbonitrile derivatives. Lat. Am. J. Pharm 2011, 30, 1492–1499. [Google Scholar]
- Krappmann, S. Tools to study molecular mechanisms of Aspergillus pathogenicity. Trends Microbiol 2006, 14, 356–364. [Google Scholar]
- Hedayati, M.T.; Pasqualotto, A.C.; Warn, P.A.; Bowyer, P.; Denning, D.W. Aspergillus flavus: Human pathogen, aleergen and mycotoxin producer. Microbiology 2007, 153, 1677–1692. [Google Scholar]
- Maggi, F.; Barboni, L.; Caprioli, G.; Papa, F.; Ricciutelli, M.; Sagratini, G.; Vittori, S. HPLC quantification of coumarin in bastard balm (Melittis melissophyllum L., Lamiaceae). Fitoterapia 2011, 82, 1215–1221. [Google Scholar]
- Olmedo, D.; Sancho, R.; Bedoya, L.M.; López-Pérez, J.L.; Olmo, E.; Muñoz, E.; Alcamí, J.; Gupta, M.P.; Feliciano, A.S. 3-Phenylcoumarins as inhibitors of HIV-1 replication. Molecules 2012, 17, 9245–9257. [Google Scholar]
- Sahebkar, A. Citrus auraptene: A potential multifunctional therapeutic agent for nonalcoholic fatty liver disease. Ann. Hepatol 2011, 10, 575–577. [Google Scholar]
- Onuma, K.; Suenaga, Y.; Sakaki, R.; Yoshitome, S.; Sato, Y.; Ogawara, S.; Suzuki, S.; Kuramitsu, Y.; Yokoyama, H.; Murakami, A.; et al. Development of a quantitative bioassay to assess preventive compounds against inflammation-based carcinogenesis. Nitric Oxide 2011, 25, 183–194. [Google Scholar]
- Hamdi, N.; Al-Ayed, A.S.; Said, R.B.; Fabienne, A. Synthesis and characterization of new thiazolidinones containing coumarin moieties and their antibacterial and antioxidant activities. Molecules 2012, 17, 9321–9334. [Google Scholar]
- Ibrahim, N.M. The behavior of certain coumarins and furanocoumarins toward sulfur reagents. Phosphorus Sulfur 2006, 181, 1773–1784. [Google Scholar]
- Benci, K.; Mandić, L.; Suhina, T.; Sedić, M.; Klobučar, M.; Pavelić, S.K.; Pavelić, K.; Wittine, K.; Mintas, M. Novel coumarin derivatives containing 1,2,4-triazole, 4,5-dicyanoimidazole and purine moieties: Synthesis and evaluation of their cytostatic activity. Molecules 2012, 17, 11010–11025. [Google Scholar]
- Mladenović, M.; Vuković, N.; Sukdolak, S.; Solujić, S. Design of novel 4-hydroxy-chromene-2-one derivatives as antimicrobial agents. Molecules 2010, 15, 4294–4308. [Google Scholar]
- Johann, S.; Mendes, B.G.; Missau, F.C.; Resende, M.A.; Pizzolatti, M.G. Antifungal activity of five species of Polygala. Braz. J. Microbiol 2011, 42, 1065–1075. [Google Scholar]
- Daoubi, M.; Durán-Patrón, R.; Hmamouchi, M.; Hernández-Galán, R.; Benharref, A.; Collado, I.G. Screening study for potential lead compounds for natural product-based fungicides: I. Synthesis and in vitro evaluation of coumarins against Botrytis cinerea. Pest Manag. Sci 2004, 60, 927–932. [Google Scholar]
- Shimizu, B.-I.; Miyagawa, H.; Ueno, T.; Sakata, K.; Watanabe, K.; Ogawa, K. Morning glory systematically accumulates scopoletin and scopolin after interaction with Fusariumoxysporum. Z. Naturforsch 2005, 60, 83–90. [Google Scholar]
- Creaven, B.S.; Egan, D.A.; Kavanagh, K.; McCann, M.; Mahon, M.; Noble, A.; Thati, B.; Walsh, M. Synthesis and antimicrobial activity of copper(II) and silver(I) complexes of hydroxyl-nitrocoumarins: X-ray crystal structures of [Cu(hnc)2(H2O)2]·2H2O and [Ag(hnc)] (hnc = 4-hydroxy-3-nitro-2H-chromen-2-one). Polyhedron 2005, 24, 949–957. [Google Scholar]
- Jurd, L.; King, A.D.; Mihara, K., Jr. Antimicrobial properties of umbelliferone derivatives. Phytochemistry 1971, 10, 2965–2970. [Google Scholar]
- Jurd, L.; Corse, J.; King, A.D.; Bayne, H., Jr; Mihara, K., Jr. Antimicrobial properties of 6,7-dihydroxy-, 7,8-dihydroxy-, 6-hydroxy- and 8-hydroxycoumarins. Phytochemistry 1971, 10, 2971–2974. [Google Scholar]
- Scotti, L.; Scotti, M.T.; Lima, E.O.; Silva, M.S.; Lima, M.C.A.; Pitta, I.R.; Moura, R.O.; Oliveira, J.G.B.; Cruz, R.M.D.; Mendonça-Junior, F.J.B. Experimental methodologies and evaluations of computer-aided drug design methodologies applied to a series of 2-aminothiophene derivatives with antifungal activities. Molecules 2012, 17, 2298–2315. [Google Scholar]
- Lima-Neto, R.G.; Cavalcante, N.N.M.; Srivastava, R.M.; Mendonça-Júnior, F.J.B.; Wanderley, A.G.; Neves, R.P.; Dos Anjos, J.V. Synthesis of 1,2,3-triazole derivatives and in vitro antifungal evaluation on Candida strains. Molecules 2012, 17, 5882–5892. [Google Scholar]
- Carmo, E.S.; Lima, E.O.; Souza, E.L.; Sousa, F.B. Effect of Cinnamomum zeylanicum Blume essential oil on the grow and morphogenesis of some potentially pathogenic Aspergillus species. Braz. J. Microbiol 2008, 39, 91–97. [Google Scholar]
- Beebe, K.R.; Pell, R.J.; Seasholtz, M.B. Chemometrics: A Practical Guide; Wiley: New York, NY, USA, 1998. [Google Scholar]
- Cohen, N.C. Guidebook on Molecular Modeling in Drug Design; Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Chung, J.W.; Lee, K.; Neikirk, C.; Nelson, C.M.; Priestley, R.D. Photoresponsive coumarin-stabilized polymeric nanoparticles as a detectable drug carrier. Small 2012, 8, 1693–1700. [Google Scholar]
- Nechifor, M. Synthesis and properties of some aromatic polyamides with coumarin chromophores. React. Funct. Polym 2009, 69, 27–35. [Google Scholar]
- Magolan, J.; Coster, M.J. Total synthesis of (+)-angelmarin. J. Org. Chem 2009, 74, 5083–5086. [Google Scholar]
- Epifano, F.; Genovese, S.; Squires, E.J.; Gray, M.A. Nelumal A, the active principle from Ligulariane lumbifolia, is a novel farnesoid X receptor agonist. Bioorg. Med. Chem. Lett 2012, 22, 3130–3135. [Google Scholar]
- Chen, Y.; Wang, T.; Tzeng, C.; Chang, N. Geiparvarin analogues: Synthesis and anticancer evaluation of α-methylidene-γ-butyrolactone-bearing coumarins. Helv. Chim. Acta 1999, 82, 191–197. [Google Scholar]
- Josudong, K. Trifoliate orange extract with anti-inflammatory activity, with potential anti-inflammatory activity of 7—including Zera Neil oxy coumarin induced Bodies and to include them for the anti-inflammatory drug. Korean Patent 10-2010-0023690, 4 March 2010. [Google Scholar]
- Adfa, M.; Hattori, Y.; Yoshimura, T.; Komura, R.; Koketsu, M. Antifeedant and termiticidal activities of 6-alkoxycoumarins and related analogs against Coptotermes formosanus Shiraki. J. Chem. Ecol 2011, 37, 598–606. [Google Scholar]
- Avetisyan, A.A.; Alvandzhyan, A.G. Synthesis on the basis of 2H-chromen-2-one and 2H-chromene-2-thione. Russ. J. Org. Chem 2006, 42, 1063–1067. [Google Scholar]
- Al-Amiery, A.A.; Musa, A.Y.; Kadhum, A.A.H.; Mohamad, A.B. The use of umbelliferone in the synthesis of new heterocyclic compounds. Molecules 2011, 16, 6833–6843. [Google Scholar]
- Martines, M.A.U.; Davolos, M.R.; Jafelicci, M., Júnior. O efeito do ultra-som em reações químicas. Quim. Nova 2000, 23, 251–256. [Google Scholar]
- Woods, L.L.; Fooladi, M. 5-Aroyl- (or -Acyl-) 4-hydroxycoumarins. J. Org. Chem 1968, 33, 2966–2968. [Google Scholar]
- He, W.; Zhang, B.; Zhou, S.; Sun, X.; Zhang, S. Facile total synthesis of xanthoxol. Synth. Commun 2007, 37, 361–367. [Google Scholar]
- Selvam, J.J.P.; Suresh, V.; Rajesh, K.; Reddy, S.R.; Venkateswarlu, Y. Highly efficient nitration of phenolic compounds by zirconyl nitrate. Tetrahedron Lett 2006, 47, 2507–2509. [Google Scholar]
- Brady, I.; Leane, D.; Hughes, H.P.; Forster, R.J.; Keyes, T.E. Electronic properties of Ru(II) complexes bound to a bisphenolate bridge with low lying π* orbitals. Dalton Trans 2004, 2, 334–341. [Google Scholar]
- Leite, A.C.L.; Silva, K.P.; Souza, I.A.; Araújo, J.M.; Brondani, D.J. Synthesis, antitumor and antimicrobial activities of new peptidyl derivatives containing the 1,3-benzodioxole system. Eur. J. Med. Chem 2004, 39, 1059–1065. [Google Scholar]
- Lei, L.; Yang, D.; Liu, Z.; Wu, L. Mono-nitration of coumarin by nitric oxide. Synth. Commun 2004, 34, 985–992. [Google Scholar]
- Sharaf, M.A.; Illman, D.L.; Kowalski, B.R. Chemometrics; John Wiley & Sons: New York, NY, USA, 1986. [Google Scholar]
- Souza, B.B.C.; De Oliveira, T.B.; Aquino, T.M.; De Lima, M.C.A.; Pitta, I.R.; Galdino, S.L.; Lima, E.O.; Gonçalves-Silva, T.; Militão, G.C.G.; Scotti, L.; et al. Preliminary antifungal and citotoxic evaluation of synthetic cycloalkyl[b]thiophene derivatives with PLS-DA analysis. Acta Pharm 2012, 62, 221–236. [Google Scholar]
- Pentacle, Version 1.5; Molecular Discovery Ltd.: Pinner, Middlesex, UK. Available online: http://www.moldiscovery.com/soft_pentacle.php accessed on 26 September 2012.
- Pastor, M.; Mclay, I.; Pickett, S.; Clementi, S. Grid-Independent descriptors (GRIND): A novel class of alignment-independent three-dimensional molecular descriptors. Med. Chem 2000, 43, 3233–3243. [Google Scholar]
- Crivori, P.; Cruciani, G.; Carrupt, P.-A.; Testa, B. Predicting blood-brain barrier permeation from three-dimensional molecular structure. J. Med. Chem 2000, 43, 2204–2216. [Google Scholar]
- Durán, Á.; Zamora, I.; Pastor, M. Suitability of GRIND-based principal properties for the description of molecular similarity and ligand-based virtual screening. J. Chem. Inf. Model. 2009, 49, 2129–2138. [Google Scholar]
- Sahin, F.; Gulluce, M.; Daferera, D.; Sokman, A.; Polissiou, M.; Agar, G.; Sharma, N.; Tripathi, A. Effects of Citrus sinensis (L.) Osbeckepicarp essential oil on growth and morphogenesis of Aspergillusniger (L.) Van Tieghem. Microbiol. Res 2006, 163, 337–344. [Google Scholar]
- Moreira, A.C.P.; Lima, E.O.; Wanderley, P.A.; Carmo, E.S.; Souza, E.S. Chemical composition and antifungal activity of Hyptissuaveolens (L.) poit leaves essential oil against Aspergillusspecies. Braz. J. Microbiol 2010, 41, 28–33. [Google Scholar]
- Pereira, F.O. Atividade antifúngica do óleo essencial de Cymbopogonwinterianus JowittexBor sobre dermatófitos do gênero Trichophyton. M.Sc. Thesis, Post-Graduate Program in Natural and Synthetic Bioactive Produts, Federal University of Paraiba, João Pessoa, Paraíba, Brasil, 2009. [Google Scholar]
- HyperChem, Version 8.0; Hybercube Inc.: Gainesville, FL, USA, 2009.
- Allinger, N.L. A hydrocarbon force field utilizing V1 and V2 torsional terms. J. Am. Chem. Soc 1977, 99, 8127–8134. [Google Scholar]
- Rocha, G.B.; Freire, R.O.; Simas, A.M.; Stewart, J.J.P. RM1: A reparameterization of AM1 for H, C, N, O, P, S, F, Cl, Br and I. J. Comput. Chem 2006, 27, 1101–1111. [Google Scholar]
- Leach, A.R. Molecular Modeling: Principles and Applications; Prentice Hall: London, UK, 2001. [Google Scholar]
- Spartan model homepage for windows, Version 8.0; Wavefunction, Inc.: Irvine, CA, USA, 2008; Available online: http://www.wavefun.com/products/windows/SpartanModel/win_model.html accessed on 10 September 2012.
| Hydrogen | Carbon | C-2 | C-3 | C-4 | C-4′ | C-5 | C-6 | C-7 | C-8 | C-8′ |
|---|---|---|---|---|---|---|---|---|---|---|
| (ppm) | 159.5 | 124.0 | 136.7 | 118.8 | 139.6 | 138.9 | 143.1 | 116.3 | 146.3 | |
| H-3 | 6.75 | α | linked | α | β | - | - | - | - | - |
| H-4 | 7.77 | β | α | linked | α | β | - | - | - | β |
| H-8 | 8.23 | - | - | - | β | - | β | α | linked | α |
| A. fumigatus | A. flavus | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | Chemical Structures | ATCC 16913 (log1/cMIC) | IPP 210 | ATCC 46913 | ATCC 40640 | LM 247 | ATCC 16013 | LM 210 | LM 26 |
| 1 | 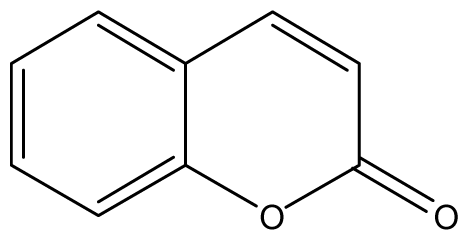 | 1024 (2.15) | 1024 | 1024 | 1024 | 1024 | 1024 | 1024 | 1024 |
| 2 | 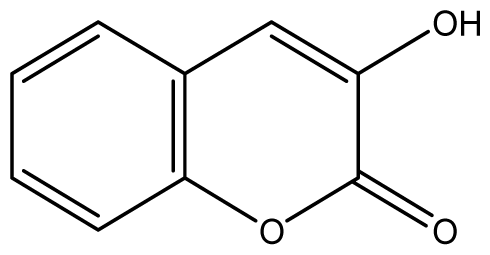 | 64 (3.40) | 64 | 64 | 64 | 128 | 128 | 128 | 128 |
| 3 | 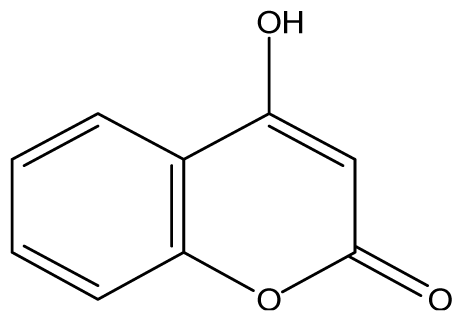 | ≥2048 (1.89) | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 |
| 4 | 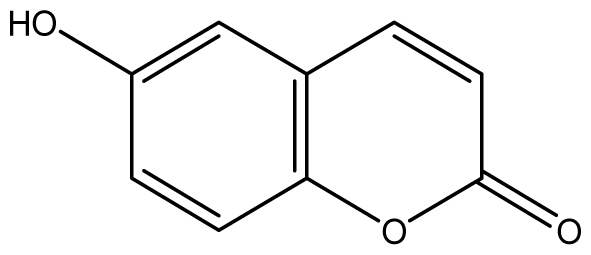 | ≥2048 (1.89) | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 |
| 5 | 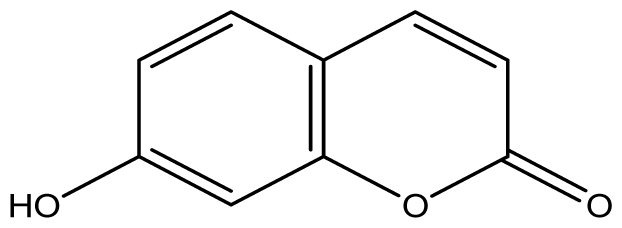 | ≥2048 (1.89) | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 |
| 6 | 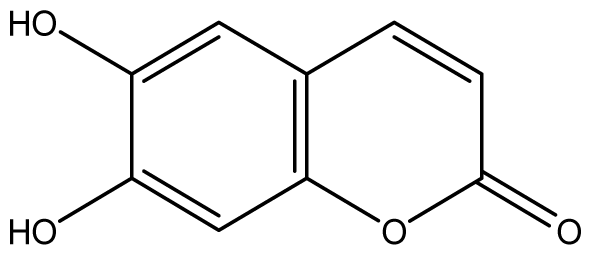 | ≥2048 (1.93) | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 |
| 7 | 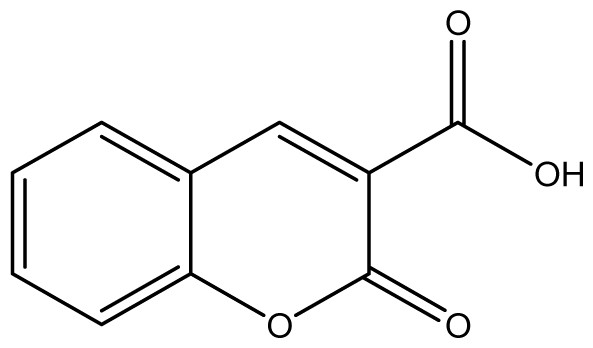 | 1024 (2.26) | 1024 | 1024 | 1024 | ≥2048 | 1024 | 1024 | 1024 |
| 8 | 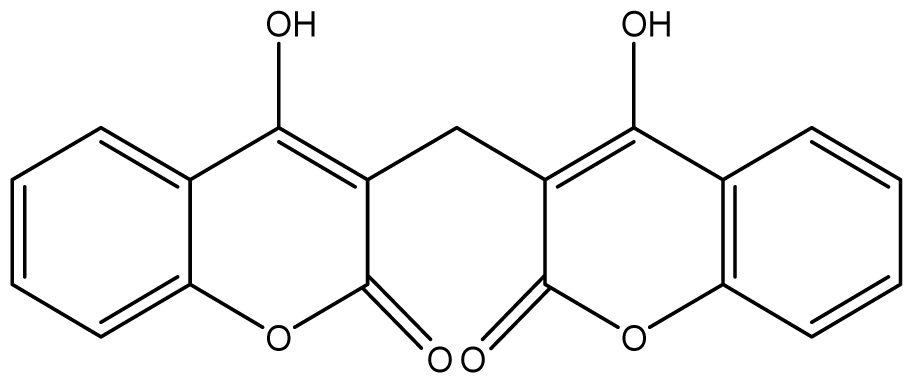 | ≥2048 (2.21) | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 |
| 9 | 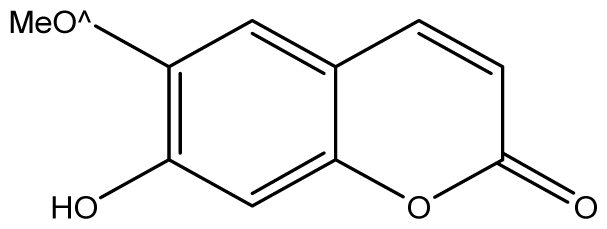 | ≥2048 (1.97) | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 |
| 10 | 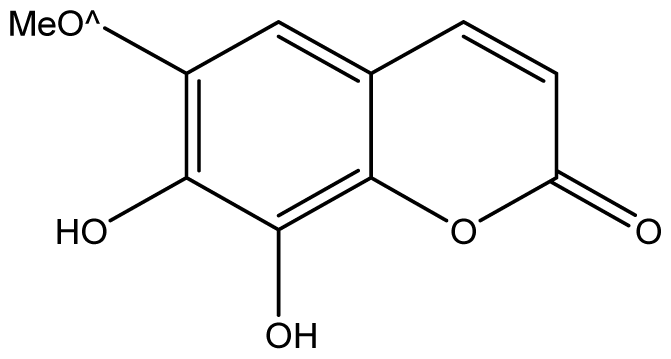 | ≥2048 (2.00) | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 |
| 11 | 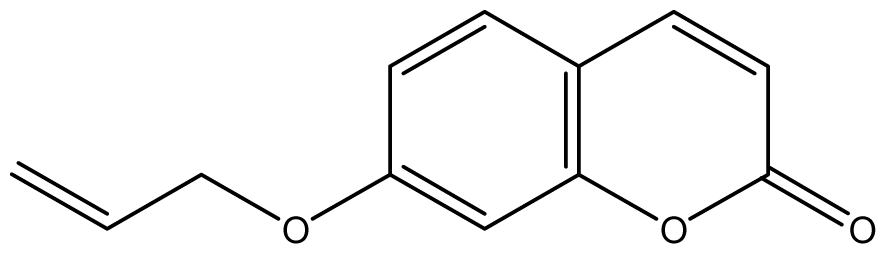 | 64 (3.49) | 64 | 64 | 64 | 256 | 64 | 64 | 64 |
| 12 | 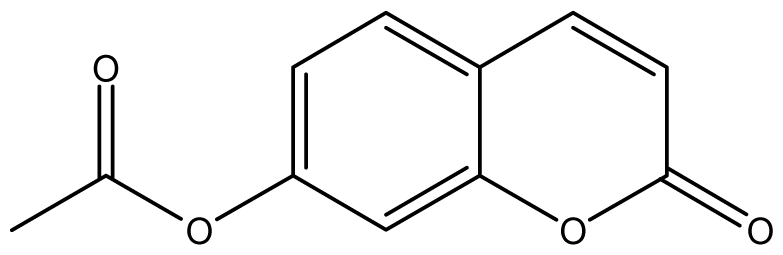 | 64 (3.50) | 64 | 64 | 64 | 128 | 64 | 64 | 64 |
| 13 | 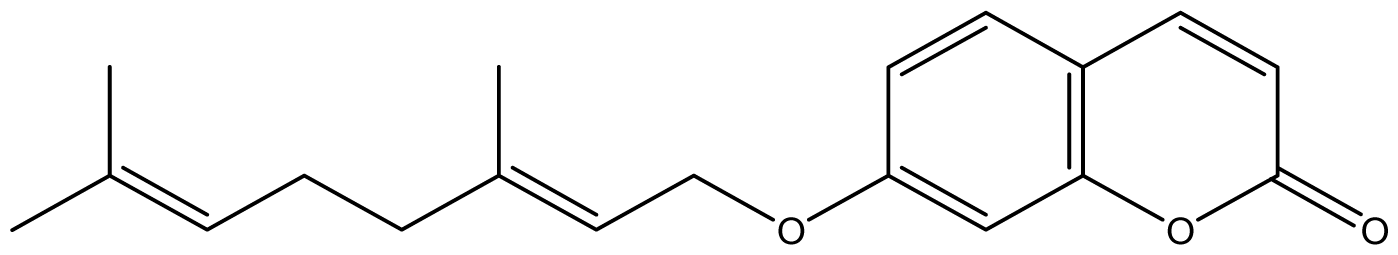 | ≥2048 (2.16) | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 |
| 14 | 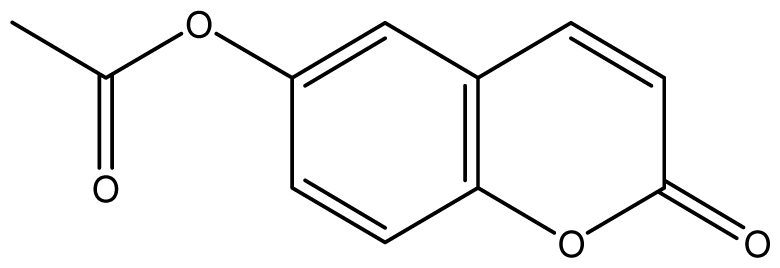 | 256 (2.89) | 256 | 256 | 256 | 256 | 256 | 256 | 256 |
| 15 | 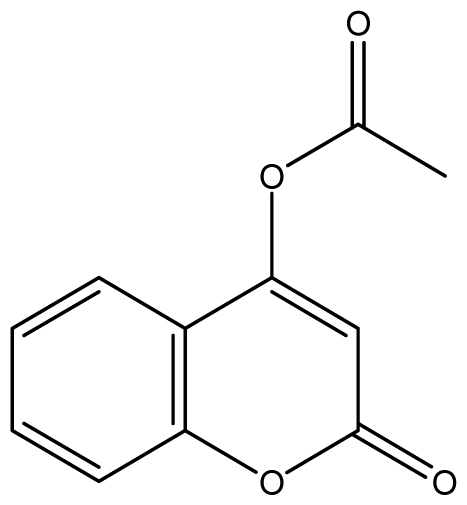 | 16 (4.10) | 16 | 16 | 16 | 16 | 16 | 16 | 16 |
| 16 | 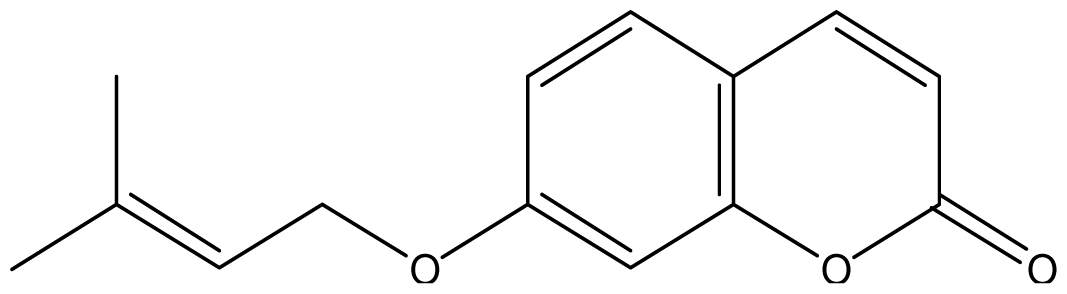 | 128 (3.25) | 128 | 128 | 128 | 1024 | 1024 | 1024 | 1024 |
| 17 | 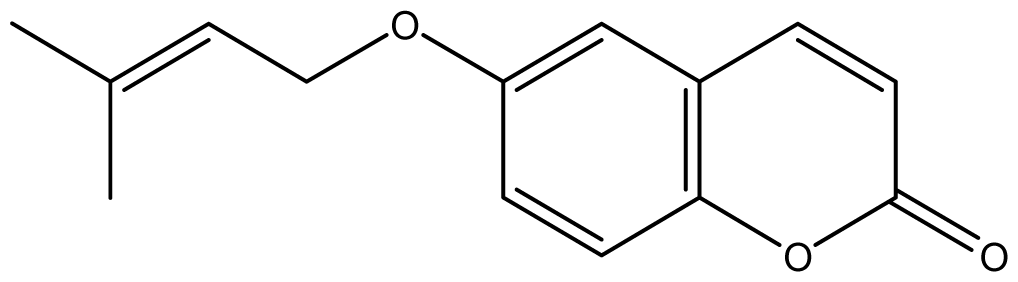 | ≥2048 (2.05) | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 |
| 18 | 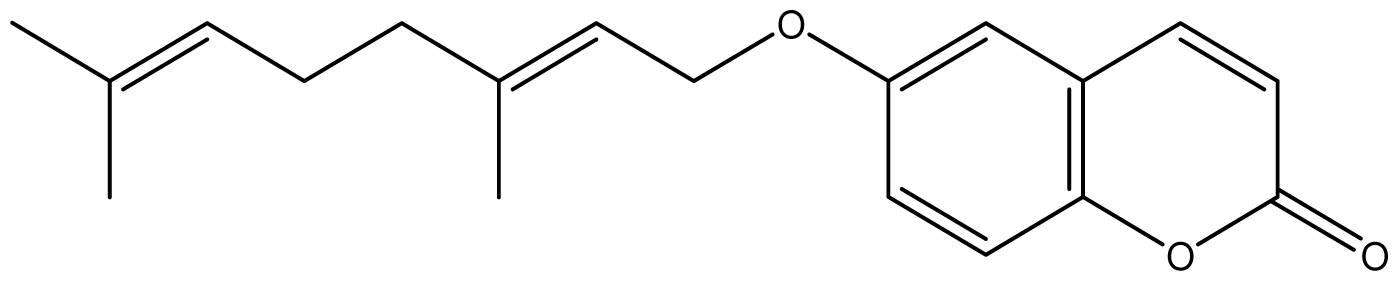 | ≥2048 (2.16) | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 |
| 19 | 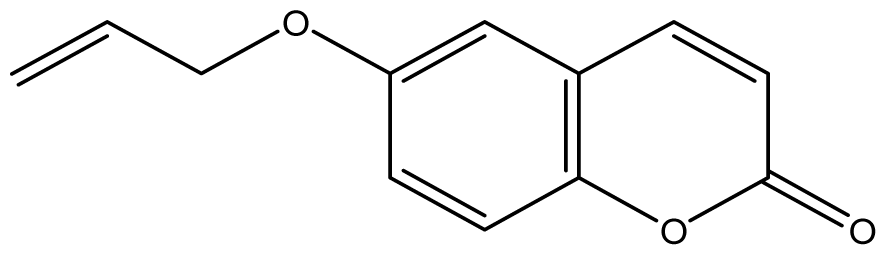 | ≥2048 (1.99) | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 |
| 20 | 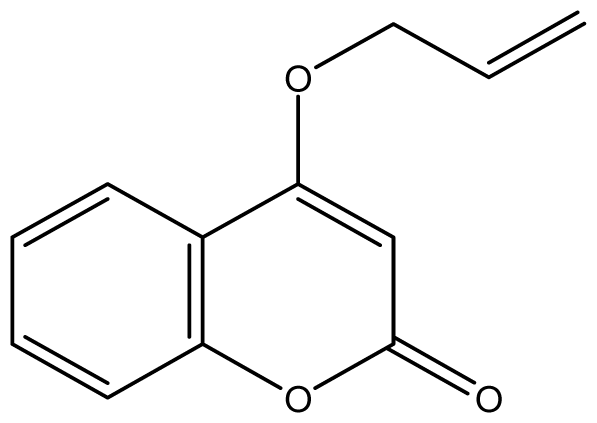 | 32 (3.80) | 64 | 32 | 32 | 1024 | 32 | 32 | 64 |
| 21 | 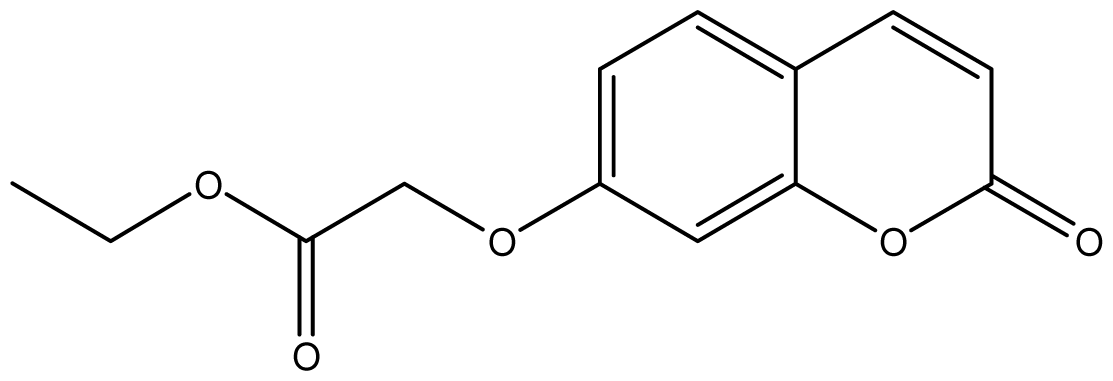 | 512 (2.68) | 512 | 512 | 512 | 512 | 512 | 512 | 512 |
| 22 | 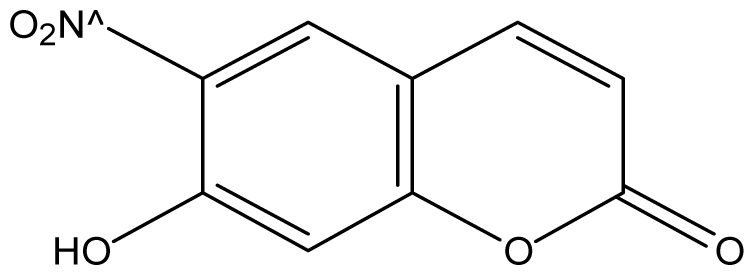 | 16 (4.11) | 16 | 16 | 16 | 16 | 16 | 16 | 16 |
| 23 | 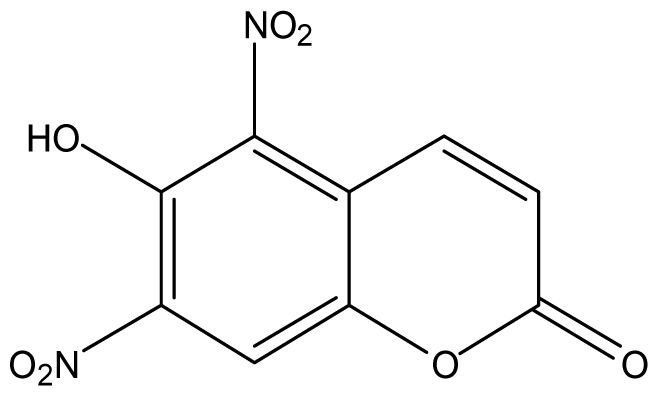 | 512 (2.69) | 512 | 512 | 512 | 512 | 512 | 512 | 512 |
| 24 | 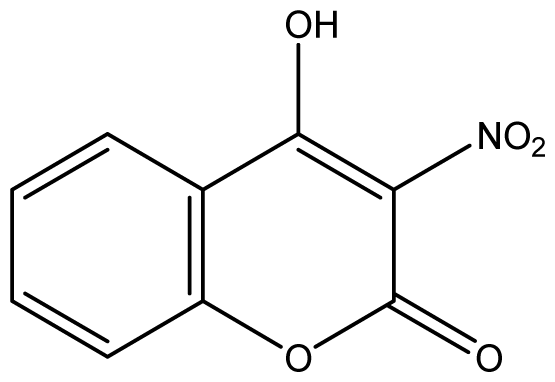 | 1024 (2.30) | 1024 | 1024 | 1024 | 1024 | 1024 | 1024 | 1024 |
| AmpB* | 2 | 2 | 2 | 2 | 8 | 2 | 2 | 512 | |
| PC | % explained variance from original data |
|---|---|
| 1 | 50.04 |
| 2 | 10.62 |
| 3 | 7.96 |
| 4 | 5.80 |
| 5 | 3.54 |
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
De Araújo, R.S.A.; Guerra, F.Q.S.; De O. Lima, E.; De Simone, C.A.; Tavares, J.F.; Scotti, L.; Scotti, M.T.; De Aquino, T.M.; De Moura, R.O.; Mendonça, F.J.B.; et al. Synthesis, Structure-Activity Relationships (SAR) and in Silico Studies of Coumarin Derivatives with Antifungal Activity. Int. J. Mol. Sci. 2013, 14, 1293-1309. https://doi.org/10.3390/ijms14011293
De Araújo RSA, Guerra FQS, De O. Lima E, De Simone CA, Tavares JF, Scotti L, Scotti MT, De Aquino TM, De Moura RO, Mendonça FJB, et al. Synthesis, Structure-Activity Relationships (SAR) and in Silico Studies of Coumarin Derivatives with Antifungal Activity. International Journal of Molecular Sciences. 2013; 14(1):1293-1309. https://doi.org/10.3390/ijms14011293
Chicago/Turabian StyleDe Araújo, Rodrigo S. A., Felipe Q. S. Guerra, Edeltrudes De O. Lima, Carlos A. De Simone, Josean F. Tavares, Luciana Scotti, Marcus T. Scotti, Thiago M. De Aquino, Ricardo O. De Moura, Francisco J. B. Mendonça, and et al. 2013. "Synthesis, Structure-Activity Relationships (SAR) and in Silico Studies of Coumarin Derivatives with Antifungal Activity" International Journal of Molecular Sciences 14, no. 1: 1293-1309. https://doi.org/10.3390/ijms14011293








